ZMPSTE24 Is Associated with Elevated Inflammation and Progerin mRNA
Abstract
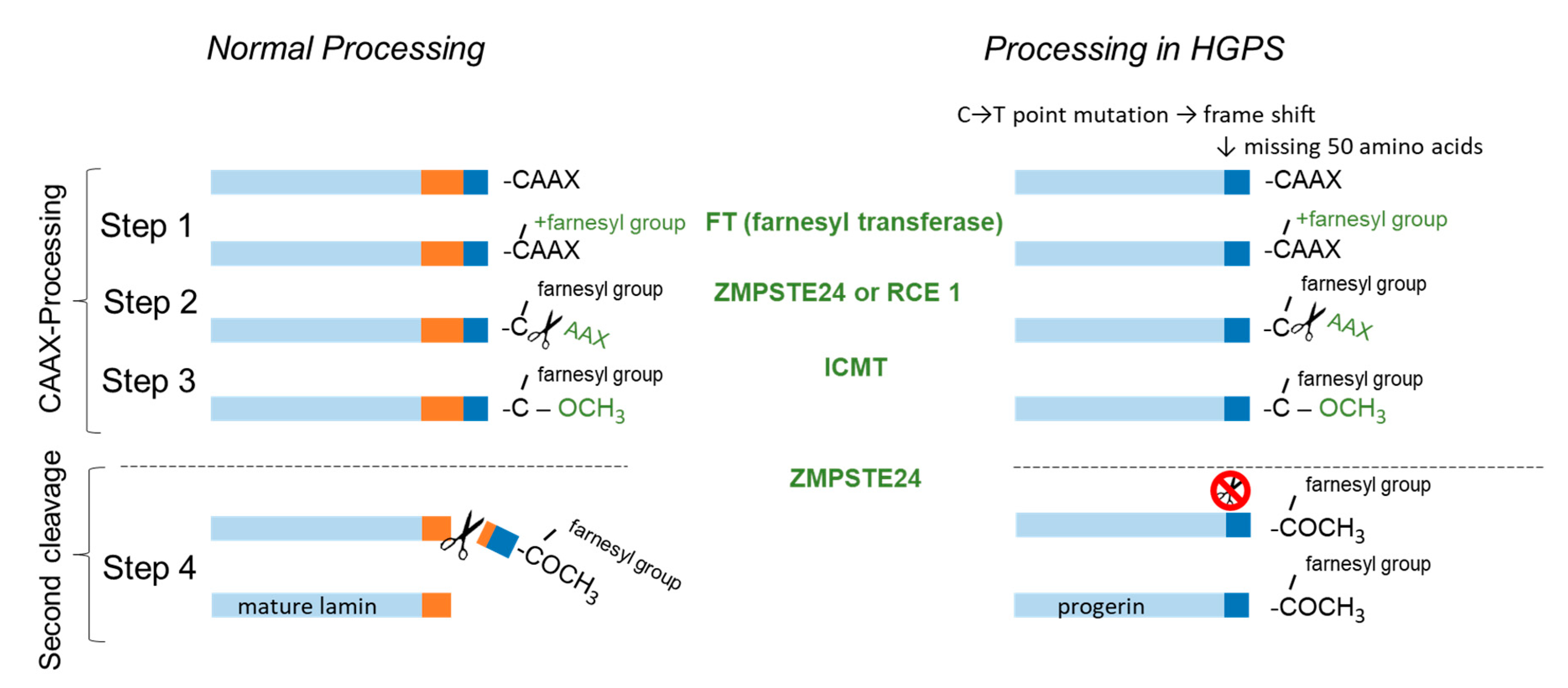
:1. Introduction
2. Materials and Methods
2.1. Study Population
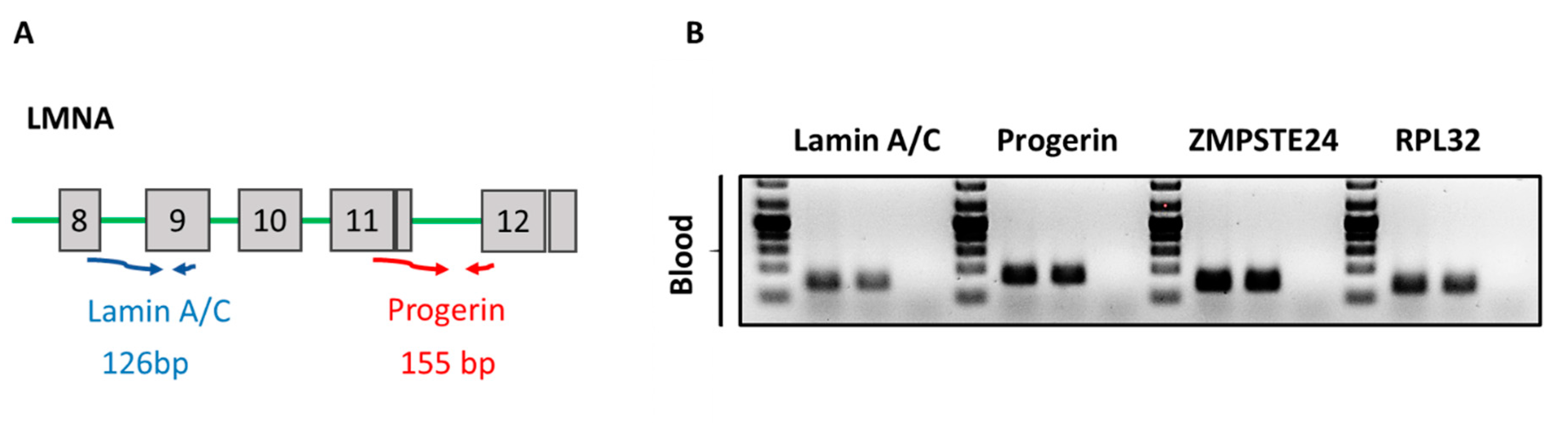
2.2. RT–PCR of mRNA
2.3. Statistical Analysis
3. Results
3.1. Detection of ZMPSTE24, Lamin A/C and Progerin mRNA in Human Blood Samples
3.2. Characteristics of Study Cohort
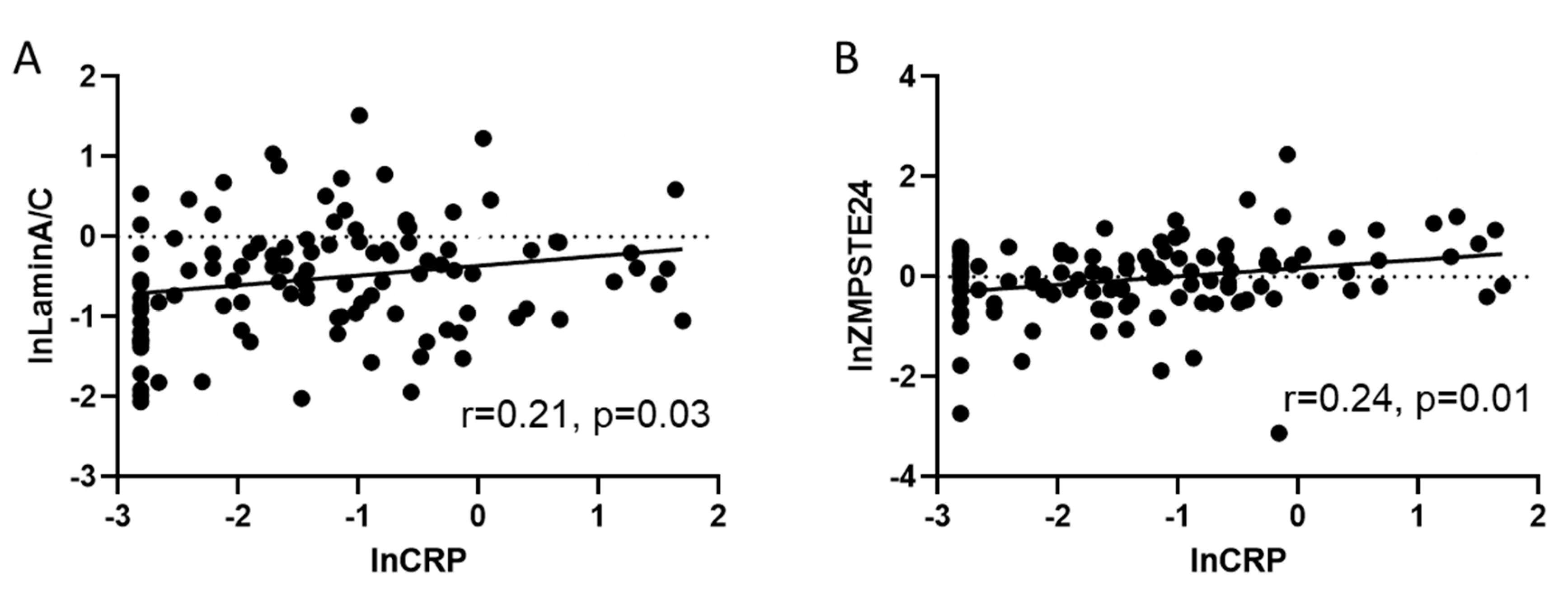
3.3. Lamin A/C and ZMPSTE24 mRNA Correlate with Progerin mRNA
3.4. CRP Correlates with Expression of Lamin A/C and ZMPSTE24 mRNA
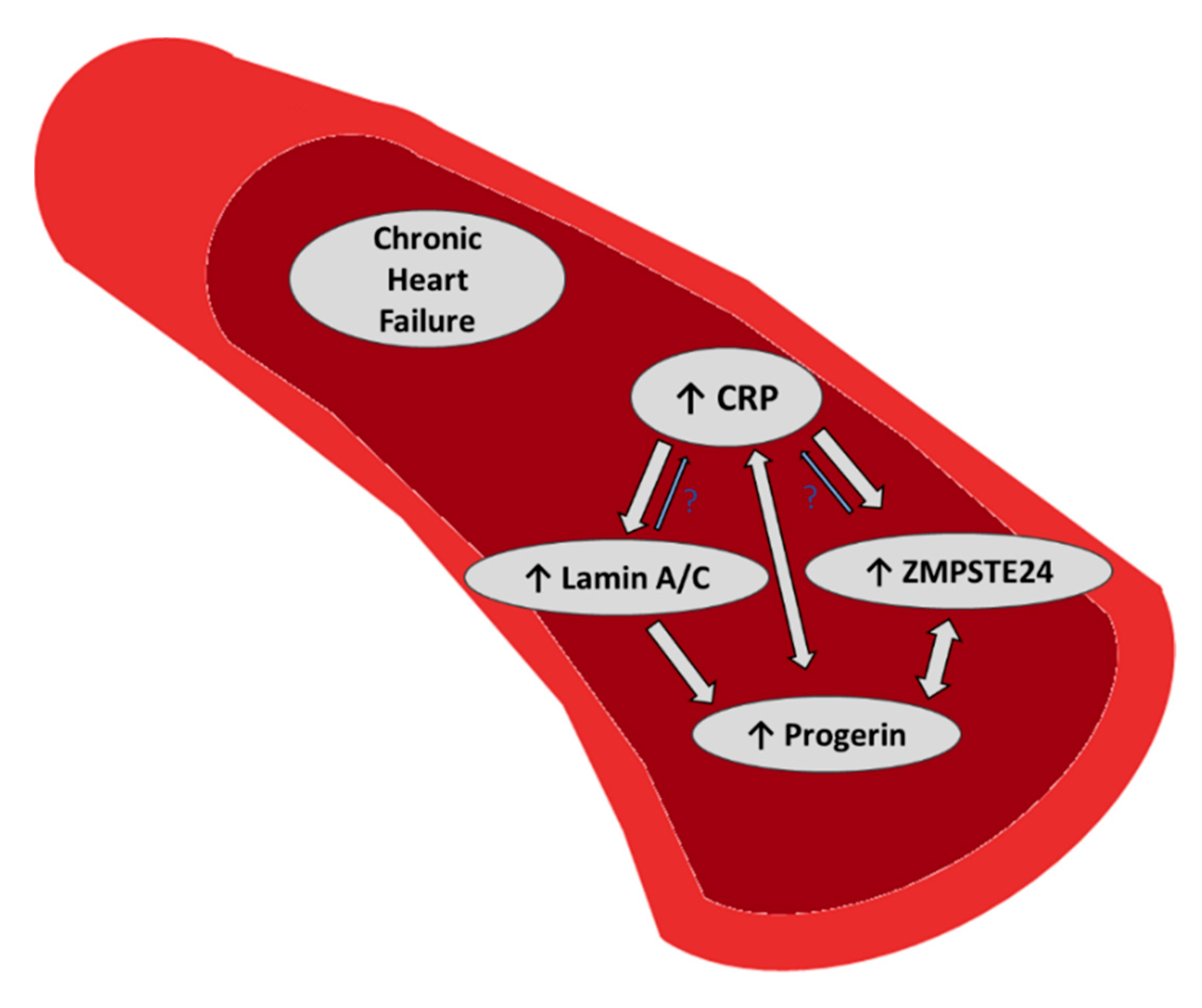
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CVS | Cardiovascular system |
| DCMP | Dilative cardiomyopathy |
| HGPS | Hutchinson–-Gilford progeria syndrome |
| Hs-CRP | High-sensitive C-reactive protein |
| HCMP | Hypertrophic cardiomyopathy |
| ICMP | Ischemic cardiomyopathy |
| NYHA | New York Heart Association |
| PCR | Polymerase chain reaction |
Appendix A
| DCMP | ICMP | HCMP | Others | Total |
|---|---|---|---|---|
| 58 (53) | 31 (28) | 12 (11) | 9 (8) | 110 |
References
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M.; et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef] [PubMed]
- Hennekam, R.C. Hutchinson-Gilford progeria syndrome: Review of the phenotype. Am. J. Med. Genet. A 2006, 140, 2603–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.M.; Jenkins, J.L.; Fedoriw, N.; Dumont, M.E. Human CaaX protease ZMPSTE24 expressed in yeast: Structure and inhibition by HIV protease inhibitors. Protein Sci. 2017, 26, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, B.S.; Fong, L.G.; Yang, S.H.; Coffinier, C.; Young, S.G. The posttranslational processing of prelamin A and disease. Annu. Rev. Genom. Hum. Genet. 2009, 10, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Messner, M.; Ghadge, S.K.; Goetsch, V.; Wimmer, A.; Dorler, J.; Polzl, G.; Zaruba, M.M. Upregulation of the aging related LMNA splice variant progerin in dilated cardiomyopathy. PLoS ONE 2018, 13, e0196739. [Google Scholar] [CrossRef] [Green Version]
- Messner, M.; Ghadge, S.K.; Schuetz, T.; Seiringer, H.; Polzl, G.; Zaruba, M.M. High Body Mass Index is Associated with Elevated Blood Levels of Progerin mRNA. Int. J. Mol. Sci. 2019, 20, 1976. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Bohm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Desgrouas, C.; Varlet, A.A.; Dutour, A.; Galant, D.; Merono, F.; Bonello-Palot, N.; Bourgeois, P.; Lasbleiz, A.; Petitjean, C.; Ancel, P.; et al. Unraveling LMNA Mutations in Metabolic Syndrome: Cellular Phenotype and Clinical Pitfalls. Cells 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shackleton, S.; Smallwood, D.T.; Clayton, P.; Wilson, L.C.; Agarwal, A.K.; Garg, A.; Trembath, R.C. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J. Med. Genet. 2005, 42, e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brayson, D.; Frustaci, A.; Verardo, R.; Chimenti, C.; Russo, M.A.; Hayward, R.; Ahmad, S.; Vizcay-Barrena, G.; Protti, A.; Zammit, P.S.; et al. Prelamin A mediates myocardial inflammation in dilated and HIV-associated cardiomyopathies. JCI Insight 2019, 4, e126315. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Arnold, R.; Henriques, G.; Djabali, K. Inhibition of JAK-STAT Signaling with Baricitinib Reduces Inflammation and Improves Cellular Homeostasis in Progeria Cells. Cells 2019, 8, 1276. [Google Scholar] [CrossRef] [Green Version]
- Osorio, F.G.; Barcena, C.; Soria-Valles, C.; Ramsay, A.J.; de Carlos, F.; Cobo, J.; Fueyo, A.; Freije, J.M.; Lopez-Otin, C. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012, 26, 2311–2324. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Chacon, M.R.; Gutierrez, C.; Vilarrasa, N.; Gomez, J.M.; Caubet, E.; Megia, A.; Vendrell, J. LMNA mRNA expression is altered in human obesity and type 2 diabetes. Obesity 2008, 16, 1742–1748. [Google Scholar] [CrossRef]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total Population N = 110 | |
|---|---|---|
| Age, years | 56 ± 15 | |
| Female sex, n (%) | 32 (29) | |
| Body mass index, kg/m2 | 26.6 ± 4.8 | |
| NYHA class, n (%) | N = 108 | 2.0 ± 0.7 |
| I | 29 (26) | |
| II | 55 (50) | |
| III | 24 (22) | |
| IV | 0 (0) | |
| Medical history, n (%) | ||
| Smoking, n (%) | N = 108 | 49 |
| Diabetes, n (%) | 19 (17) | |
| Atrial fibrillation, n (%) | 42 (38) | |
| LVEF, n (%) | 38.2 ± 14.9 | |
| ACE-inhibitor, n (%) | 55 (50) | |
| ARB, n (%) | 33 (30) | |
| Beta blocker | 90 (81) | |
| Clinical features | ||
| NT-proBNP, ng/L | N = 108 | 2094 ± 4421 |
| Troponin T, ng/L | N = 109 | 28 ± 53 |
| Serum creatinine, mg/dl | 1.2 ± 0.46 | |
| GFR, mL/min | 54 ± 10 | |
| Urea, mg/dl | 50 ± 37 | |
| HbA1c,% | N = 110 | 5.9 ± 1.0 |
| Triglycerides, mg/dl | 135 ± 68 | |
| Total cholesterol, mg/dl | 172 ± 44 | |
| LDL, mg/dl | N = 109 | 107 ± 37 |
| HDL, mg/dl | N = 109 | 52 ± 19 |
| Hs-CRP, mg/dl | N = 110 | 0.64 ± 1.07 |
| GGT, U/I | 86 ± 146 | |
| GOT, U/I | 29.2 ± 12.4 | |
| Potassium | 4.2 ± 0.5 | |
| Sodium | 138 ± 13 | |
| Leucocyte count, G/L | 7.7 ± 2.2 | |
| Thrombocytes, G/I | 217 ± 71 | |
| Hemoglobin, g/L | 140 ± 15 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messner, M.; Ghadge, S.K.; Maurer, T.; Graber, M.; Staggl, S.; Christine Maier, S.; Pölzl, G.; Zaruba, M.-M. ZMPSTE24 Is Associated with Elevated Inflammation and Progerin mRNA. Cells 2020, 9, 1981. https://doi.org/10.3390/cells9091981
Messner M, Ghadge SK, Maurer T, Graber M, Staggl S, Christine Maier S, Pölzl G, Zaruba M-M. ZMPSTE24 Is Associated with Elevated Inflammation and Progerin mRNA. Cells. 2020; 9(9):1981. https://doi.org/10.3390/cells9091981
Chicago/Turabian StyleMessner, Moritz, Santhosh Kumar Ghadge, Thomas Maurer, Michael Graber, Simon Staggl, Sarah Christine Maier, Gerhard Pölzl, and Marc-Michael Zaruba. 2020. "ZMPSTE24 Is Associated with Elevated Inflammation and Progerin mRNA" Cells 9, no. 9: 1981. https://doi.org/10.3390/cells9091981





