Exercise-Induced Changes in Bioactive Lipids Might Serve as Potential Predictors of Post-Exercise Hypotension. A Pilot Study in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
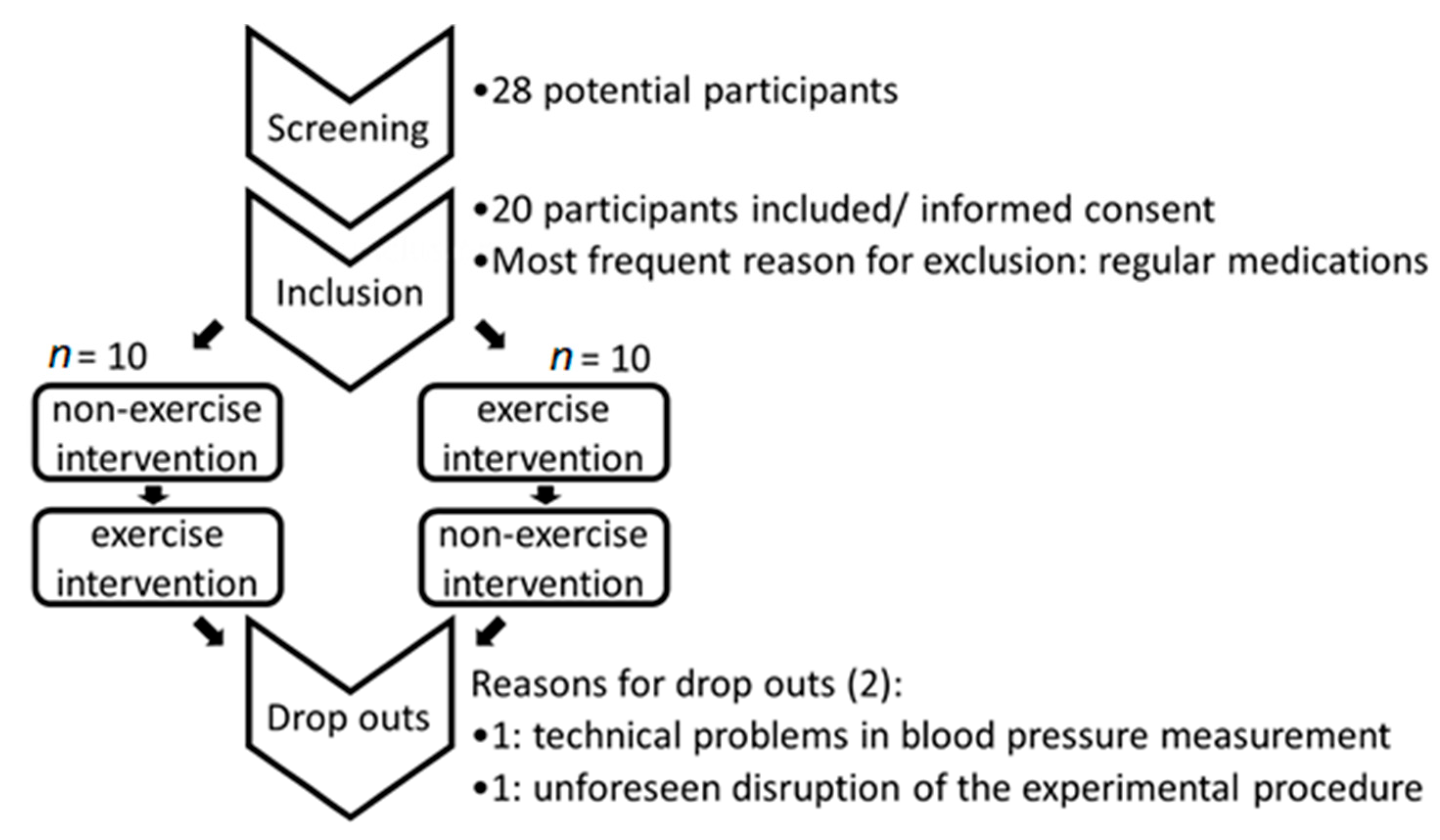
2.1. Study Design
2.2. Endpoints of the Study
2.3. Inclusion Criteria
- Age between 18 and 35 years.
- Caucasian.
- Written consent.
- Sufficient body fitness to perform exercise intervention.
2.4. Exclusion Criteria
- Existing diseases that are associated with a health risk in combination with exercise at 80% of maximal heart rate (e.g., Angina pectoris, malign arrhythmia; bronchial asthma).
- Existing diseases which hinder exercise performance (e.g., peripheral arterial occlusion, severe respiratory disease, painful disease of musculoskeletal system).
- Existing disease with increased risk of injury during exercise (e.g., epilepsy; disturbance of equilibrium).
- Pathological changes in ECG.
- Pathological blood count, increased inflammatory parameters in screening examination.
- Pregnancy.
- Acute disease in the last 14 days before exercise intervention.
- Psychiatric disorder which does not allow voluntary oral consent.
- Chronic drug administration (except oral contraceptives) or drug administration in the last 48 h prior to exercise intervention.
- Known hypertonia or hypertonia as diagnosed in screening examination.
- Consumption of caffeine in the last 12 h before intervention.
- Intake of licorice or grapefruit 48 h before intervention.
2.5. Exercise Protocol, Blood Pressure Determination and Blood Sample Preparation
2.6. Blood Withdrawal and Plasma Preparation
2.7. Measurement of Hematocrit
2.8. Sample Preparation and LC-MS/MS Analysis of Lipid Mediators
2.9. Statistics
3. Results
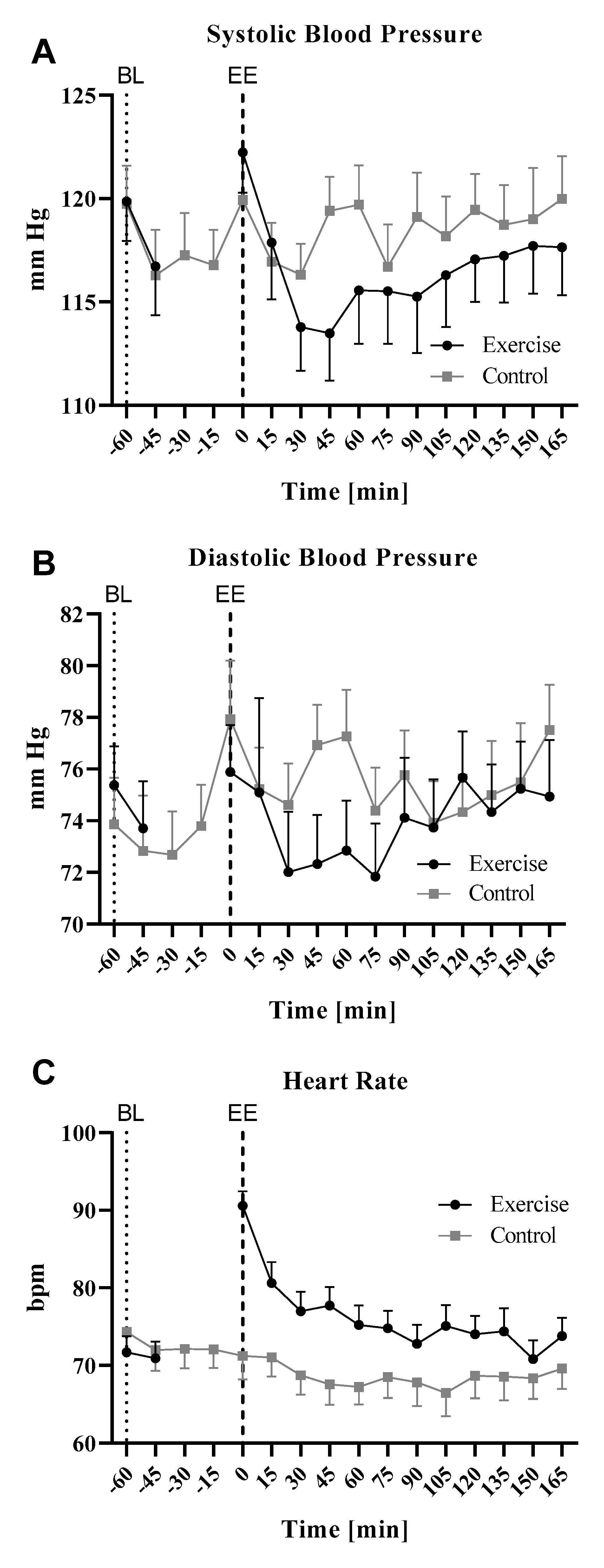
3.1. Effects of Cycling Exercise on Heart Rate and Blood Pressure
3.2. Effects of Exercise on Bioactive Plasma Lipids
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Ozemek, C.; Tiwari, S.C.; Sabbahi, A.; Carbone, S.; Lavie, C.J. Impact of therapeutic lifestyle changes in resistant hypertension. Prog. Cardiovasc. Dis. 2020, 63, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Prescribing exercise as preventive therapy. Can. Med. Assoc. J. 2006, 174, 961–974. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, P.F.; Narayan, P.; Papademetriou, V. Exercise as Hypertension Therapy. Cardiol. Clin. 2001, 19, 507–516. [Google Scholar] [CrossRef]
- Tedla, Y.G.; Bautista, L.E. Drug Side Effect Symptoms and Adherence to Antihypertensive Medication. Am. J. Hypertens. 2015, 29, 772–779. [Google Scholar] [CrossRef]
- Hagberg, J.M.; Park, J.-J.; Brown, M.D. The Role of Exercise Training in the Treatment of Hypertension. Sports Med. 2000, 30, 193–206. [Google Scholar] [CrossRef]
- Liu, S.; Goodman, J.; Nolan, R.; Lacombe, S.; Thomas, S. Blood Pressure Responses to Acute and Chronic Exercise Are Related in Prehypertension. Med. Sci. Sports Exerc. 2012, 44, 1644–1652. [Google Scholar] [CrossRef] [Green Version]
- Hecksteden, A.; Grütters, T.; Meyer, T. Associations between Acute and Chronic Effects of Exercise on Indicators of Metabolic Health: A Pilot Training Trial. PLoS ONE 2013, 8, e81181. [Google Scholar] [CrossRef]
- Wegmann, M.; Hecksteden, A.; Poppendieck, W.; Steffen, A.; Kraushaar, J.; Morsch, A.; Meyer, T. Postexercise Hypotension as a Predictor for Long-Term Training-Induced Blood Pressure Reduction: A Large-Scale Randomized Controlled Trial. Clin. J. Sport Med. 2017, 28, 509–515. [Google Scholar]
- Halliwill, J.R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev. 2001, 29, 65–70. [Google Scholar]
- Capdevila, J.H. Regulation of ion transport and blood pressure by cytochrome p450 monooxygenases. Curr. Opin. Nephrol. Hypertens. 2007, 16, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rocic, P.; Schwartzman, M.L. 20-HETE in the regulation of vascular and cardiac function. Pharmacol. Ther. 2018, 192, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Gupta, T.; Garcia, V.; Ding, Y.; Schwartzman, M.L. 20-HETE and Blood Pressure Regulation. Cardiol. Rev. 2014, 22, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, E.E.; Gordon, J.A.; Spector, A.A. HETEs and coronary artery endothelial cells: Metabolic and functional interactions. Am. J. Physiol. Physiol. 1991, 261, C623–C633. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Gauthier, K.M.; Campbell, W.B. 15-Lipoxygenase metabolites contribute to age-related reduction in acetylcholine-induced hypotension in rabbits. Am. J. Physiol. Circ. Physiol. 2008, 295, H89–H96. [Google Scholar] [CrossRef] [Green Version]
- Chawengsub, Y.; Aggarwal, N.T.; Nithipatikom, K.; Gauthier, K.M.; Anjaiah, S.; Hammock, B.D.; Falck, J.R.; Campbell, W.B. Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta. Am. J. Physiol. Circ. Physiol. 2008, 294, H1348–H1356. [Google Scholar] [CrossRef]
- Gauthier, K.M.; Goldman, D.H.; Aggarwal, N.T.; Chawengsub, Y.; Falck, J.R.; Campbell, W.B. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries. Am. J. Physiol. Circ. Physiol. 2010, 300, H725–H735. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.B.; Gauthier, K.M. Inducible Endothelium-derived Hyperpolarizing Factor. J. Cardiovasc. Pharmacol. 2013, 61, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Van Diest, M.J.; Verbeuren, T.J.; Herman, A.G. 15-lipoxygenase metabolites of arachidonic acid evoke contractions and relaxations in isolated canine arteries: Role of thromboxane receptors, endothelial cells and cyclooxygenase. J. Pharmacol. Exp. Ther. 1991, 256, 194–203. [Google Scholar]
- Matsuda, H.; Miyatake, K.; Dahlen, S.-E. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J. Pharmacol. Exp. Ther. 1995, 273, 1182–1189. [Google Scholar]
- Moncada, S. Adventures in vascular biology: A tale of two mediators. Philos. Trans. R Soc. B Biol. Sci. 2006, 361, 735–759. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.M.; Newman, J.W.; Pedersen, T.L.; Ramos, M.I.; Stebbins, C.L. Effects of dynamic exercise on plasma arachidonic acid epoxides and diols in human volunteers. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 471–479. [Google Scholar] [CrossRef]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef] [Green Version]
- Gollasch, B.; Dogan, I.; Rothe, M.; Gollasch, M.; Luft, F.C. Maximal exercise and plasma cytochrome P450 and lipoxygenase mediators: A lipidomics study. Physiol. Rep. 2019, 7, e14165. [Google Scholar] [CrossRef] [Green Version]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.Y.; Zhang, Q.; Sakaguchi, C.A.; Stephan, E.H. Carbohydrate intake attenuates post-exercise plasma levels of cytochrome P450-generated oxylipins. PLoS ONE 2019, 14, e0213676. [Google Scholar]
- Wilson, J.R.; Kapoor, S.C. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am. J. Physiol. Circ. Physiol. 1993, 265, H171–H175. [Google Scholar] [CrossRef]
- Lockwood, J.M.; Pricher, M.P.; Wilkins, B.W.; Holowatz, L.A.; Halliwill, J.R. Postexercise hypotension is not explained by a prostaglandin-dependent peripheral vasodilation. J. Appl. Physiol. 2005, 98, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Suo, J.; Ulshöfer, T.; Jordan, H.; De Bruin, N.; Scholich, K.; Geisslinger, G.; Ferreirós, N. Nano-LC-MS/MS for the quantitation of prostanoids in immune cells. Anal. Bioanal. Chem. 2014, 406, 7103–7116. [Google Scholar] [CrossRef]
- Gurke, R.; Thomas, D.; Schreiber, Y.; Schäfer, S.; Fleck, S.; Geisslinger, G.; Ferreirós, N. Determination of endocannabinoids and endocannabinoid-like substances in human K3EDTA plasma—LC-MS/MS method validation and pre-analytical characteristics. Talanta 2019, 204, 386–394. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R.; MacDougall, J.D.; Hogben, C.D. The effects of exercise duration on post-exercise hypotension. J. Hum. Hypertens. 2000, 14, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keese, F.; Farinatti, P.D.T.V.; Pescatello, L.; Cunha, F.; Monteiro, W.D. Aerobic Exercise Intensity Influences Hypotension Following Concurrent Exercise Sessions. Int. J. Sports Med. 2011, 33, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Cote, A.T.; Bredin, S.S.D.; Phillips, A.A.; Koehle, M.S.; Warburton, D.E. Greater autonomic modulation during post-exercise hypotension following high-intensity interval exercise in endurance-trained men and women. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 115, 81–89. [Google Scholar] [CrossRef]
- Efron, B. Better Bootstrap Confidence-Intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Wilcox, R.R. Comparing dependent robust correlations. Br. J. Math. Stat. Psychol. 2016, 69, 215–224. [Google Scholar] [CrossRef]
- DiCiccio, T.J.; Romano, J.P. A Review of Bootstrap Confidence Intervals. J. R. Stat. Soc. Ser. B Stat. Methodol. 1988, 50, 338–354. [Google Scholar] [CrossRef]
- Angadi, S.S.; Bhammar, D.M.; Gaesser, G.A.; Gaessser, G.A. Postexercise Hypotension After Continuous, Aerobic Interval, and Sprint Interval Exercise. J. Strength Cond. Res. 2015, 29, 2888–2893. [Google Scholar] [CrossRef]
- Jones, H.; George, K.; Edwards, B.; Atkinson, G. Is the magnitude of acute post-exercise hypotension mediated by exercise intensity or total work done? Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 102, 33–40. [Google Scholar] [CrossRef]
- Macdonald, J.R. Potential causes, mechanisms, and implications of post exercise hypotension. J. Hum. Hypertens. 2002, 16, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Halliwill, J.R.; Minson, C.T.; Joyner, M.J. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J. Appl. Physiol. 2000, 89, 1830–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- New, K.J.; Reilly, M.E.; Templeton, K.; Ellis, G.; James, P.E.; McEneny, J.; Penney, M.; Hooper, J.; Hullin, D.; Davies, B.; et al. Free Radical-Mediated Lipid Peroxidation and Systemic Nitric Oxide Bioavailability: Implications for Postexercise Hemodynamics. Am. J. Hypertens. 2012, 26, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Gimbrone, M.A.; Schafer, A.I. Vascular lipoxygenase activity: Synthesis of 15-hydroxyeicosatetraenoic acid from arachidonic acid by blood vessels and cultured vascular endothelial cells. Thromb. Res. 1987, 45, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Uotila, P.; Vargas, R.; Wroblewska, B.; D’Alarcao, M.; Matsuda, S.P.; Corey, E.J.; Cunard, C.M.; Ramwell, P.W. Relaxing effects of 15-lipoxygenase products of arachidonic acid on rat aorta. J. Pharmacol. Exp. Ther. 1987, 242, 945–949. [Google Scholar] [PubMed]
- Lefebvre, B.; Caron, F.; Bessard, G.; Stanke-Labesque, F. Effect of 5-lipoxygenase blockade on blood pressure and acetylcholine-evoked endothelium-dependent contraction in aorta from spontaneously hypertensive rats. J. Hypertens. 2006, 24, 85–93. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Holmes, B.B.; Cui, L.; Viita, H.; Ylä-Herttuala, S.; Campbell, W.B. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: Role in arachidonic acid-induced relaxation. Am. J. Physiol. Circ. Physiol. 2007, 292, H1033–H1041. [Google Scholar] [CrossRef] [Green Version]
- Harder, D.; Campbell, W.; Roman, R. Role of Cytochrome P-450 Enzymes and Metabolites of Arachidonic Acid in the Control of Vascular Tone. J. Vasc. Res. 1995, 32, 79–92. [Google Scholar] [CrossRef]
- Rahman, M.; Wright, J.T.; Douglas, J.G. The Role of the Cytochrome P450-Dependent Metabolites of Arachidonic Acid in Blood Pressure Regulation and Renal Function A Review. Am. J. Hypertens. 1997, 10, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Imig, J.D. Epoxyeicosatrienoic Acids and 20-Hydroxyeicosatetraenoic Acid on Endothelial and Vascular Function. HIV-1: Mol. Biol. Pathog. 2016, 77, 105–141. [Google Scholar] [CrossRef] [Green Version]
- Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of dietary Docosahexaenoic, training and acute exercise on lipid mediators. J. Int. Soc. Sports Nutr. 2016, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Halliwill, J.R.; Buck, T.M.; Lacewell, A.N.; Romero, S.A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 2012, 98, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Hellsten, Y. Muscle blood flow and oxygen uptake in recovery from exercise. Acta Physiol. Scand. 1998, 162, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.L.; Spitzbarth, N.; Nithipatikom, K.; Edgemond, W.S.; Falck, J.R.; Campbell, W.B. Identification of the 11,14,15- and 11,12,15-Trihydroxyeicosatrienoic Acids as Endothelium-derived Relaxing Factors of Rabbit Aorta. J. Biol. Chem. 1998, 273, 30879–30887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, N.T.; Chawengsub, Y.; Gauthier, K.M.; Viita, H.; Ylä-Herttuala, S.; Campbell, W.B. Endothelial 15-Lipoxygenase-1 Overexpression Increases Acetylcholine-Induced Hypotension and Vasorelaxation in Rabbits. Hypertension 2008, 51, 246–251. [Google Scholar] [CrossRef] [Green Version]




| Demographic Data | Mean | SE |
|---|---|---|
| Male | 5 (3) | |
| Female | 15 | |
| Age [years] | 25.10 | 3.14 |
| Height [meters] | 1.72 | 0.07 |
| Weight [kg] | 65.38 | 8.88 |
| BMI | 22.12 | 2.43 |
| Activity Index | 55.60 | 26.91 |
| SBP ± SE | ||
|---|---|---|
| Time [Min] | Control | Exercise |
| −60 | 1.00 ± 0 | 1.00 ± 0 |
| 0 | 1.00 ± 0.01 | 1.02 ± 0.01 |
| 30 | 0.97± 0.01 | 0.95 ± 0.008 *** |
| 60 | 1.00 ± 0.01 | 0.96 ± 0.01 # |
| 120 | 1.00 ± 0.01 | 0.98 ± 0.01 |
| Variable 1 | Variable 2 | Control Variable | Pearson r | Significance | n |
|---|---|---|---|---|---|
| PEH | 15-HETE | no | 0.505 | 0.032 | 18 |
| PEH | 15-HETE | AA | 0.744 | 0.001 | 18 |
| PEH | 15-HETE | 5-HETE | 0.758 | 0.000423 | 18 |
| PEH | 15-HETE | 12-HETE | 0.057 | 0.038 | 18 |
| PEH | 15-HETE | 20-HETE | 0.539 | 0.026 | 18 |
| PEH | 15-HETE | BMI | 0.438 | 0.079 | 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolters, M.C.; Schmetzer, J.; Möser, C.V.; Hahnefeld, L.; Angioni, C.; Thomas, D.; Ferreirós, N.; Geisslinger, G.; Niederberger, E. Exercise-Induced Changes in Bioactive Lipids Might Serve as Potential Predictors of Post-Exercise Hypotension. A Pilot Study in Healthy Volunteers. Cells 2020, 9, 2111. https://doi.org/10.3390/cells9092111
Wolters MC, Schmetzer J, Möser CV, Hahnefeld L, Angioni C, Thomas D, Ferreirós N, Geisslinger G, Niederberger E. Exercise-Induced Changes in Bioactive Lipids Might Serve as Potential Predictors of Post-Exercise Hypotension. A Pilot Study in Healthy Volunteers. Cells. 2020; 9(9):2111. https://doi.org/10.3390/cells9092111
Chicago/Turabian StyleWolters, Miriam C., Julia Schmetzer, Christine V. Möser, Lisa Hahnefeld, Carlo Angioni, Dominique Thomas, Nerea Ferreirós, Gerd Geisslinger, and Ellen Niederberger. 2020. "Exercise-Induced Changes in Bioactive Lipids Might Serve as Potential Predictors of Post-Exercise Hypotension. A Pilot Study in Healthy Volunteers" Cells 9, no. 9: 2111. https://doi.org/10.3390/cells9092111
APA StyleWolters, M. C., Schmetzer, J., Möser, C. V., Hahnefeld, L., Angioni, C., Thomas, D., Ferreirós, N., Geisslinger, G., & Niederberger, E. (2020). Exercise-Induced Changes in Bioactive Lipids Might Serve as Potential Predictors of Post-Exercise Hypotension. A Pilot Study in Healthy Volunteers. Cells, 9(9), 2111. https://doi.org/10.3390/cells9092111






