The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD
Abstract
1. Introduction
2. Materials and Methods
2.1. MAO Inhibitor Tranylcypromine
2.2. Cell Lines
2.3. Wild-Type Mice and 5xFAD Mice
2.4. MTT Assay
2.5. Cell Counting Kit-8 (CCK-8) Assay
2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.7. Real-Time PCR (Q-PCR)
2.8. Western Blotting
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Immunocytochemistry
2.11. Subcellular Fractionation (Cytosol vs. Nuclear)
2.12. Statistical Analyses
3. Results
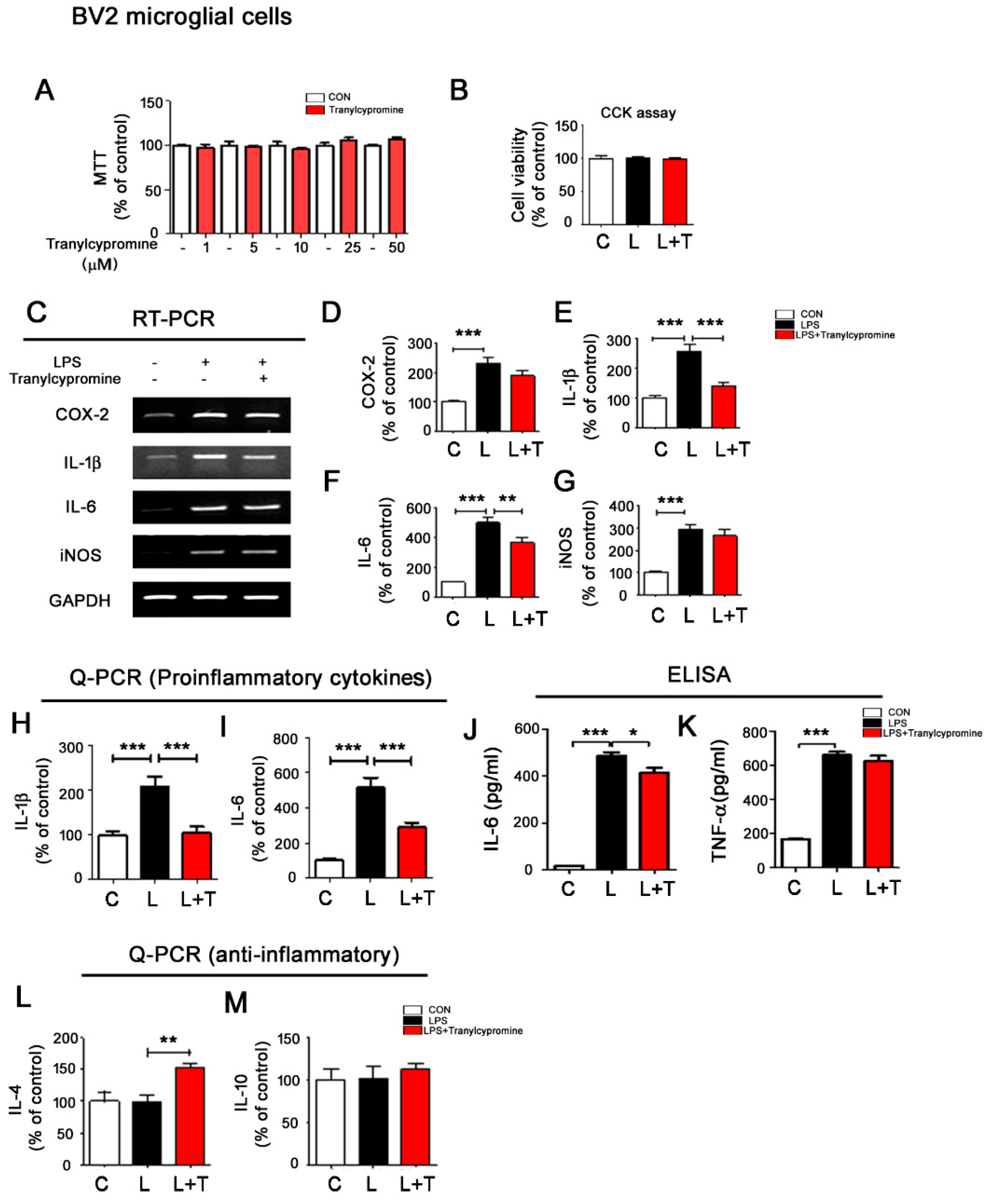
3.1. Tranylcypromine Regulates LPS-Mediated Proinflammatory Cytokine Levels in BV2 Microglial Cells
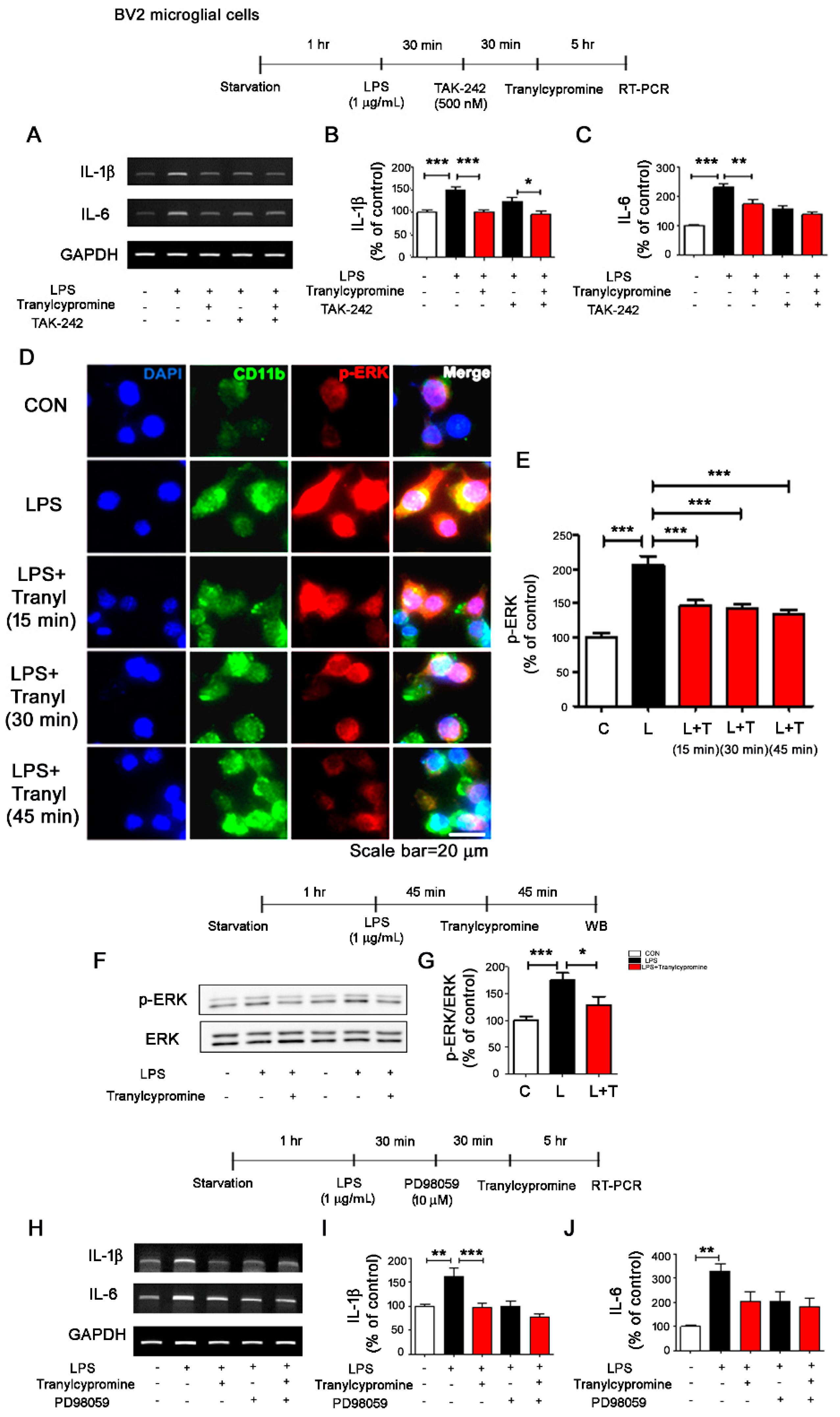
3.2. Tranylcypromine Affects LPS-Induced TLR4/ERK Signaling in BV2 Microglial Cells
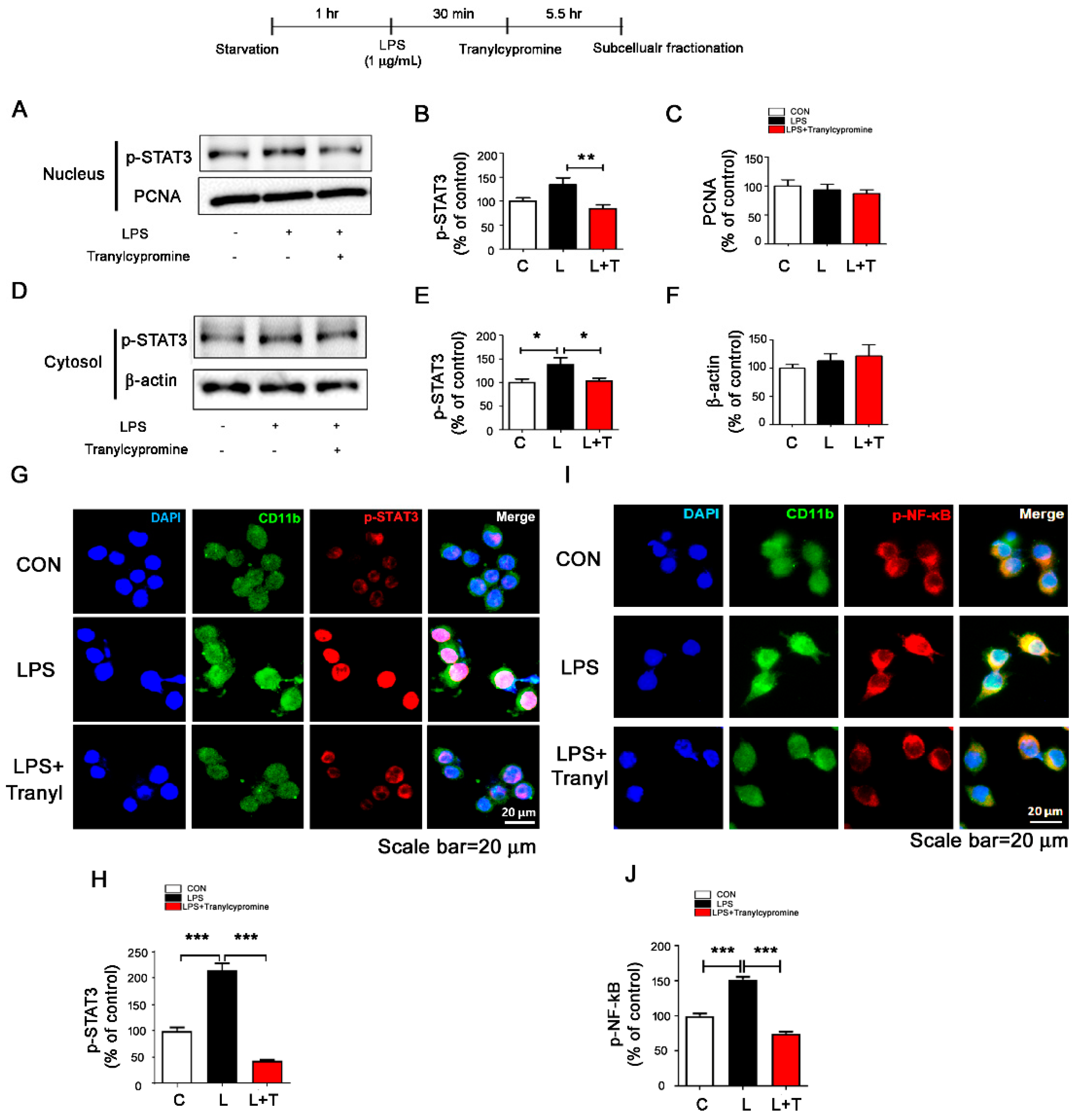
3.3. Tranylcypromine Regulates LPS-Evoked Microglial Nuclear p-STAT3 and p-NF-κB Levels
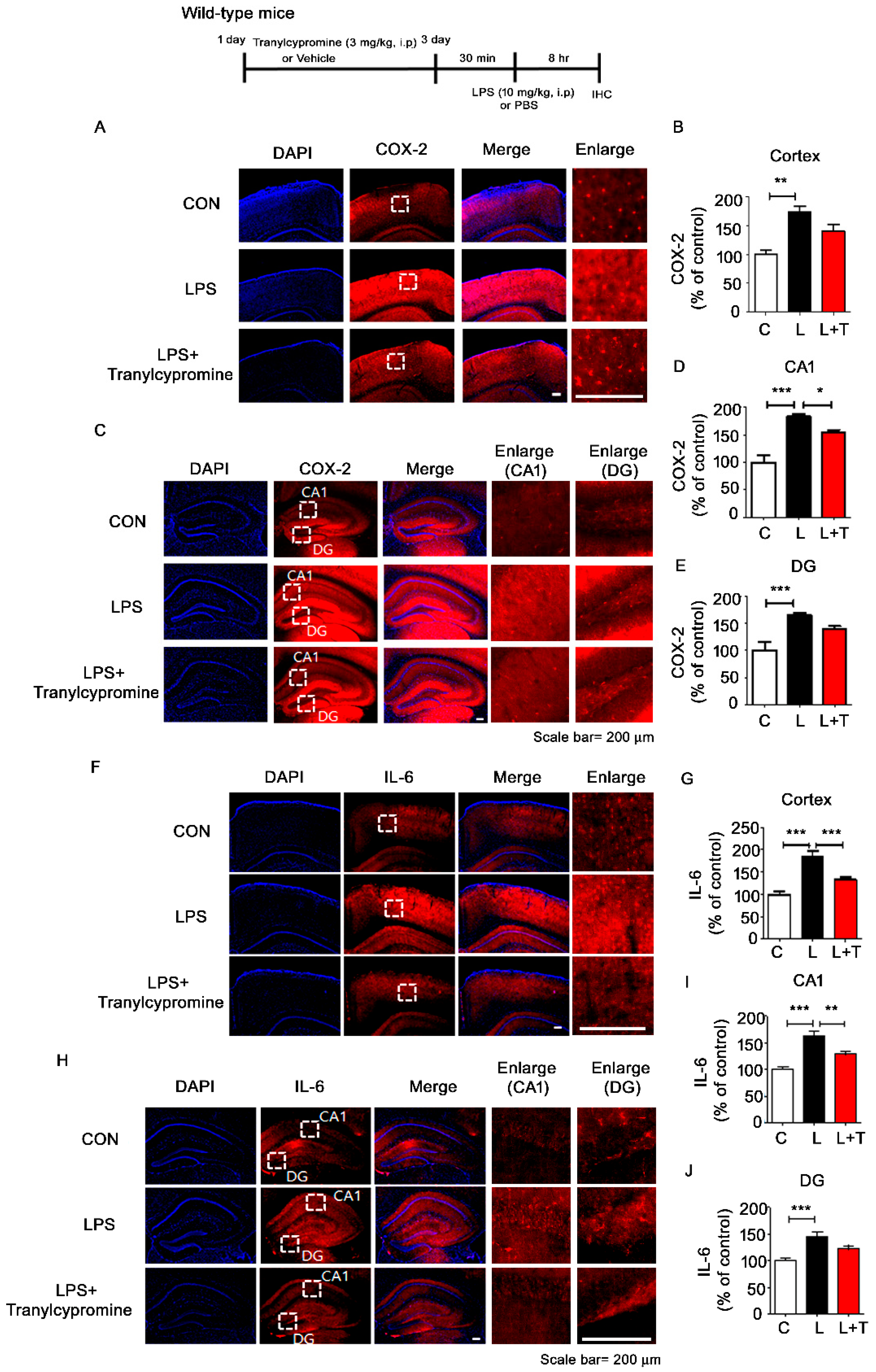
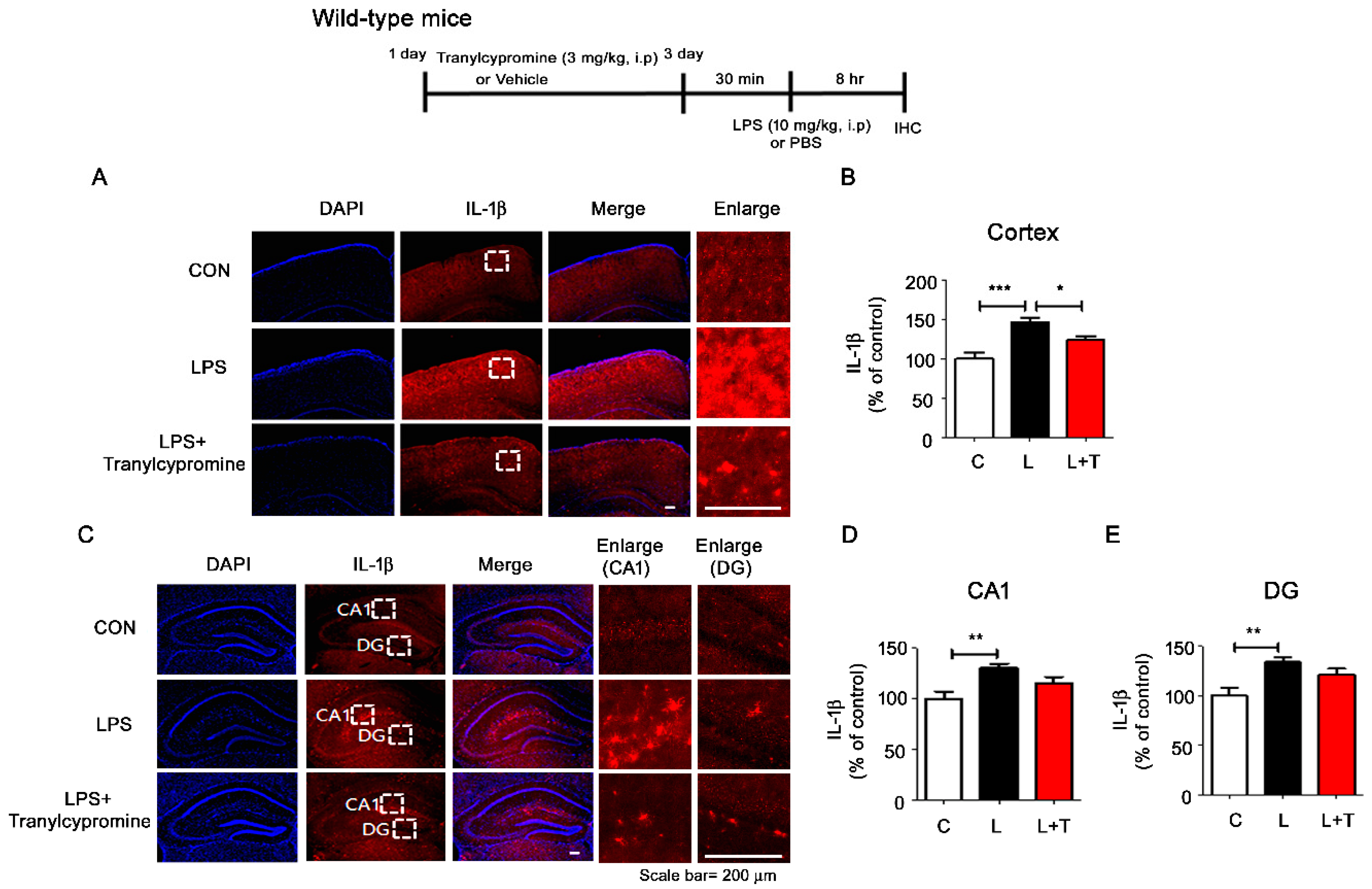
3.4. Tranylcypromine Treatment Decreases LPS-Mediated Microglial Activation in Wild-Type Mice
3.5. Tranylcypromine Treatment Reduces LPS-Mediated Proinflammatory Cytokine COX-2 and IL-6 Levels in Wild-Type Mice
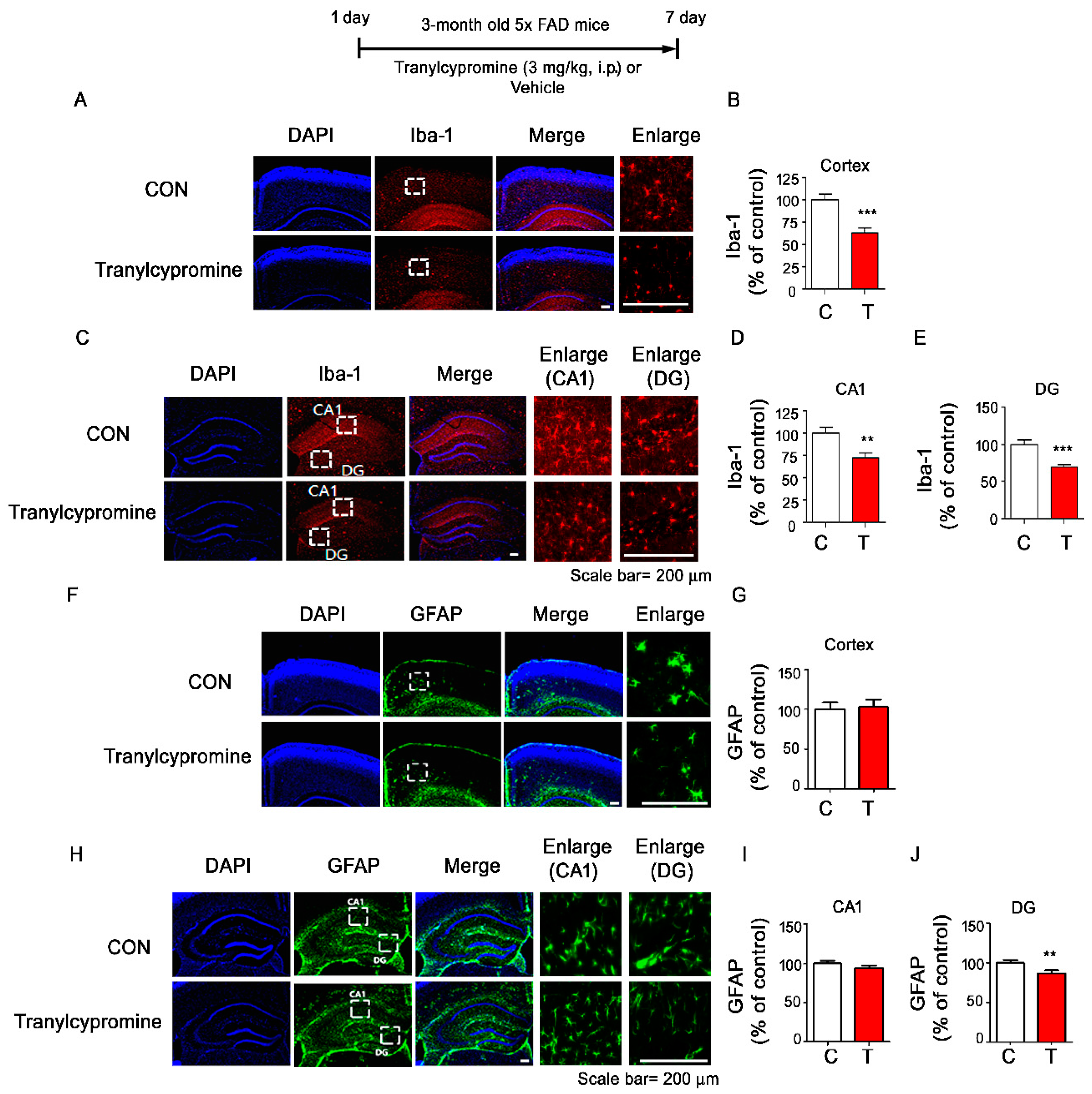
3.6. Tranylcypromine-Injected 5xFAD Mice Exhibit Downregulated Aβ-Mediated Microglial Activation
4. Discussion
4.1. Tranylcypromine Alters LPS-Induced Proinflammatory Cytokine Production
4.2. Tranylcypromine Regulates LPS-Induced Microglial Neuroinflammatory Signaling Pathways
4.3. Tranylcypromine Inhibits LPS-Induced Microglial Neuroinflammatory Responses in Wild-Type Mice
4.4. Tranylcypromine Modulates the Aβ-Induced Neuroinflammatory Response in 5xFAD Mice
4.5. Potential Therapeutic Effects of Tranylcypromine on Neurodegenerative Diseases and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- Bachiller, S.; Jimenez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Koenigsknecht-Talboo, J.; Landreth, G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005, 25, 8240–8249. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, A.M.; Paul-Samojedny, M.; Obuchowicz, E. Moclobemide exerts anti-inflammatory effect in lipopolysaccharide-activated primary mixed glial cell culture. Naunyn Schmiedebergs Arch. Pharm. 2010, 382, 409–417. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Akao, Y.; Yi, H.; Yamaoka, Y. Involvement of type A monoamine oxidase in neurodegeneration: Regulation of mitochondrial signaling leading to cell death or neuroprotection. J. Neural. Transm. Suppl. 2006. [Google Scholar] [CrossRef]
- Rodriguez, S.; Ito, T.; He, X.J.; Uchida, K.; Nakayama, H. Resistance of the golden hamster to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-neurotoxicity is not only related with low levels of cerebral monoamine oxidase-B. Exp. Toxicol. Pathol. 2013, 65, 127–133. [Google Scholar] [CrossRef]
- Zheng, H.; Youdim, M.B.; Fridkin, M. Site-activated chelators targeting acetylcholinesterase and monoamine oxidase for Alzheimer’s therapy. Acs. Chem. Biol. 2010, 5, 603–610. [Google Scholar] [CrossRef]
- Youdim, M.B.; Lavie, L. Selective MAO-A and B inhibitors, radical scavengers and nitric oxide synthase inhibitors in Parkinson’s disease. Life Sci. 1994, 55, 2077–2082. [Google Scholar] [CrossRef]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharm. 2006, 147 (Suppl. 1), 287–296. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Lewy body diseases with dementia: Pathophysiology and treatment. Brain Cogn. 1995, 28, 266–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delumeau, J.C.; Bentue-Ferrer, D.; Gandon, J.M.; Amrein, R.; Belliard, S.; Allain, H. Monoamine oxidase inhibitors, cognitive functions and neurodegenerative diseases. J. Neural. Transm. Suppl. 1994, 41, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Grailhe, R.; Cardona, A.; Even, N.; Seif, I.; Changeux, J.P.; Cloez-Tayarani, I. Regional changes in the cholinergic system in mice lacking monoamine oxidase A. Brain Res. Bull. 2009, 78, 283–289. [Google Scholar] [CrossRef]
- Huang, L.; Lu, C.; Sun, Y.; Mao, F.; Luo, Z.; Su, T.; Jiang, H.; Shan, W.; Li, X. Multitarget-directed benzylideneindanone derivatives: Anti-beta-amyloid (Abeta) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer’s disease. J. Med. Chem. 2012, 55, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Fridkin, M.; Youdim, M.B. From antioxidant chelators to site-activated multi-target chelators targeting hypoxia inducing factor, beta-amyloid, acetylcholinesterase and monoamine oxidase A/B. Mini Rev. Med. Chem. 2012, 12, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C. Epigenetic drug discovery for Alzheimer’s disease. Expert Opin. Drug Discov. 2014, 9, 1059–1086. [Google Scholar] [CrossRef]
- Ji, Y.Y.; Lin, S.D.; Wang, Y.J.; Su, M.B.; Zhang, W.; Gunosewoyo, H.; Yang, F.; Li, J.; Tang, J.; Zhou, Y.B.; et al. Tying up tranylcypromine: Novel selective histone lysine specific demethylase 1 (LSD1) inhibitors. Eur. J. Med. Chem. 2017, 141, 101–112. [Google Scholar] [CrossRef]
- Gooden, D.M.; Schmidt, D.M.; Pollock, J.A.; Kabadi, A.M.; McCafferty, D.G. Facile synthesis of substituted trans-2-arylcyclopropylamine inhibitors of the human histone demethylase LSD1 and monoamine oxidases A and B. Bioorg. Med. Chem. Lett. 2008, 18, 3047–3051. [Google Scholar] [CrossRef]
- Barth, J.; Abou-El-Ardat, K.; Dalic, D.; Kurrle, N.; Maier, A.M.; Mohr, S.; Schutte, J.; Vassen, L.; Greve, G.; Schulz-Fincke, J.; et al. LSD1 inhibition by tranylcypromine derivatives interferes with GFI1-mediated repression of PU.1 target genes and induces differentiation in AML. Leukemia 2019, 33, 1411–1426. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, K.; Yan, X.; Wang, T.; Jiang, T.; Zhou, Q.; Qi, J.; Qian, N.; Zhou, H.; Chen, B.; et al. The effects of tranylcypromine on osteoclastogenesis in vitro and in vivo. Faseb J. 2019, 33, 9828–9841. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, V.S.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Juca, P.M.; Soares, M.V.R.; Barroso, P.N.; Cristino, L.M.F.; Jiang, W.; Teixeira, A.L.; de Lucena, D.F.; et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J. Affect. Disord. 2020, 268, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Pappalardo, G.; Basile, L.; Giuffrida, A.; Copani, A.; Tosto, R.; Sinopoli, A.; Giuffrida, M.L.; Pirrone, E.; Drago, F.; et al. Neuroprotective effects of the monoamine oxidase inhibitor tranylcypromine and its amide derivatives against Abeta(1-42)-induced toxicity. Eur J. Pharm. 2015, 764, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.Y.; Lee, H.J.; Woo, H.; Kang, R.J.; Han, K.M.; Park, H.; Lee, S.M.; Lee, J.Y.; Jeong, Y.J.; Nam, H.W.; et al. Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J. Neuroinflammation 2019, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Nam, J.H.; Nam, Y.; Nam, H.Y.; Yoon, G.; Ko, E.; Kim, S.B.; Bautista, M.R.; Capule, C.C.; Koyanagi, T.; et al. The small molecule CA140 inhibits the neuroinflammatory response in wild-type mice and a mouse model of AD. J. Neuroinflammation 2018, 15, 286. [Google Scholar] [CrossRef]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflammation 2018, 15, 271. [Google Scholar] [CrossRef]
- Kang, C.H.; Jayasooriya, R.G.; Dilshara, M.G.; Choi, Y.H.; Jeong, Y.K.; Kim, N.D.; Kim, G.Y. Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-kappaB activation and ERK phosphorylation. Food Chem. Toxicol. 2012, 50, 4270–4276. [Google Scholar] [CrossRef]
- Reed, A.L.; Happe, H.K.; Petty, F.; Bylund, D.B. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacol. (Berl) 2008, 197, 433–441. [Google Scholar] [CrossRef]
- Villegier, A.S.; Gallager, B.; Heston, J.; Belluzzi, J.D.; Leslie, F.M. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacol. (Berl) 2010, 208, 593–601. [Google Scholar] [CrossRef]
- Villegier, A.S.; Salomon, L.; Granon, S.; Changeux, J.P.; Belluzzi, J.D.; Leslie, F.M.; Tassin, J.P. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology 2006, 31, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Sturza, A.; Popoiu, C.M.; Ionica, M.; Duicu, O.M.; Olariu, S.; Muntean, D.M.; Boia, E.S. Monoamine Oxidase-Related Vascular Oxidative Stress in Diseases Associated with Inflammatory Burden. Oxid. Med. Cell. Longev. 2019, 2019, 8954201. [Google Scholar] [CrossRef] [PubMed]
- Sturza, A.; Olariu, S.; Ionica, M.; Duicu, O.M.; Vaduva, A.O.; Boia, E.; Muntean, D.M.; Popoiu, C.M. Monoamine oxidase is a source of oxidative stress in obese patients with chronic inflammation (1). Can. J. Physiol. Pharm. 2019, 97, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Dhabal, S.; Das, P.; Biswas, P.; Kumari, P.; Yakubenko, V.P.; Kundu, S.; Cathcart, M.K.; Kundu, M.; Biswas, K.; Bhattacharjee, A. Regulation of monoamine oxidase A (MAO-A) expression, activity, and function in IL-13-stimulated monocytes and A549 lung carcinoma cells. J. Biol. Chem. 2018, 293, 14040–14064. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Chacon, P.; Monteseirin, J.; El Bekay, R.; Alvarez, M.; Alba, G.; Conde, J.; Martin-Nieto, J.; Bedoya, F.J.; Pintado, E.; et al. A new role for monoamine oxidases in the modulation of macrophage-inducible nitric oxide synthase gene expression. J. Leukoc. Biol. 2004, 75, 1093–1101. [Google Scholar] [CrossRef]
- Ratiu, C.; Utu, D.; Petrus, A.; Norbert, P.; Olariu, S.; Duicu, O.; Sturza, A.; Muntean, D.M. Monoamine oxidase inhibition improves vascular function and reduces oxidative stress in rats with lipopolysaccharide-induced inflammation. Gen. Physiol. Biophys. 2018, 37, 687–694. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, H.; Bae, H. Phenelzine (monoamine oxidase inhibitor) increases production of nitric oxide and proinflammatory cytokines via the NF-kappaB pathway in lipopolysaccharide-activated microglia cells. Neurochem. Res. 2012, 37, 2117–2124. [Google Scholar] [CrossRef]
- Nowakowska, E.; Chodera, A. [Inhibitory monoamine oxidases of the new generation]. Pol. Merkur. Lek. 1997, 3, 1–4. [Google Scholar]
- Mann, J.J.; Aarons, S.F.; Frances, A.J.; Brown, R.D. Studies of selective and reversible monoamine oxidase inhibitors. J. Clin. Psychiatry 1984, 45, 62–66. [Google Scholar]
- Lee, J.D.; Kato, K.; Tobias, P.S.; Kirkland, T.N.; Ulevitch, R.J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J. Exp. Med. 1992, 175, 1697–1705. [Google Scholar] [CrossRef]
- Vindis, C.; Seguelas, M.H.; Bianchi, P.; Parini, A.; Cambon, C. Monoamine oxidase B induces ERK-dependent cell mitogenesis by hydrogen peroxide generation. Biochem. Biophys. Res. Commun. 2000, 271, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C.J.; Rose-John, S.; Lissilaa, R.; Ferlin, W.; Ernst, M.; Hertzog, P.J.; Mansell, A.; Jenkins, B.J. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J. Immunol. 2011, 186, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Niemand, C.; Nimmesgern, A.; Haan, S.; Fischer, P.; Schaper, F.; Rossaint, R.; Heinrich, P.C.; Muller-Newen, G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. 2003, 170, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflammation 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Hennessy, E.; Dansereau, M.A.; Cunningham, C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Yates, S.L.; Burgess, L.H.; Kocsis-Angle, J.; Antal, J.M.; Dority, M.D.; Embury, P.B.; Piotrkowski, A.M.; Brunden, K.R. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 2000, 74, 1017–1025. [Google Scholar] [CrossRef]
- Fu, W.; Vukojevic, V.; Patel, A.; Soudy, R.; MacTavish, D.; Westaway, D.; Kaur, K.; Goncharuk, V.; Jhamandas, J. Role of microglial amylin receptors in mediating beta amyloid (Abeta)-induced inflammation. J. Neuroinflammation 2017, 14, 199. [Google Scholar] [CrossRef]
- Park, J.H.; Ju, Y.H.; Choi, J.W.; Song, H.J.; Jang, B.K.; Woo, J.; Chun, H.; Kim, H.J.; Shin, S.J.; Yarishkin, O.; et al. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Fagervall, I.; Ross, S.B. Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors. Biochem. Pharm. 1986, 35, 1381–1387. [Google Scholar] [CrossRef]
- McKenna, K.F.; McManus, D.J.; Baker, G.B.; Coutts, R.T. Chronic administration of the antidepressant phenelzine and its N-acetyl analogue: Effects on GABAergic function. J. Neural. Transm. Suppl. 1994, 41, 115–122. [Google Scholar] [CrossRef]
- Hill, M.N.; Ho, W.S.; Hillard, C.J.; Gorzalka, B.B. Differential effects of the antidepressants tranylcypromine and fluoxetine on limbic cannabinoid receptor binding and endocannabinoid contents. J. Neural. Transm. (Vienna) 2008, 115, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Ricken, R.; Ulrich, S.; Schlattmann, P.; Adli, M. Tranylcypromine in mind (Part II): Review of clinical pharmacology and meta-analysis of controlled studies in depression. Eur. Neuropsychopharmacol. 2017, 27, 714–731. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Han, K.-M.; Jeon, H.; Lee, J.-S.; Lee, H.; Jeon, S.G.; Park, J.-H.; Kim, Y.G.; Lin, Y.; Lee, Y.-H.; et al. The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD. Cells 2020, 9, 1982. https://doi.org/10.3390/cells9091982
Park H, Han K-M, Jeon H, Lee J-S, Lee H, Jeon SG, Park J-H, Kim YG, Lin Y, Lee Y-H, et al. The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD. Cells. 2020; 9(9):1982. https://doi.org/10.3390/cells9091982
Chicago/Turabian StylePark, HyunHee, Kyung-Min Han, Hyongjun Jeon, Ji-Soo Lee, Hyunju Lee, Seong Gak Jeon, Jin-Hee Park, Yu Gyung Kim, Yuxi Lin, Young-Ho Lee, and et al. 2020. "The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD" Cells 9, no. 9: 1982. https://doi.org/10.3390/cells9091982
APA StylePark, H., Han, K.-M., Jeon, H., Lee, J.-S., Lee, H., Jeon, S. G., Park, J.-H., Kim, Y. G., Lin, Y., Lee, Y.-H., Jeong, Y. H., & Hoe, H.-S. (2020). The MAO Inhibitor Tranylcypromine Alters LPS- and Aβ-Mediated Neuroinflammatory Responses in Wild-type Mice and a Mouse Model of AD. Cells, 9(9), 1982. https://doi.org/10.3390/cells9091982




