Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes?
Abstract
1. Introduction
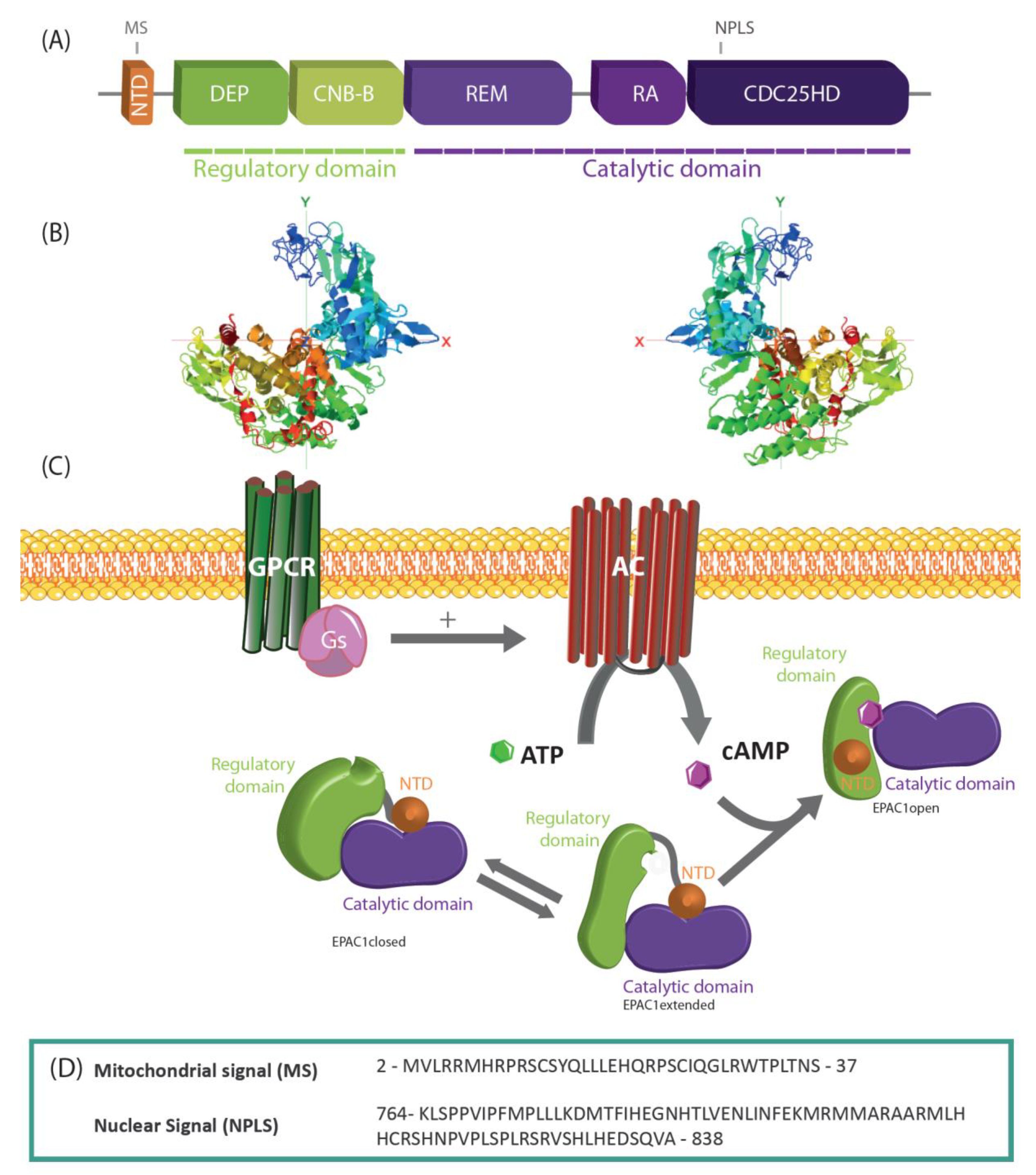
2. Gene, Expression, and Structure
3. EPAC1 Pharmacological Modulators
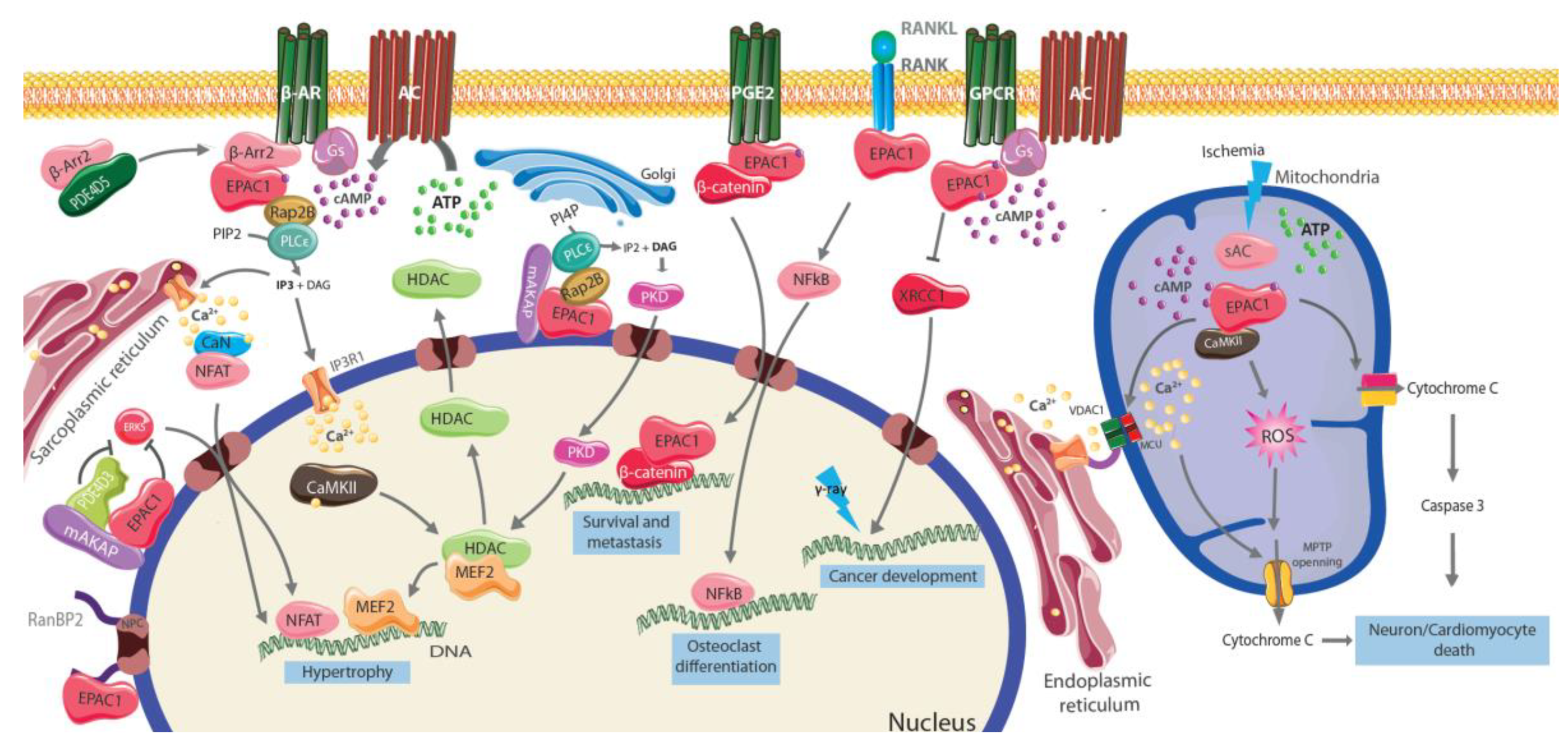
4. EPAC Compartmentation
4.1. Integration of Membrane Signaling
4.2. EPAC Presence and Functional Relevance on the Nucleus
4.3. EPAC Presence and Functional Relevance in the Mitochondria
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chao, Y.C.; Surdo, N.C.; Pantano, S.; Zaccolo, M. Imaging cAMP nanodomains in the heart. Biochem. Soc. Trans. 2019, 47, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. The Cyclic AMP Pathway. Cold Spring Harb. Perspect. Biol. 2012, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pozdniakova, S.; Ladilov, Y. Functional Significance of the Adcy10-Dependent Intracellular cAMP Compartments. J. Cardiovasc. Dev. Dis. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Torres-Quesada, O.; Mayrhofer, J.E.; Stefan, E. The many faces of compartmentalized PKA signalosomes. Cell Signal. 2017, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, J.; Zwartkruis, F.J.T.; Verheijen, M.H.G.; Cool, R.H.; Nijman, S.M.B.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Schleicher, K.; Zaccolo, M. Defining a cellular map of cAMP nanodomains. Mol. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Liu, W.; Ha, Y.; Xia, F.; Zhu, S.; Li, Y.; Shi, S.; Mei, F.C.; Merkley, K.; Vizzeri, G.; Motamedi, M.; et al. Neuronal Epac1 mediates retinal neurodegeneration in mouse models of ocular hypertension. J. Exp. Med. 2020, 1–19. [Google Scholar] [CrossRef]
- Robichaux, W.G.; Cheng, X.X. Intracellular cAMP Sensor EPAC: Physiology, Pathophys-iology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Kumar, N.; Prasad, P.; Jash, E.; Saini, M.; Husain, A.; Goldman, A.; Sehrawat, S. Insights into exchange factor directly activated by cAMP (EPAC) as potential target for cancer treatment. Mol. Cell. Biochem. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Luo, C.; Cheng, X.; Lu, M. Lithium and an EPAC-specific inhibitor ESI-09 synergistically suppress pancreatic cancer cell proliferation and survival. Acta Biochim. Biophys. Sin. 2017, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.-F.; Xu, R. Function of cancer cell-derived extracellular matrix in tumor progression. J. Cancer Metastasis Treat. 2016, 357–364. [Google Scholar] [CrossRef]
- Lezoualc’H, F.; Fazal, L.; Laudette, M.; Conte, C.; Lezoualch, F.; Lezoualc’H, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Bouvet, M.; Blondeau, J.; Lezoualch, F. The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome Pathophysiology. Cells 2019, 8, 1543. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Mei, F.C.; Popov, V.L.; Vergara, L.A.; Cheng, X. Cell Cycle-dependent Subcellular Localization of Exchange Factor Directly Activated by cAMP*. J. Biol. Chem. 2002, 277, 26581–26586. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Lin, S.-Z.; Lee, H.-T.; Fan, J.-R.; Hsu, Y.-H.; Wang, H.-J.; Yu, Y.-L.; Shyu, W.-C. HIF-1α binding to the Epac1 promoter recruits hematopoietic stem cells to the ischemic brain following stroke. J. Mol. Cell Biol. 2012, 4, 184–187. [Google Scholar] [CrossRef]
- Ebrahimighaei, R.; McNeill, M.; Smith, S.A.; Wray, J.P.; Ford, K.L.; Newby, A.C.; Bond, M. Elevated cyclic-AMP represses expression of exchange protein activated by cAMP (EPAC1) by inhibiting YAP-TED activity and HDAC-mediated histone deacetylation. BBA Mol. Cell Res. 2019, 1866, 1634–1649. [Google Scholar] [CrossRef]
- Ponsioen, B.; Gloerich, M.; Ritsma, L.; Rehmann, H.; Bos, J.L.; Jalink, K. Direct Spatial Control of Epac1 by Cyclic AMP. Mol. Cell. Biol. 2009, 29, 2521–2531. [Google Scholar] [CrossRef]
- Enserink, J.M.; Christensen, A.E.; de Rooij, J.; van Triest, M.; Schwede, F.; Genieser, H.G.; Døskeland, S.O.; Blank, J.L.; Bos, J.L. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 2002, 4, 1–6. [Google Scholar] [CrossRef]
- Dao, K.K.; Teigen, K.; Kopperud, R.; Hodneland, E.; Schwede, F.; Christensen, A.E.; Martinez, A.; Døskeland, S.O. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J. Biol. Chem. 2006, 281, 21500–21511. [Google Scholar] [CrossRef]
- Niimura, M.; Miki, T.; Shibasaki, T.; Fujimoto, W.; Iwanaga, T.; Seino, S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J. Cell. Physiol. 2009, 219, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Parnell, E.; Smith, B.O.; Yarwood, S.J. The cAMP sensors, EPAC1 and EPAC2, display distinct subcellular distributions despite sharing a common nuclear pore localisation signal. Cell Signal. 2015, 27, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER: Fully automated protein structure prediction in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, H.; Das, J.; Knipscheer, P.; Wittinghofer, A.; Bos, J.L. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 2006, 439, 625–628. [Google Scholar] [CrossRef]
- Rehmann, H.; Arias-Palomo, E.; Hadders, M.A.; Schwede, F.; Llorca, O.; Bos, J.L. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 2008, 455, 124–127. [Google Scholar] [CrossRef]
- White, M.A.; Tsalkova, T.; Mei, F.C.; Cheng, X. Conformational States of Exchange Protein Directly Activated by cAMP (EPAC1) Revealed by Ensemble Modeling and Integrative Structural Biology. Cells 2020, 9, 35. [Google Scholar] [CrossRef]
- Luchowska-Stańska, U.; Morgan, D.; Yarwood, S.J.; Barker, G. Selective small-molecule EPAC activators. Biochem. Soc. Trans. 2019, 47, 1415–1427. [Google Scholar] [CrossRef]
- Courilleau, D.; Bisserier, M.; Jullian, J.C.; Lucas, A.; Bouyssou, P.; Fischmeister, R.; Blondeau, J.P.; Lezoualc’h, F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J. Biol. Chem. 2012, 287, 44192–44202. [Google Scholar] [CrossRef]
- Schwede, F.; Bertinetti, D.; Langerijs, C.N.; Hadders, M.A.; Wienk, H.; Ellenbroek, J.H.; de Koning, E.J.P.; Bos, J.L.; Herberg, F.W.; Genieser, H.-G.; et al. Structure-Guided Design of Selective Epac1 and Epac2 Agonists. PLoS Biol. 2015, 13, e1002038. [Google Scholar] [CrossRef]
- Vliem, M.J.; Ponsioen, B.; Schwede, F.; Pannekoek, W.J.; Riedl, J.; Kooistra, M.R.H.; Jalink, K.; Genieser, H.G.; Bos, J.L.; Rehmann, H. 8-pCPT-2′-O-Me-cAMP-AM: An improved Epac-selective cAMP analogue. ChemBioChem 2008, 9, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Parnell, E.; McElroy, S.P.; Wiejak, J.; Baillie, G.L.; Porter, A.; Adams, D.R.; Rehmann, H.; Smith, B.O.; Yarwood, S.J. Identification of a novel, small molecule partial agonist for the cyclic AMP sensor, EPAC1. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wiejak, J.; van Basten, B.; Luchowska-Stańska, U.; Hamilton, G.; Yarwood, S.J. The novel exchange protein activated by cyclic AMP 1 (EPAC1) agonist, I942, regulates inflammatory gene expression in human umbilical vascular endothelial cells (HUVECs). Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 264–276. [Google Scholar] [CrossRef]
- Shao, H.; Mohamed, H.; Boulton, S.; Huang, J.; Wang, P.; Chen, H.; Zhou, J.; Luchowska-Stańska, U.; Jentsch, N.G.; Armstrong, A.L.; et al. Mechanism of Action of an EPAC1-Selective Competitive Partial Agonist. J. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luchowska-Stańska, U.; van Basten, B.; Chen, H.; Liu, Z.; Wiejak, J.; Whelan, P.; Morgan, D.; Lochhead, E.; Barker, G.; et al. Synthesis and Biochemical Evaluation of Non-Cyclic Nucleotide Exchange Protein Directly Activated by cAMP 1 (EPAC1) Regulators. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Beck, E.M.; Parnell, E.; Cowley, A.; Porter, A.; Gillespie, J.; Robinson, J.; Robinson, L.; Pannifer, A.D.; Hamon, V.; Jones, P.; et al. Identification of A Novel Class of Benzofuran Oxoacetic Acid-Derived Ligands that Selectively Activate Cellular EPAC1. Cells 2019, 8, 1425. [Google Scholar] [CrossRef]
- Chen, H.; Tsalkova, T.; Mei, F.C.; Hu, Y.; Cheng, X.; Zhou, J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 2012, 22, 4038–4043. [Google Scholar] [CrossRef]
- Tsalkova, T.; Mei, F.C.; Cheng, X. A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS ONE 2012, 7, e30441. [Google Scholar] [CrossRef]
- Almahariq, M.; Tsalkova, T.; Mei, F.C.; Chen, H.; Zhou, J.; Sastry, S.K.; Schwede, F.; Cheng, X. A Novel EPAC-Specific Inhibitor Suppresses Pancreatic Cancer Cell Migration and Invasions. Mol. Pharmacol. 2013, 83, 122–128. [Google Scholar] [CrossRef]
- Gong, B.; Shelite, T.; Mei, F.C.; Ha, T.; Hu, Y.; Xu, G.; Chang, Q.; Wakamiya, M.; Ksiazek, T.G.; Boor, P.J.; et al. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc. Natl. Acad. Sci. USA 2013, 110, 19615–19620. [Google Scholar] [CrossRef]
- Rehmann, H. Epac-Inhibitors: Facts and Artefacts. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Zhu, Y.; Chen, H.; Liu, Z.; Mei, F.C.; Wild, C.; Chen, H.; Cheng, X.; Zhou, J. Structure-Activity Relationship Studies of Substituted 2-(Isoxazol-3-yl)-2-oxo-N′-phenyl-acetohydrazonoyl Cyanide Analogues: Identification of Potent Exchange Proteins Directly Activated by cAMP (EPAC) Antagonists. J. Med. Chem. 2015, 58, 6033–6047. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Rogers, K.E.; Aroonsakool, N.; McCammon, J.A.; Insel, P.A. Allosteric Inhibition of Epac computational modeling and experimental validation to identify allosteric sites and inhibitors. J. Biol. Chem. 2014, 289, 29148–29157. [Google Scholar] [CrossRef] [PubMed]
- Courilleau, D.; Bouyssou, P.; Fischmeister, R.; Lezoualc’h, F.; Blondeau, J.P. The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1). Biochem. Biophys. Res. Commun. 2013, 440, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Boulton, S.; Selvaratnam, R.; Blondeau, J.P.; Lezoualc’H, F.; Melacini, G. Mechanism of Selective Enzyme Inhibition through Uncompetitive Regulation of an Allosteric Agonist. J. Am. Chem. Soc. 2018, 140, 9624–9637. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Boulton, S.; Shao, H.; Akimoto, M.; Natarajan, A.; Cheng, X.; Melacini, G. Recent Advances in EPAC-Targeted Therapies: A Biophysical Perspective. Cells 2019, 8, 1462. [Google Scholar] [CrossRef]
- Laudette, M.; Zuo, H.; 1, F.L.; Schmidt, M. Epac Function and cAMP Scaffolds in the Heart and Lung. J. Cardiovasc. Dev. Dis. 2018, 5, 9. [Google Scholar] [CrossRef]
- Laudette, M.; Coluccia, A.; Sainte-Marie, Y.; Solari, A.; Fazal, L.; Sicard, P.; Silvestri, R.; Mialet-Perez, J.; Pons, S.; Ghaleh, B.; et al. Identification of a pharmacological inhibitor of Epac1 that protects the heart against acute and chronic models of cardiac stress. Cardiovasc. Res. 2019, 115, 1766–1777. [Google Scholar] [CrossRef]
- Bufano, M.; Laudette, M.; Blondeau, J.P.; Lezoualc’h, F.; Nalli, M.; Silvestri, R.; Brancale, A.; Coluccia, A. Modeling Epac1 interactions with the allosteric inhibitor AM-001 by co-solvent molecular dynamics. J. Comput. Aided. Mol. Des. 2020. [Google Scholar] [CrossRef]
- Ali, D.C.; Naveed, M.; Gordon, A.; Majeed, F.; Saeed, M.; Ogbuke, M.I.; Atif, M.; Zubair, H.M.; Changxing, L. β-Adrenergic receptor, an essential target in cardiovascular diseases. Heart Fail. Rev. 2019, 25, 343–354. [Google Scholar] [CrossRef]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Balligand, J.-L. Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovasc. Res. 2016, 111, 1286133. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, C.; Femminella, G.D.; Gambino, G.; Pagano, G.; Allocca, E.; Rengo, C.; Silvestri, C.; Leosco, D.; Ferrara, N.; Rengo, G. Adrenal adrenoceptors in heart failure. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Berthouze-Duquesnes, M.; Lucas, A.; Saulière, A.; Sin, Y.Y.; Laurent, A.-C.; Galés, C.; Baillie, G.; Lezoualc’h, F. Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtype differential effects on cardiac hypertro-phic signaling. Cell Signal. 2013, 25, 970–980. [Google Scholar] [CrossRef]
- Laurent, A.-C.; Bisserier, M.; Lucas, A.; Tortosa, F.; Roumieux, M.; de régibus, A.; Swiader, A.; Sainte-Marie, C.; Heymes, Y.; Vindis, C.; et al. Exchange Protein Directly Activated by cAMP 1 Promotes Autophagy During Cardiomyocyte Hypertroph. 2015. Available online: https://pubmed.ncbi.nlm.nih.gov/25411381/?from_term=laurent+epac+hypertrophy&from_pos=1 (accessed on 17 April 2020).
- Métrich, M.; Lucas, A.; Gastineau, M.; Samuel, J.-L.; Heymes, C.; Morel, E.; Lezoualc’h, F. Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 2008, 102, 959–965. [Google Scholar] [CrossRef]
- Mangmool, S.; Shukla, A.; Rockman, H. B-Arrestin–dependent activation of Ca2+/calmodulin kinase II after B1–adrenergic receptor stimulation. J. Cell Biol. 2010. [Google Scholar] [CrossRef]
- Métrich, M.; Berthouze, M.; Morel, E.; Crozatier, B.; Gomez, A.M.; Lezoualc’h, F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. Eur. J. Physiol. 2010, 459, 535–546. [Google Scholar] [CrossRef]
- Pereira, L.; Ruiz-Hurtado, G.; Morel, E.; Laurent, A.-C.; Métrich, M.; Domínguez, A.; Lauton, S.; Lucas, A.; Benitah, J.-P.; Bers, D.M.; et al. Epac enhances excitation-transcription coupling in cardiac myocytes. J. Mol. Cell. Cardiol. 2012, 52, 283–291. [Google Scholar] [CrossRef]
- Oestreich, E.A.; Malik, S.; Goonasekera, S.A.; Blaxall, B.C.; Kelley, G.G.; Dirksen, R.T.; Smrcka, A.V. Epac and phospholipase Cε regulate Ca2+ release in the heart by activation of protein kinase Cε and calcium-calmodulin kinase II. J. Biol. Chem. 2009, 284, 1514–1522. [Google Scholar] [CrossRef]
- Oestreich, E.A.; Wang, H.; Malik, S.; Kaproth-Joslin, K.A.; Blaxall, B.C.; Kelley, G.G.; Dirksen, R.T.; Smrcka, A.V. Epac-mediated activation of phospholipase Cε plays a critical role in β-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J. Biol. Chem. 2007, 282, 5488–5495. [Google Scholar] [CrossRef]
- Morel, E.; Marcantoni, A.; Gastineau, M.; Birkedal, R.; Rochais, F.; Garnier, A.; Lompré, A.M.; Vandecasteele, G.; Lezoualc’h, F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ. Res. 2005, 97, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Métrich, M.; Laurent, A.C.; Breckler, M.; Duquesnes, N.; Hmitou, I.; Courillau, D.; Blondeau, J.P.; Crozatier, B.; Lezoualc’h, F.; Morel, E. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell Signal. 2010, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Berlin, M.; Schürger, A.; Mathar, I.; Bacmeister, L.; Medert, R.; Frede, W.; Marx, A.; Segin, S.; Londoño, J.E.C. TRP Channels in the Heart. In Neurobiol; TRP Channels; CRC Press: Boca Raton, FL, USA, 2017; pp. 149–185. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A.; Ruiz-Hurtado, G.; Sabourin, J.; Gómez, A.M.; Alvarez, J.L.; Benitah, J.P. Proarrhythmic effect of sustained EPAC activation on TRPC3/4 in rat ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 87, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Aflaki, M.; Qi, X.-Y.; Xiao, L.; Ordog, B.; Tadevosyan, A.; Luo, X.; Maguy, A.; Shi, Y.; Tardif, J.-C.; Nattel, S. Exchange protein directly activated by cAMP mediates slow delayed-rectifier current remodeling by sustained β-adrenergic activation in guinea pig hearts. Circ. Res. 2014, 114, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Clapham, K.R.; Singh, I.; Capuano, I.S.; Rajagopal, S.; Chun, H.J. MEF2 and the Right Ventricle: From Development to Disease. Front. Cardiovasc. Med. 2019, 6, 29. [Google Scholar] [CrossRef]
- Sun, L.; Kondeti, V.K.; Xie, P.; Raparia, K.; Kanwar, Y.S. Epac1-Mediated, High Glucose-Induced Renal Proximal Tubular Cells Hypertrophy via the Akt/p21 Pathway. Am. J. Pathol. 2011, 179, 1706–1718. [Google Scholar] [CrossRef]
- Mediero, A.; Perez-Aso, M.; Cronstein, B.N. Activation of EPAC1/2 is essential for osteoclast formation by modulating NFκB nuclear translocation and actin cytoskeleton rearrangements. FASEB J. 2014, 28, 4901–4913. [Google Scholar] [CrossRef]
- Gloerich, M.; Vliem, M.J.; Prummel, E.; Meijer, L.A.T.; Rensen, M.G.A.; Rehmann, H.; Bos, J.L. The nucleoporin RanBP2 tethers the cAMP effector Epac1 and inhibits its catalytic activity. J. Cell Biol. 2011, 193, 1009–1020. [Google Scholar] [CrossRef]
- Liu, C.; Takahashi, M.; Li, Y.; Dillon, T.J.; Kaech, S.; Stork, P.J.S. The Interaction of Epac1 and Ran Promotes Rap1 Activation at the Nuclear Envelope. Mol. Cell. Biol. 2010, 30, 3956–3969. [Google Scholar] [CrossRef]
- Baameur, F.; Singhmar, P.; Zhou, Y.; Hancock, J.F.; Cheng, X.; Heijnen, C.J.; Kavelaars, A. Epac1 interacts with importin β1 and controls neurite outgrowth independently of cAMP and Rap1 OPEN. Nat. Publ. Gr. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Pereira, L.; Rehmann, H.; Lao, D.H.; Erickson, J.R.; Bossuyt, J.; Chen, J.; Bers, D.M. Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 3991–3996. [Google Scholar] [CrossRef] [PubMed]
- Dodge-Kafka, K.L.; Soughayer, J.; Pare, G.C.; Michel, J.J.C.; Langeberg, L.K.; Kapiloff, M.S.; Scott, J.D. The protein kinase A anchoring protein mAKAP co-ordinates two integrated cAMP effector pathways. Nature 2005, 437, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Evellin, S.; Weernink, P.A.O.; Dorp, F.V.; Rehmann, H.; Lomasney, J.W.; Jakobs, K.H. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 2001, 3, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Malik, S.; Kelley, G.G.; Kapiloff, M.S.; Smrcka, A.V. Phospholipase Cε scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J. Biol. Chem. 2011, 286, 23012–23021. [Google Scholar] [CrossRef]
- Zhang, L.; Malik, S.; Pang, J.; Wang, H.; Park, K.M.; Yule, D.I.; Blaxall, B.C.; Smrcka, A.V. Phospholipase Cε Hydrolyzes Perinuclear Phosphatidylinositol 4-Phosphate to Regulate Cardiac Hypertrophy. Cell 2013, 153, 216–227. [Google Scholar] [CrossRef]
- Jansen, S.R.; Poppinga, W.J.; de Jager, W.; Lezoualc’h, F.; Cheng, X.; Wieland, T.; Yarwood, S.J.; Gosens, R.; Schmidt, M. Epac1 links prostaglandin E 2 to β-catenin-dependent transcription during epithelial-to-mesenchymal transition. Oncotarget 2016, 7, 46354. [Google Scholar] [CrossRef][Green Version]
- Huston, E.; Lynch, M.J.; Mohamed, A.; Collins, D.M.; Hill, E.V.; Macleod, R.; Krause, E.; Baillie, G.S.; Houslay, M.D. EPAC and PKA Allow cAMP Dual Control Over DNA-PK Nuclear Translocation. 2008. Available online: www.pnas.org/cgi/content/full/ (accessed on 30 April 2020).
- Wang, Z.; Liu, D.; Varin, A.; Nicolas, V.; Courilleau, D.; Mateo, P.; Caubere, C.; Rouet, P.; Gomez, A.-M.; Vandecasteele, G.; et al. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef]
- Fazal, L.; Laudette, M.; Paula-Gomes, S.; Pons, S.; Conte, C.; Tortosa, F.; Sicard, P.; Sainte-Marie, Y.; Bisserier, M.; Lairez, O.; et al. Multifunctional mitochondrial Epac1 controls myocardial cell death. Circ. Res. 2017, 120, 645–657. [Google Scholar] [CrossRef]
- Wang, H.; Iii, W.G.R.; Wang, Z.; Mei, F.C.; Cai, M.; Du, G.; Chen, J.; Cheng, X. Inhibition of Epac1 suppresses mitochondrial fission and reduces neointima formation induced by vascular injury OPEN. Sci. Rep. 2016, 6, 36552. [Google Scholar] [CrossRef]
- Del, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Mangmool, S.; Hemplueksa, P.; Parichatikanond, W.; Chattipakorn, N. Epac is Required for GLP-1R-Mediated Inhibition of Oxidative Stress and Apoptosis in Cardiomyocytes. Mol. Endocrinol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, C.I.; Khaliulin, I.; Bond, M.; James, A.F.; Dyar, Z.; Amini, R.; Johnson, J.L.; Suleiman, M.-S. Functional and cardioprotective effects of simultaneous and individual activation of protein kinase A and Epac. Br. J. Pharmacol. 2017, 174, 438. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, V.; Appukuttan, A.; Aslam, M.; Reusch, P.; Regitz-Zagrosek, V.; Ladilov, Y. Regulation of AMPK activity by type 10 adenylyl cyclase: Contribution to the mitochondrial biology, cellular redox and energy homeostasis. Cell. Mol. Life Sci. 2019, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Stokman, G.; Yan, K.; Ramaiahgari, S.; Verbeek, F.; de Graauw, M.; van de Water, B.; Price, L.S. cAMP signalling protects proximal tubular epithelial cells from cisplatin-induced apoptosis via activation of Epac. Br. J. Pharmacol. 2011, 165, 1137–1150. [Google Scholar] [CrossRef]
- Szanda, G.; Wisniewski, É.; Rajki, A.; Spät, A. Mitochondrial cAMP exerts positive feedback on mitochondrial Ca2+ uptake via the recruitment of Epac1. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Okumura, S.; Fujita, T.; Cai, W.; Jin, M.; Namekata, I.; Mototani, Y.; Jin, H.; Ohnuki, Y.; Tsuneoka, Y.; Kurotani, R.; et al. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J. Clin. Investig. 2014, 124. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokoyama, U.; Abe, T.; Kiyonari, H.; Yamashita, N.; Kato, Y.; Kurotani, R.; Sato, M.; Okumura, S.; Ishikawa, Y. Differential Roles of Epac in Regulating Cell Death in Neuronal and Myocardial Cells. J. Biol. Chem. 2010, 285, 24248–24259. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, S.; Dabral, S.; Singh, S.; Sehrawat, S. Role of exchange protein directly activated by cAMP (EPAC1) in breast cancer cell migration and apoptosis. Mol. Cell. Biochem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Moon, E.Y.; Lee, G.H.; Lee, M.S.; Kim, H.M.; Lee, J.W. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci. 2012, 90, 373–380. [Google Scholar] [CrossRef]
- Cho, E.A.; Juhnn, Y.S. The cAMP signaling system inhibits the repair of γ-ray-induced DNA damage by promoting Epac1-mediated proteasomal degradation of XRCC1 protein in human lung cancer cells. Biochem. Biophys. Res. Commun. 2012, 422, 256–262. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formoso, K.; Lezoualc’h, F.; Mialet-Perez, J. Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes? Cells 2020, 9, 1954. https://doi.org/10.3390/cells9091954
Formoso K, Lezoualc’h F, Mialet-Perez J. Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes? Cells. 2020; 9(9):1954. https://doi.org/10.3390/cells9091954
Chicago/Turabian StyleFormoso, Karina, Frank Lezoualc’h, and Jeanne Mialet-Perez. 2020. "Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes?" Cells 9, no. 9: 1954. https://doi.org/10.3390/cells9091954
APA StyleFormoso, K., Lezoualc’h, F., & Mialet-Perez, J. (2020). Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes? Cells, 9(9), 1954. https://doi.org/10.3390/cells9091954






