Diversity and Horizontal Transfer of Antarctic Pseudomonas spp. Plasmids
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Culture Conditions
2.2. DNA Manipulations and Introduction of Plasmid DNA into Bacterial Cells
2.3. Testing of the Host Range of the Pseudomonas Plasmids
2.4. Testing of Bacterial Adherence to an Artificial Surface
2.5. Statistical Analysis
2.6. Live Cell Confocal Microscopy (Biofilm Analysis)
2.7. Testing of the Functionality of Transposable Elements (TEs) Carried within the pA62H2 Plasmid
2.8. DNA Sequencing
2.9. Bioinformatics
2.10. Nucleotide Sequence Accession Numbers
3. Results and Discussion
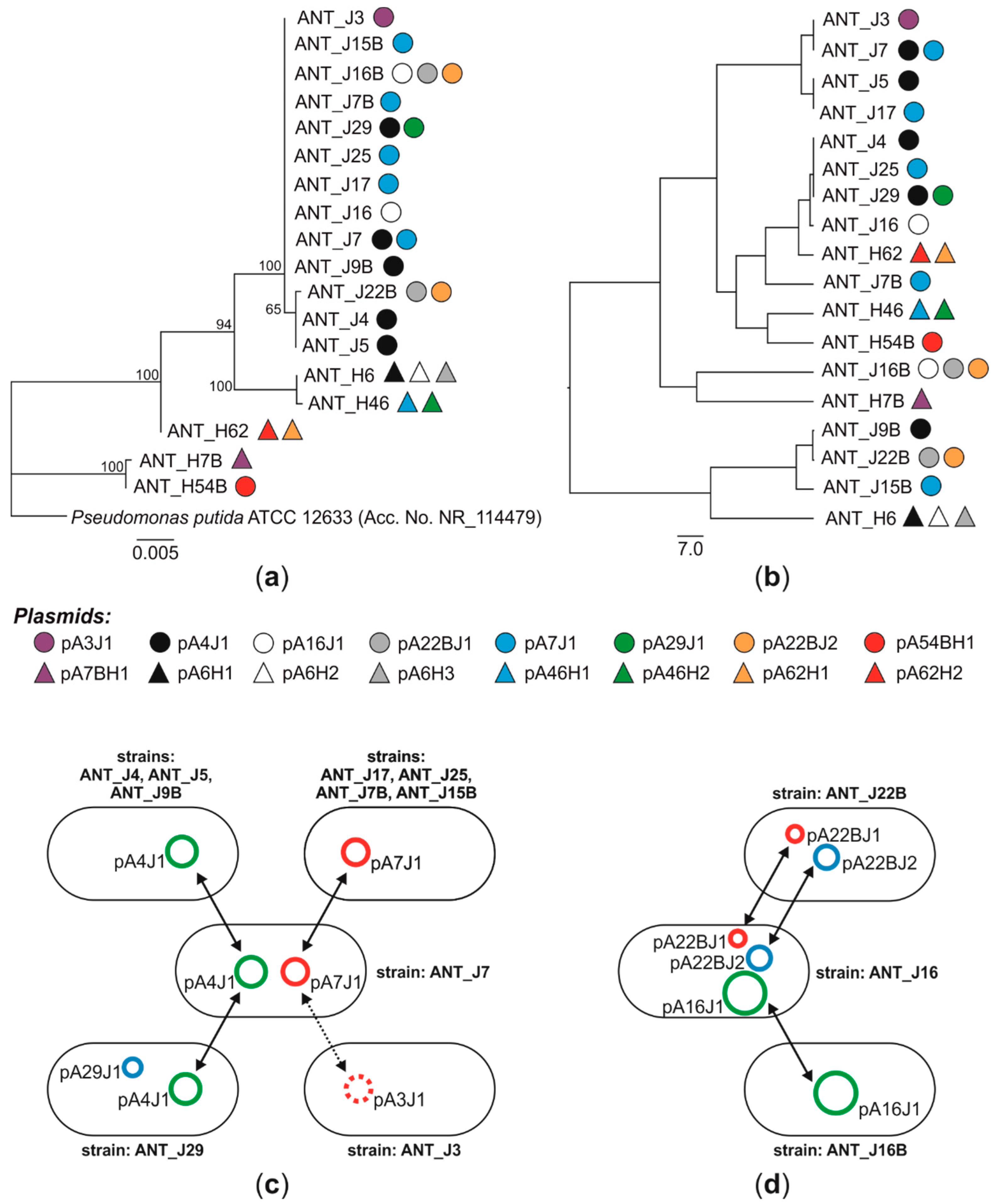
3.1. Distribution of Plasmids in Antarctic Pseudomonas spp.—Evidences for the Plasmid Horizontal Transfer
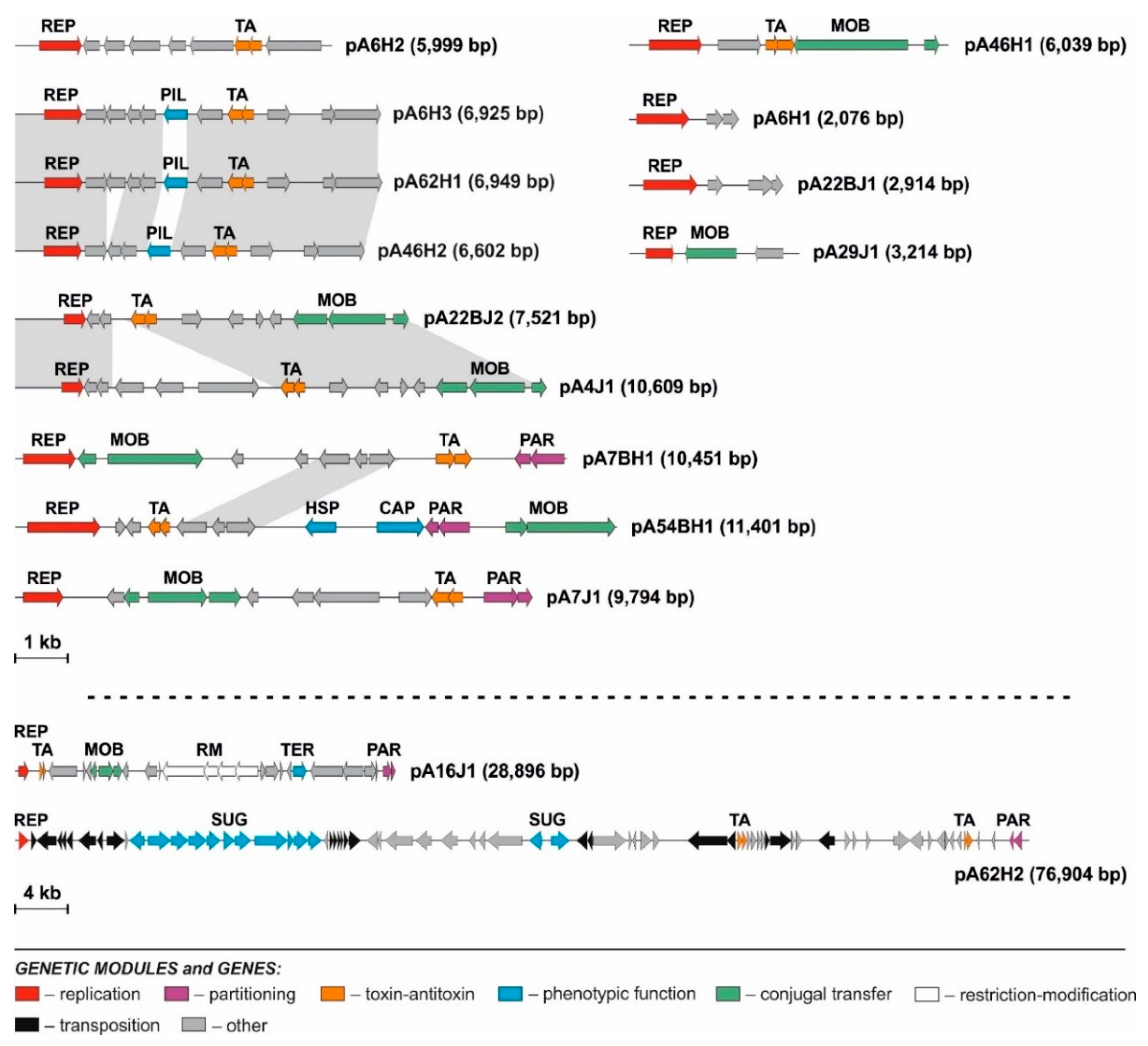
3.2. Diversity of Plasmids of Antarctic Pseudomonas spp.
3.2.1. Replication Modules
3.2.2. Mobilization to Conjugal Transfer Modules
3.2.3. Stabilization Modules
3.2.4. Predicted Phenotypic Modules
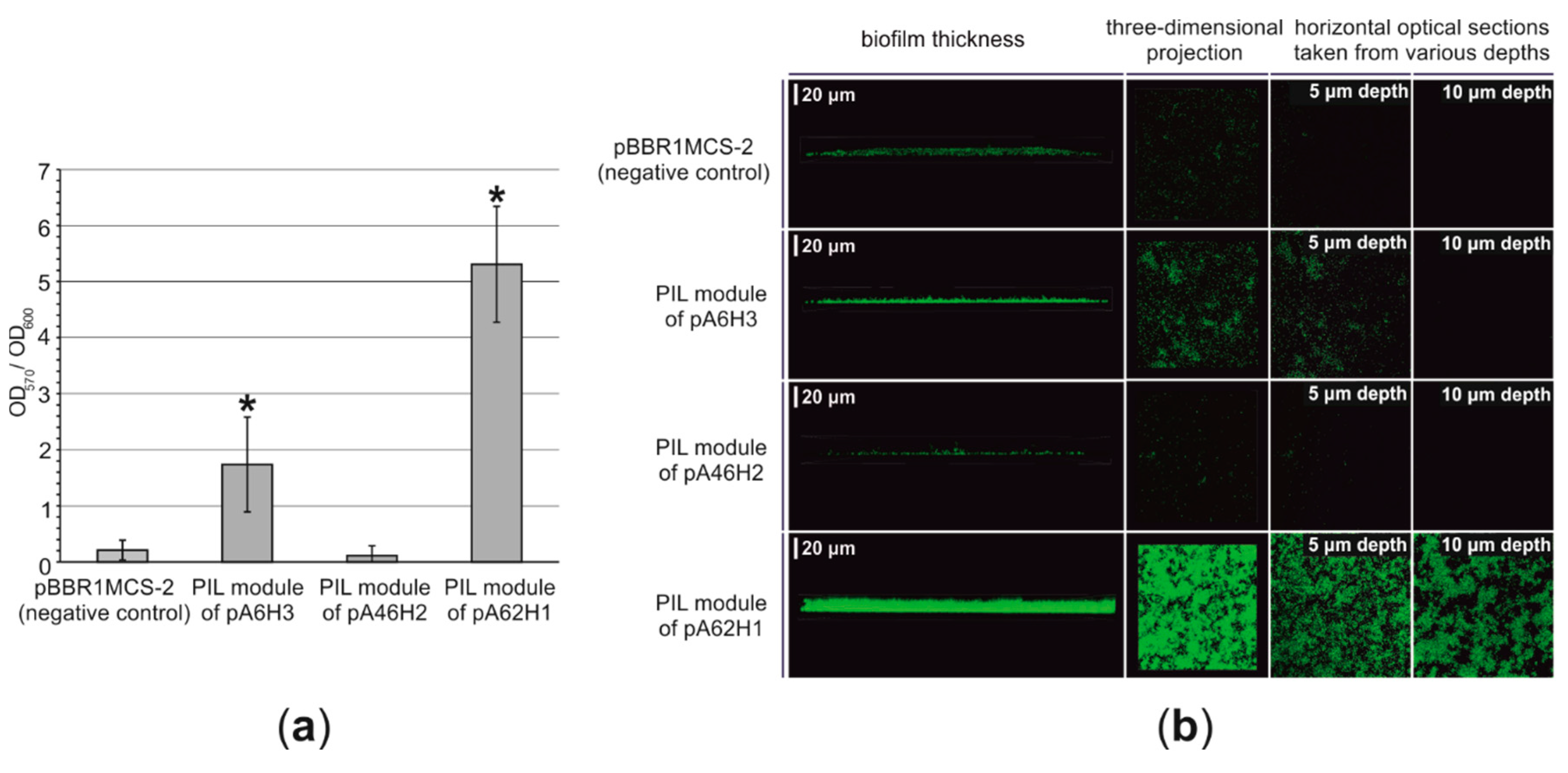
3.2.5. Functional Analysis of the PIL Modules—Biofilm Synthesis Testing
3.2.6. Transposable Elements Present in the Pseudomonas Plasmids
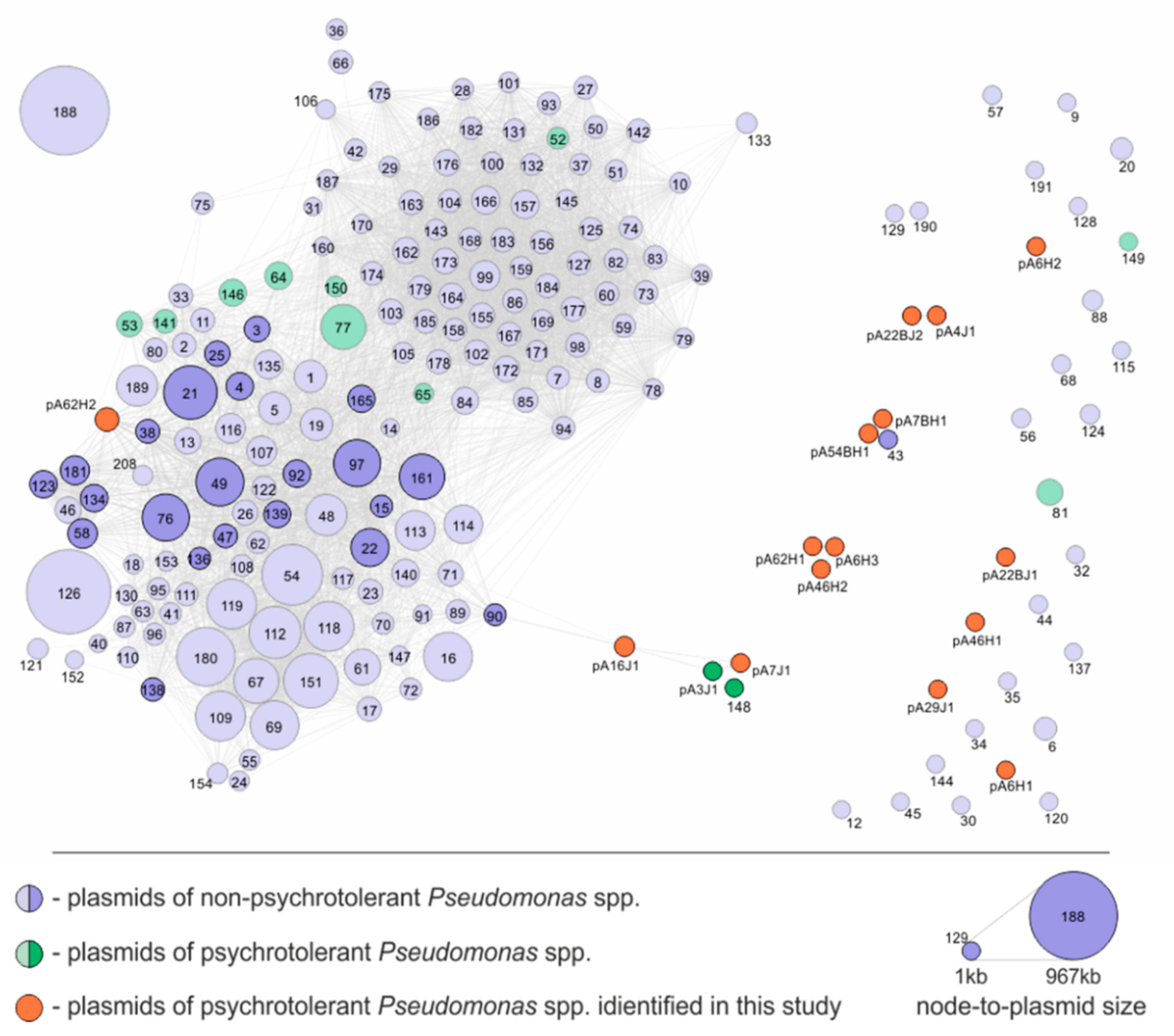
3.3. Comparative Analysis of the Pseudomonas spp. Plasmids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özen, A.I.; Ussery, D.W. Defining the Pseudomonas genus: Where do we draw the line with Azotobacter? Microb. Ecol. 2012, 63, 239–248. [Google Scholar] [CrossRef]
- Nitisakulkan, T.; Oku, S.; Kudo, D.; Nakashimada, Y.; Tajima, T.; Vangnai, A.S.; Kato, J. Degradation of chloroanilines by toluene dioxygenase from Pseudomonas putida T57. J. Biosci. Bioeng. 2014, 117, 292–297. [Google Scholar] [CrossRef]
- Pinjari, A.B.; Pandey, J.P.; Kamireddy, S.; Siddavattam, D. Expression and subcellular localization of organophosphate hydrolase in acephate-degrading Pseudomonas sp. strain Ind01 and its use as a potential biocatalyst for elimination of organophosphate insecticides. Lett. Appl. Microbiol. 2013, 57, 63–68. [Google Scholar] [CrossRef]
- Nielsen, M.N.; Sorensen, J.; Fels, J.; Pedersen, H.C. Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl. Environ. Microbiol. 1998, 64, 3563–3569. [Google Scholar]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Romaniuk, K.; Ciok, A.; Decewicz, P.; Uhrynowski, W.; Budzik, K.; Nieckarz, M.; Pawlowska, J.; Zdanowski, M.K.; Bartosik, D.; Dziewit, L. Insight into heavy metal resistome of soil psychrotolerant bacteria originating from King George Island (Antarctica). Polar Biol. 2018, 41, 1319–1333. [Google Scholar] [CrossRef]
- Kristoffersen, V.; Rämä, T.; Isaksson, J.; Andersen, J.H.; Gerwick, W.H.; Hansen, E. Characterization of rhamnolipids produced by an Arctic marine bacterium from the Pseudomonas fluorescence group. Mar. Drugs 2018, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Master, E.R.; Mohn, W.W. Psychrotolerant bacteria isolated from arctic soil that degrade polychlorinated biphenyls at low temperatures. Appl. Environ. Microbiol. 1998, 64, 4823–4829. [Google Scholar] [PubMed]
- Chauhan, A.; Bharti, P.K.; Goyal, P.; Varma, A.; Jindal, T. Psychrophilic Pseudomonas in antarctic freshwater lake at stornes peninsula, larsemann hills over east Antarctica. Springerplus 2015, 4, 582. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.M.; Singh, R.N.; Naik, S.; Roy, U.; Srivastava, A.; Bolter, M. Bacterial communities in ancient permafrost profiles of Svalbard, Arctic. J. Basic Microbiol. 2017, 57, 1018–1036. [Google Scholar] [CrossRef]
- González-Rocha, G.; Muñoz-Cartes, G.; Canales-Aguirre, C.B.; Lima, C.A.; Domínguez-Yévenes, M.; Bello-Toledo, H.; Hernández, C.E. Diversity structure of culturable bacteria isolated from the Fildes Peninsula (King George Island, Antarctica): A phylogenetic analysis perspective. PLoS ONE 2017, 12, e0179390. [Google Scholar] [CrossRef] [PubMed]
- Baltrus, D.A.; Nishimura, M.T.; Romanchuk, A.; Chang, J.H.; Mukhtar, M.S.; Cherkis, K.; Roach, J.; Grant, S.R.; Jones, C.D.; Dangl, J.L. Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19 Pseudomonas syringae isolates. PLoS Pathog. 2011, 7, e1002132. [Google Scholar] [CrossRef] [PubMed]
- Greated, A.; Lambertsen, L.; Williams, P.A.; Thomas, C.M. Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol. 2002, 4, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Dunn, N.W.; Gunsalus, I.C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 1973, 114, 974–979. [Google Scholar]
- Hernández-Ramírez, K.C.; Reyes-Gallegos, R.I.; Chávez-Jacobo, V.M.; Díaz-Magaña, A.; Meza-Carmen, V.; Ramírez-Díaz, M.I. A plasmid-encoded mobile genetic element from Pseudomonas aeruginosa that confers heavy metal resistance and virulence. Plasmid 2018, 98, 15–21. [Google Scholar] [CrossRef]
- Dziewit, L.; Grzesiak, J.; Ciok, A.; Nieckarz, M.; Zdanowski, M.K.; Bartosik, D. Sequence determination and analysis of three plasmids of Pseudomonas sp. GLE121, a psychrophile isolated from surface ice of Ecology Glacier (Antarctica). Plasmid 2013, 70, 254–262. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.-J.; Yang, J.Y.; Jung, Y.-J.; Hong, S.G.; Kim, O.-S. Complete genome sequence of Pseudomonas antarctica PAMC 27494, a bacteriocin-producing psychrophile isolated from Antarctica. J. Biotechnol. 2017, 259, 15–18. [Google Scholar] [CrossRef]
- Park, A.K.; Kim, H.; Kim, I.-S.; Roh, S.J.; Shin, S.C.; Lee, J.H.; Park, H.; Kim, H.-W. Crystal structure of cis-dihydrodiol naphthalene dehydrogenase (NahB) from Pseudomonas sp. MC1: Insights into the early binding process of the substrate. Biochem. Biophys. Res. Commun. 2017, 491, 403–408. [Google Scholar] [CrossRef]
- Dziewit, L.; Pyzik, A.; Szuplewska, M.; Matlakowska, R.; Mielnicki, S.; Wibberg, D.; Schlüter, A.; Pühler, A.; Bartosik, D. Diversity and role of plasmids in adaptation of bacteria inhabiting the Lubin copper mine in Poland, an environment rich in heavy metals. Front. Microbiol. 2015, 6, 152. [Google Scholar] [CrossRef]
- Hooykaas, P.J.; den Dulk-Ras, H.; Schilperoort, R.A. Molecular mechanism of Ti plasmid mobilization by R plasmids: Isolation of Ti plasmids with transposon-insertions in Agrobacterium tumefaciens. Plasmid 1980, 4, 64–75. [Google Scholar] [CrossRef]
- Ludtke, D.N.; Eichorn, B.G.; Austin, S.J. Plasmid-partition functions of the P7 prophage. J. Mol. Biol. 1989, 209, 393–406. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Bartosik, A.A.; Glabski, K.; Jecz, P.; Mikulska, S.; Fogtman, A.; Koblowska, M.; Jagura-Burdzy, G. Transcriptional profiling of ParA and ParB mutants in actively dividing cells of an opportunistic human pathogen Pseudomonas aeruginosa. PLoS ONE 2014, 9, e87276. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, W.D.; Pehl, M.J.; Gregory, G.A.; Orwin, P.M. Coordinated surface activities in Variovorax paradoxus EPS. BMC Microbiol. 2009, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, D.; Bialkowska, A.; Baj, J.; Wlodarczyk, M. Construction of mobilizable cloning vectors derived from pBGS18 and their application for analysis of replicator region of a pTAV202 mini-derivative of Paracoccus versutus pTAV1 plasmid. Acta Microbiol. Pol. 1997, 46, 387–392. [Google Scholar]
- Romaniuk, K.; Krucon, T.; Decewicz, P.; Gorecki, A.; Dziewit, L. Molecular characterization of the pA3J1 plasmid from the psychrotolerant Antarctic bacterium Pseudomonas sp. ANT_J3. Plasmid 2017, 92, 49–56. [Google Scholar] [CrossRef]
- Kovach, M.E.; Phillips, R.W.; Elzer, P.H.; Roop 2nd, R.M.; Peterson, K.M. pBBR1MCS: A broad-host-range cloning vector. Biotechniques 1994, 16, 800–802. [Google Scholar]
- Szuplewska, M.; Bartosik, D. Identification of a mosaic transposable element of Paracoccus marcusii composed of insertion sequence ISPmar4 (ISAs1 family) and an IS1247a-driven transposable module (TMo). FEMS Microbiol. Lett. 2009, 292, 216–221. [Google Scholar] [CrossRef]
- Ditta, G.; Stanfield, S.; Corbin, D.; Helinski, D.R. Broad host range DNA cloning system for gram-negative bacteria: Construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 1980, 77, 7347–7351. [Google Scholar] [CrossRef]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Bartosik, D.; Szymanik, M.; Wysocka, E. Identification of the partitioning site within the repABC-type replicon of the composite Paracoccus versutus plasmid pTAV1. J. Bacteriol. 2001, 183, 6234–6243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kushner, S.R. An improved method for transformation of E. coli with ColE1 derived plasmids. In Genetic Engineering; Boyer, H.B., Nicosia, S., Eds.; Elsevier/North-Holland: Amsterdam, The Netherlands, 1978; pp. 17–23. [Google Scholar]
- Irani, V.R.; Rowe, J.J. Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. Biotechniques 1997, 22, 54–56. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, A.; Bacal, P.; Wasiluk, A.; Trybunko, A.; Adamczyk-Poplawska, M. The dam replacing gene product enhances Neisseria gonorrhoeae FA1090 viability and biofilm formation. Front. Microbiol. 2014, 5, 712. [Google Scholar] [CrossRef]
- Gay, P.; Le Coq, D.; Steinmetz, M.; Berkelman, T.; Kado, C.I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 1985, 164, 918–921. [Google Scholar]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 22 October 2019).
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Dodd, I.B.; Egan, J.B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990, 18, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Martin, M.J.; O’Donovan, C. UniProt Protein Knowledgebase. Methods. Mol. Biol. 2017, 1558, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Darzentas, N. Circoletto: Visualizing sequence similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef]
- Poszytek, K.; Karczewska-Golec, J.; Ciok, A.; Decewicz, P.; Dziurzynski, M.; Gorecki, A.; Jakusz, G.; Krucon, T.; Lomza, P.; Romaniuk, K.; et al. Genome-guided characterization of Ochrobactrum sp. POC9 enhancing sewage sludge utilization—Biotechnological potential and biosafety considerations. Int. J. Environ. Res. Public Health 2018, 15, 1501. [Google Scholar] [CrossRef]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Zhou, D.; Feng, J.; Zhan, Z.; Xiong, Z.; Yang, B.; Liu, Z.; Li, T.; Tong, Y.; et al. Comparative genomics of five different resistance plasmids coexisting in a clinical multi-drug resistant Citrobacter freundii isolate. Infect. Drug Resist. 2018, 11, 1447–1460. [Google Scholar] [CrossRef]
- Maj, A.; Dziewit, L.; Czarnecki, J.; Wlodarczyk, M.; Baj, J.; Skrzypczyk, G.; Giersz, D.; Bartosik, D. Plasmids of carotenoid-producing Paracoccus spp. (Alphaproteobacteria)—Structure, diversity and evolution. PLoS ONE 2013, 8, e80258. [Google Scholar] [CrossRef]
- Martinez-Rosales, C.; Fullana, N.; Musto, H.; Castro-Sowinski, S. Antarctic DNA moving forward: Genomic plasticity and biotechnological potential. FEMS Microbiol. Lett. 2012, 331, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, S.; Tschitschko, B.; Zhong, L.; Raftery, M.J.; Cavicchioli, R. A plasmid from an Antarctic haloarchaeon uses specialized membrane vesicles to disseminate and infect plasmid-free cells. Nat. Microbiol. 2017, 2, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Grossi, C.; Nereo, S.; Michaud, L.; Lo Giudice, A.; Bruni, V.; Baldi, F.; Fani, R. Molecular and physiological characterisation of psychrotrophic hydrocarbon-degrading bacteria isolated from Terra Nova Bay (Antarctica). Eur. J. Soil Biol. 2007, 43, 368–379. [Google Scholar] [CrossRef]
- Giménez, M.; Azziz, G.; Gill, P.R.; Batista, S. Horizontal Gene Transfer Elements: Plasmids in Antarctic Microorganisms. In The Ecological Role of Micro-Organisms in the Antarctic Environment; Castro-Sowinski, S., Ed.; Springer Polar Sciences; Springer: Cham, Switzerland, 2019; pp. 85–107. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Shao, Z. Pseudomonas, the dominant polycyclic aromatic hydrocarbon-degrading bacteria isolated from Antarctic soils and the role of large plasmids in horizontal gene transfer. Environ. Microbiol. 2006, 8, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Haines, A.S.; Kosheleva, I.A.; Boronin, A. Pseudomonas plasmids. In Pseudomonas: Model Organism, Pathogen, Cell Factory; Rehm, B.H.A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Thomas, C.M. Paradigms of plasmid organization. Mol. Microbiol. 2000, 37, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
- del Solar, G.; Giraldo, R.; Ruiz-Echevarria, M.J.; Espinosa, M.; Diaz-Orejas, R. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 1998, 62, 434–464. [Google Scholar]
- Konieczny, I.; Bury, K.; Wawrzycka, A.; Wegrzyn, K. Iteron Plasmids. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Dziewit, L.; Cegielski, A.; Romaniuk, K.; Uhrynowski, W.; Szych, A.; Niesiobedzki, P.; Zmuda-Baranowska, M.J.; Zdanowski, M.K.; Bartosik, D. Plasmid diversity in arctic strains of Psychrobacter spp. Extremophiles 2013, 17, 433–444. [Google Scholar] [CrossRef][Green Version]
- Ciok, A.; Budzik, K.; Zdanowski, M.K.; Gawor, J.; Grzesiak, J.; Decewicz, P.; Gromadka, R.; Bartosik, D.; Dziewit, L. Plasmids of psychrotolerant Polaromonas spp. isolated from arctic and antarctic glaciers—diversity and role in adaptation to polar environments. Front. Microbiol. 2018, 9, 1285. [Google Scholar] [CrossRef]
- Ciok, A.; Dziewit, L.; Grzesiak, J.; Budzik, K.; Gorniak, D.; Zdanowski, M.K.; Bartosik, D. Identification of miniature plasmids in psychrophilic Arctic bacteria of the genus Variovorax. FEMS Microbiol. Ecol. 2016, 92, fiw043. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, T.; Misevic, D.; Lotton, C.; Brown, S.P.; Lindner, A.B.; Taddei, F. Indirect fitness benefits enable the spread of host genes promoting costly transfer of beneficial plasmids. PLoS Biol. 2016, 14, e1002478. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, M.; Virta, M.; Fani, R.; Fondi, M. Large-scale analysis of plasmid relationships through gene-sharing networks. Mol. Biol. Evol. 2012, 29, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Mc Ginty, S.É.; Lehmann, L.; Brown, S.P.; Rankin, D.J. The interplay between relatedness and horizontal gene transfer drives the evolution of plasmid-carried public goods. Proceed. Biol. Sci. 2013, 280, 20130400. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, T.; Marchant, L.; Buckling, A.; Raymond, B. Bacteria from natural populations transfer plasmids mostly towards their kin. Proceed. Biol. Sci. 2019, 286, 20191110. [Google Scholar] [CrossRef] [PubMed]
- Francia, M.V.; Varsaki, A.; Garcillan-Barcia, M.P.; Latorre, A.; Drainas, C.; de la Cruz, F. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 2004, 28, 79–100. [Google Scholar] [CrossRef]
- Garcillan-Barcia, M.P.; Francia, M.V.; de la Cruz, F. The diversityof conjugative relaxases and its application in plasmidclassification. FEMS Microbiol. Rev. 2009, 33, 657–687. [Google Scholar] [CrossRef]
- Moller-Jensen, J.; Jensen, R.B.; Gerdes, K. Plasmid and chromosome segregation in prokaryotes. Trends Microbiol. 2000, 8, 313–320. [Google Scholar] [CrossRef]
- Hayes, F. Toxins-antitoxins: Plasmid maintenance, programmed cell death, and cell cycle arrest. Science 2003, 301, 1496–1499. [Google Scholar] [CrossRef]
- Pedersen, K.; Zavialov, A.V.; Pavlov, M.Y.; Elf, J.; Gerdes, K.; Ehrenberg, M. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 2003, 112, 131–140. [Google Scholar] [CrossRef]
- Munoz-Villagran, C.M.; Mendez, K.N.; Cornejo, F.; Figueroa, M.; Undabarrena, A.; Morales, E.H.; Arenas-Salinas, M.; Arenas, F.A.; Castro-Nallar, E.; Vasquez, C.C. Comparative genomic analysis of a new tellurite-resistant Psychrobacter strain isolated from the Antarctic Peninsula. PeerJ 2018, 6, e4402. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Gonzalez, M.A.; Diaz-Vasquez, W.A.; Ruiz-Leon, D.; Becerra, A.A.; Aguayo, D.R.; Perez-Donoso, J.M.; Vasquez, C.C. A comparative analysis of tellurite detoxification by members of the genus Shewanella. Arch. Microbiol. 2018, 200, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rojas, F.; Tapia, P.; Castro-Nallar, E.; Undabarrena, A.; Munoz-Diaz, P.; Arenas-Salinas, M.; Diaz-Vasquez, W.; Valdes, J.; Vasquez, C. Draft genome sequence of a multi-metal resistant bacterium Pseudomonas putida ATH-43 isolated from Greenwich Island, Antarctica. Front. Microbiol. 2016, 7, 1777. [Google Scholar] [CrossRef]
- Arenas, F.A.; Pugin, B.; Henríquez, N.A.; Arenas-Salinas, M.A.; Diaz-Vasquez, W.A.; Pozo, M.F.; Munoz, C.M.; Chasteen, T.G.; Perez-Donoso, J.M.; Vasquez, C.C. Isolation, identification and characterization of highly tellurite-resistant, tellurite-reducing bacteria from Antarctica. Polar Sci. 2014, 8, 40–52. [Google Scholar] [CrossRef]
- Romaniuk, K.; Golec, P.; Dziewit, L. Insight into the diversity and possible role of plasmids in the adaptation of psychrotolerant and metalotolerant Arthrobacter spp. to extreme Antarctic environments. Front. Microbiol. 2018, 9, 3144. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Potential applications of the Escherichia coli heat shock response in synthetic biology. Trends Biotechnol. 2018, 36, 186–198. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, A.; Wang, M. Polysaccharide export outer membrane proteins in Gram-negative bacteria. Future Microbiol. 2013, 8, 525–535. [Google Scholar] [CrossRef]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Ciok, A.; Cegielski, A.; Bartosik, D.; Dziewit, L. Benefits and drawbacks of harboring plasmid pP32BP2, identified in arctic psychrophilic bacterium Psychrobacter sp. DAB_AL32B. Int. J. Mol. Sci. 2019, 20, 2015. [Google Scholar] [CrossRef] [PubMed]
- Hinsa-Leasure, S.M.; Koid, C.; Tiedje, J.M.; Schultzhaus, J.N. Biofilm formation by Psychrobacter arcticus and the role of a large adhesin in attachment to surfaces. Appl. Environ. Microbiol. 2013, 79, 3967–3973. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C. Biofilms: Microbial strategies for surviving UV exposure. Adv. Exp. Med. Biol. 2017, 996, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vorkapic, D.; Pressler, K.; Schild, S. Multifaceted roles of extracellular DNA in bacterial physiology. Curr. Genet. 2016, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lauf, U.; Müller, C.; Herrmann, H. The transposable elements resident on the plasmids of Pseudomonas putida strain H, Tn5501 and Tn5502, are cryptic transposons of the Tn3 family. Mol. Gen. Genet. 1998, 259, 674–678. [Google Scholar] [CrossRef]
- Molina, L.; Udaondo, Z.; Duque, E.; Fernández, M.; Molina-Santiago, C.; Roca, A.; Porcel, M.; de la Torre, J.; Segura, A.; Plesiat, P.; et al. Antibiotic resistance determinants in a Pseudomonas putida strain isolated from a hospital. PLoS ONE 2014, 9, e81604. [Google Scholar] [CrossRef]
- Li, W.; Shi, J.; Wang, X.; Han, Y.; Tong, W.; Ma, L.; Liu, B.; Cai, B. Complete nucleotide sequence and organization of the naphthalene catabolic plasmid pND6-1 from Pseudomonas sp. strain ND6. Gene 2004, 336, 231–240. [Google Scholar] [CrossRef]
- Dziewit, L.; Jazurek, M.; Drewniak, L.; Baj, J.; Bartosik, D. The SXT conjugative element and linear prophage N15 encode toxin-antitoxin-stabilizing systems homologous to the tad-ata module of the Paracoccus aminophilus plasmid pAMI2. J. Bacteriol. 2007, 189, 1983–1997. [Google Scholar] [CrossRef]
- Nagy, Z.; Chandler, M. Regulation of transposition in bacteria. Res. Microbiol. 2004, 155, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, M.; Ludwiczak, M.; Lyzwa, K.; Czarnecki, J.; Bartosik, D. Mobility and generation of mosaic non-autonomous transposons by Tn3-derived inverted-repeat miniature elements (TIMEs). PLoS ONE 2014, 9, e105010. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Plasmid Name | Characteristics 1 | Reference |
|---|---|---|
| pABW1 | Kmr; ori pMB1; Mob+; oriT RK2; lacZα; MCS | [25] |
| pABW1-R | pABW1 carrying the REP module of pA3J1 (SphI/EcoRI restriction fragment) | [26] |
| pABW1-REP4J1 | pABW1 carrying the REP module of pA4J1 (XbaI restriction fragment) | This work |
| pABW1-REP6H1 | pABW1 carrying the REP module of pA6H1 (PCR- amplified with the primers LREP6H1 and RREP6H1) | This work |
| pABW1-REP6H2 | pABW1 carrying the REP module of pA6H2 (PCR- amplified with primers LREP6H2 and RREP6H2) | This work |
| pABW1-REP6H3 | pABW1 carrying the REP module of pA6H3 (PCR- amplified with the primers LREP6H3 and RREP6H3) | This work |
| pABW1-REP7J1 | pABW1 carrying the REP module of pA7J1 (PCR- amplified with the primers LREP7J1 and RREP7J1) | This work |
| pABW1-REP16J1 | pABW1 carrying the REP module of pA16J1 (PCR- amplified with the primers LREP16J1 and RREP16J1) | This work |
| pABW1-REP22BJ1 | pABW1 carrying the REP module of pA22BJ1 (PCR- amplified with the primers LREP22BJ1 and RREP22BJ1) | This work |
| pABW1-REP22BJ2 | pABW1 carrying REP the module of pA22BJ2 (PCR- amplified with the primers LREP22BJ2 and RREP22BJ2) | This work |
| pABW1-REP29J1 | pABW1 carrying the REP module of pA29J1 (PCR- amplified with the primers LREP29J1 and RREP29J1) | This work |
| pABW1-REP46H1 | pABW1 carrying the REP module of pA46H1 (PCR- amplified with the primers LREP46H1 and RREP46H1) | This work |
| pABW1-REP46H2 | pABW1 carrying the REP module of pA46H2 (PCR- amplified with the primers LREP46H2 and RREP46H2) | This work |
| pABW1-REP62H1 | pABW1 carrying the REP module of pA62H1 (PCR- amplified with the primers LREP62H1 and RREP62H1) | This work |
| pABW1-REP62H2 | pABW1 carrying the REP module of pA62H2 (PCR- amplified with the primers LREP62H2 and RREP62H2) | This work |
| pABW1-REP7BH1 | pABW1 carrying the REP module of pA7BH1 (PCR- amplified with the primers LREP7BH1 and RREP7BH1) | This work |
| pABW1-REP54BH1 | pABW1 carrying the REP module of pA54BH1 (PCR- amplified with the primers LREP54BH1 and RREP54BH1) | This work |
| pBBR1MCS-2 | Kmr; ori pBBR1; lacZα; MCS | [27] |
| pBBR1-Pil6 | pBBR1MCS-2 carrying the PIL module of pA6H3 (PCR- amplified with the primers LPIL and RPIL) | This work |
| pBBR1-Pil46 | pBBR1MCS-2 carrying the PIL module of pA46H2 (PCR- amplified with the primers LPIL and RPIL) | This work |
| pBBR1-Pil62 | pBBR1MCS-2 carrying the PIL module of pA62H1 (PCR- amplified with the primers LPIL and RPIL) | This work |
| pMAT1 | Kmr; ori pBBR1; sacB; entrapment plasmid | [28] |
| pRK2013 | Kmr; helper plasmid carrying genes for conjugal transfer of RK2 | [29] |
| 9 | GenBank Accession Number | Host Strain | Plasmid Size (bp) | GC Content (%) | No. of Genes | Genetic Modules 1 |
|---|---|---|---|---|---|---|
| pA4J1 | MK376337 | ANT_J4, ANT_J5, ANT_J7, ANT_J29, ANT_J9B | 10,609 | 53.77 | 15 | MOB, REP, TA |
| pA6H1 | MK376338 | ANT_H6 | 2076 | 53.37 | 3 | REP |
| pA6H2 | MK376339 | ANT_H6 | 5999 | 43.69 | 9 | REP, TA |
| pA6H3 | MK376340 | ANT_H6 | 6925 | 54.69 | 12 | PIL, REP, TA |
| pA7BH1 | MK376341 | ANT_H7B | 10,451 | 53.19 | 12 | MOB, PAR, REP, TA |
| pA7J1 | MK376342 | ANT_J7, ANT_J17, ANT_J25, ANT_J7B, ANT_J15B | 9794 | 50.61 | 13 | MOB, PAR, REP, TA |
| pA16J1 | MK376343 | ANT_J16, ANT_J16B | 28,896 | 56.00 | 27 | MOB, PAR, REP, RM, TA, TER |
| pA22BJ1 | MK376344 | ANT_J16B, ANT_J22B | 2914 | 50.72 | 4 | REP |
| pA22BJ2 | MK376345 | ANT_J16B, ANT_J22B | 7521 | 53.56 | 12 | MOB, REP, TA |
| pA29J1 | MK376346 | ANT_J29 | 3214 | 58.03 | 3 | MOB, REP |
| pA46H1 | MK376347 | ANT_H46 | 5039 | 57.63 | 6 | MOB, REP, TA |
| pA46H2 | MK376348 | ANT_H46 | 6602 | 54.18 | 11 | PIL, REP, TA |
| pA54BH1 | MK376349 | ANT_H54B | 11,401 | 52.36 | 14 | CAP, HSP, MOB, PAR, REP, TA |
| pA62H1 | MK376350 | ANT_H62 | 6949 | 54.94 | 12 | PIL, REP, TA |
| pA62H2 | MK376351 | ANT_H62 | 76,906 | 53.35 | 74 | PAR, REP, SUG, TA, TE |
| TE | Family (Group) | Location in pA62H2 (Coordinates) | TE Length (bp) | IR (bp) 1 | DR Sequence |
|---|---|---|---|---|---|
| ISPsp13 | IS4 (IS4) | 6917–8340 | 1424 | 17/18 | 5’-GCTCCAAGAAC-3’ |
| ISPsp14 | IS3 (IS3) | 42,584–43,820 | 1237 | 22/27 | 5’-AAT-3’ |
| Tn5501 | Tn3 | 50,967–56,596 | 5630 | 36/38 | Not determined |
| ISPsp15 | IS256 | 60,847–62,210 | 1364 | 23/27 | 5’-CTT-3’ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaniuk, K.; Styczynski, M.; Decewicz, P.; Buraczewska, O.; Uhrynowski, W.; Fondi, M.; Wolosiewicz, M.; Szuplewska, M.; Dziewit, L. Diversity and Horizontal Transfer of Antarctic Pseudomonas spp. Plasmids. Genes 2019, 10, 850. https://doi.org/10.3390/genes10110850
Romaniuk K, Styczynski M, Decewicz P, Buraczewska O, Uhrynowski W, Fondi M, Wolosiewicz M, Szuplewska M, Dziewit L. Diversity and Horizontal Transfer of Antarctic Pseudomonas spp. Plasmids. Genes. 2019; 10(11):850. https://doi.org/10.3390/genes10110850
Chicago/Turabian StyleRomaniuk, Krzysztof, Michal Styczynski, Przemyslaw Decewicz, Oliwia Buraczewska, Witold Uhrynowski, Marco Fondi, Marcin Wolosiewicz, Magdalena Szuplewska, and Lukasz Dziewit. 2019. "Diversity and Horizontal Transfer of Antarctic Pseudomonas spp. Plasmids" Genes 10, no. 11: 850. https://doi.org/10.3390/genes10110850
APA StyleRomaniuk, K., Styczynski, M., Decewicz, P., Buraczewska, O., Uhrynowski, W., Fondi, M., Wolosiewicz, M., Szuplewska, M., & Dziewit, L. (2019). Diversity and Horizontal Transfer of Antarctic Pseudomonas spp. Plasmids. Genes, 10(11), 850. https://doi.org/10.3390/genes10110850







