AMPK-Dependent Mechanisms but Not Hypothalamic Lipid Signaling Mediates GH-Secretory Responses to GHRH and Ghrelin
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Procedure
2.2. Experimental Design
2.3. Hormone Assays
2.4. Statistical Analysis
3. Results
3.1. Blockade of Hypothalamic AMPK blunts GHRH- and Ghrelin-Stimulated GH Secretion
3.2. Hypothalamic Lipid Metabolism does not Affect GHRH- or Ghrelin-Stimulated GH Secretion
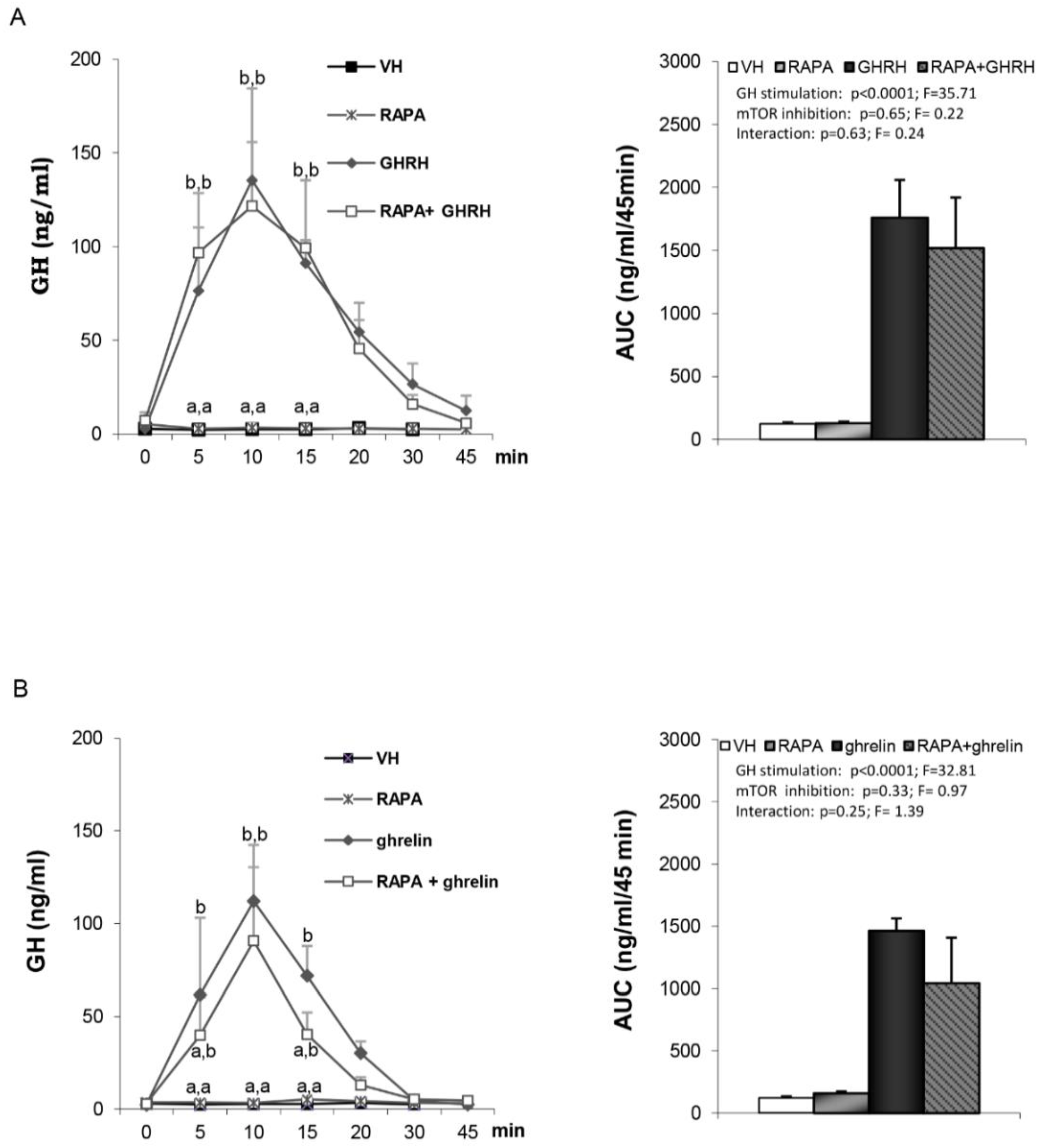
3.3. Hypothalamic mTOR is not Involved in GHRH- and Ghrelin-Stimulated GH Secretion
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dieguez, C.; Casanueva, F.F. Influence of metabolic substrates and obesity on growth hormone secretion. Trends Endocrinol. Metab. 1995, 6, 55–59. [Google Scholar] [CrossRef]
- Oh, T.S.; Cho, H.; Cho, J.H.; Yu, S.W.; Kim, E.K. Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy 2016, 12, 2009–2025. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Varela, L.; Vazquez, M.J.; Da Boit, K.; Saha, A.K.; Cordido, F.; Dieguez, C.; Lopez, M. Influence of ghrelin and growth hormone deficiency on AMP-activated protein kinase and hypothalamic lipid metabolism. J. Neuroendocr. 2010, 22, 543–556. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Liu, Z.W.; Walllingford, N.; Erion, D.M.; Borok, E.; Friedman, J.M.; Tschop, M.H.; Shanabrough, M.; Cline, G.; Shulman, G.I.; et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008, 454, 846–851. [Google Scholar] [CrossRef]
- Wen, J.P.; Liu, C.E.; Hu, Y.T.; Chen, G.; Lin, L.X. Globular adiponectin regulates energy homeostasis through AMP-activated protein kinase-acetyl-CoA carboxylase (AMPK/ACC) pathway in the hypothalamus. Mol. Cell. Biochem. 2010, 344, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Nogueiras, R.; Tena-Sempere, M.; Dieguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Lu, N.; Hu, C.; Liu, W.; Sun, Y.; Wang, X.; You, Q.; Gu, C.; Xi, T.; Guo, Q. Beclin 1-mediated autophagy in hepatocellular carcinoma cells: Implication in anticancer efficiency of oroxylin A via inhibition of mTOR signaling. Cell Signal. 2012, 24, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller-Herzog, C.; Hall, M.N. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012, 22, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xu, Y.; Liu, F. Hypothalamic roles of mTOR complex I: Integration of nutrient and hormone signals to regulate energy homeostasis. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E994–E1002. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Saucisse, N.; Cota, D. Influence of mTOR in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 67–77. [Google Scholar] [CrossRef]
- Leprivier, G.; Rotblat, B. How does mTOR sense glucose starvation? AMPK is the usual suspect. Cell Death Discov. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Cork, G.K.; Thompson, J.; Slawson, C. Real Talk: The Inter-play Between the mTOR, AMPK, and Hexosamine Biosynthetic Pathways in Cell Signaling. Front. Endocrinol. (Lausanne) 2018, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Lage, R.; Vazquez, M.J.; Varela, L.; Saha, A.K.; Vidal-Puig, A.; Nogueiras, R.; Dieguez, C.; Lopez, M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010, 24, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Lei, Q.Y. Metabolite sensing and signaling in cell metabolism. Signal Transduct. Target. 2018, 3, 30. [Google Scholar] [CrossRef]
- Martins, L.; Fernandez-Mallo, D.; Novelle, M.G.; Vazquez, M.J.; Tena-Sempere, M.; Nogueiras, R.; Lopez, M.; Dieguez, C. Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS ONE 2012, 7, e46923. [Google Scholar] [CrossRef]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef]
- Kola, B.; Hubina, E.; Tucci, S.A.; Kirkham, T.C.; Garcia, E.A.; Mitchell, S.E.; Williams, L.M.; Hawley, S.A.; Hardie, D.G.; Grossman, A.B.; et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J. Biol. Chem. 2005, 280, 25196–25201. [Google Scholar] [CrossRef]
- Lopez, M.; Lage, R.; Saha, A.K.; Perez-Tilve, D.; Vazquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodriguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef]
- Roa, J.; Tena-Sempere, M. Energy balance and puberty onset: Emerging role of central mTOR signaling. Trends Endocrinol. Metab. 2010, 21, 519–528. [Google Scholar] [CrossRef]
- Roa, J.; Garcia-Galiano, D.; Varela, L.; Sanchez-Garrido, M.A.; Pineda, R.; Castellano, J.M.; Ruiz-Pino, F.; Romero, M.; Aguilar, E.; Lopez, M.; et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 2009, 150, 5016–5026. [Google Scholar] [CrossRef]
- Roa, J.; Barroso, A.; Ruiz-Pino, F.; Vazquez, M.J.; Seoane-Collazo, P.; Martinez-Sanchez, N.; Garcia-Galiano, D.; Ilhan, T.; Pineda, R.; Leon, S.; et al. Metabolic regulation of female puberty via hypothalamic AMPK-kisspeptin signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E10758–E10767. [Google Scholar] [CrossRef] [PubMed]
- Seoane, L.M.; Lopez, M.; Tovar, S.; Casanueva, F.F.; Senaris, R.; Dieguez, C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology 2003, 144, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Seoane, L.M.; Tovar, S.; Baldelli, R.; Arvat, E.; Ghigo, E.; Casanueva, F.F.; Dieguez, C. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. Eur. J. Endocrinol. 2000, 143, R7–R9. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef]
- Casanueva, F.F.; Dieguez, C. Neuroendocrine regulation and actions of leptin. Front. Neuroendocr. 1999, 20, 317–363. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, K.K. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. Int. J. Mol. Sci. 2018, 19, 3552. [Google Scholar] [CrossRef]
- Palomo-Guerrero, M.; Fado, R.; Casas, M.; Perez-Montero, M.; Baena, M.; Helmer, P.O.; Dominguez, J.L.; Roig, A.; Serra, D.; Hayen, H.; et al. Sensing of nutrients by CPT1C regulates late endosome/lysosome anterograde transport and axon growth. Elife 2019, 8. [Google Scholar] [CrossRef]
- Mir, J.F.; Zagmutt, S.; Lichtenstein, M.P.; Garcia-Villoria, J.; Weber, M.; Gracia, A.; Fabrias, G.; Casas, J.; Lopez, M.; Casals, N.; et al. Ghrelin Causes a Decline in GABA Release by Reducing Fatty Acid Oxidation in Cortex. Mol. Neurobiol. 2018, 55, 7216–7228. [Google Scholar] [CrossRef]
- Ramirez, S.; Martins, L.; Jacas, J.; Carrasco, P.; Pozo, M.; Clotet, J.; Serra, D.; Hegardt, F.G.; Dieguez, C.; Lopez, M.; et al. Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 2013, 62, 2329–2337. [Google Scholar] [CrossRef]
- Gao, S.; Moran, T.H.; Lopaschuk, G.D.; Butler, A.A. Hypothalamic malonyl-CoA and the control of food intake. Physiol. Behav. 2013, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vazquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef]
- Martinez de Morentin, P.B.; Gonzalez-Garcia, I.; Martins, L.; Lage, R.; Fernandez-Mallo, D.; Martinez-Sanchez, N.; Ruiz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buque, X.; Aurrekoetxea, I.; Delgado, T.C.; Vazquez-Martinez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 Axis Mediates the Central Actions of Thyroid Hormones on Energy Balance. Cell Metab. 2017, 26, 212–229.e212. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Miralpeix, C.; Fosch, A.; Pozo, M.; Calderon-Dominguez, M.; Perpinya, X.; Vellvehi, M.; Lopez, M.; Herrero, L.; Serra, D.; et al. CPT1C in the ventromedial nucleus of the hypothalamus is necessary for brown fat thermogenesis activation in obesity. Mol. Metab. 2019, 19, 75–85. [Google Scholar] [CrossRef]
- Avendano, M.S.; Vazquez, M.J.; Tena-Sempere, M. Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update 2017, 23, 737–763. [Google Scholar] [CrossRef]
- Roa-Mansergas, X.; Fado, R.; Atari, M.; Mir, J.F.; Muley, H.; Serra, D.; Casals, N. CPT1C promotes human mesenchymal stem cells survival under glucose deprivation through the modulation of autophagy. Sci. Rep. 2018, 8, 6997. [Google Scholar] [CrossRef]
- Tannenbaum, G.S.; Martin, J.B. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 1976, 98, 562–570. [Google Scholar] [CrossRef]
- Plotsky, P.M.; Vale, W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science 1985, 230, 461–463. [Google Scholar] [CrossRef]
- Kawano, H.; Daikoku, S.; Saito, S. Immunohistochemical studies of intrahypothalamic somatostatin-containing neurons in rat. Brain Res. 1982, 242, 227–232. [Google Scholar] [CrossRef]
- Broglio, F.; Prodam, F.; Riganti, F.; Muccioli, G.; Ghigo, E. Ghrelin: From somatotrope secretion to new perspectives in the regulation of peripheral metabolic functions. Front. Horm. Res. 2006, 35, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Benso, A.; Gottero, C.; Prodam, F.; Grottoli, S.; Tassone, F.; Maccario, M.; Casanueva, F.F.; Dieguez, C.; Deghenghi, R.; et al. Effects of glucose, free fatty acids or arginine load on the GH-releasing activity of ghrelin in humans. Clin. Endocrinol. (Oxf.) 2002, 57, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, R.; Bellone, S.; Castellino, N.; Petri, A.; Rapa, A.; Vivenza, D.; Bellone, J.; Broglio, F.; Ghigo, E.; Bona, G. Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin. Endocrinol. (Oxf.) 2006, 64, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef]
- Velasquez, D.A.; Martinez, G.; Romero, A.; Vazquez, M.J.; Boit, K.D.; Dopeso-Reyes, I.G.; Lopez, M.; Vidal, A.; Nogueiras, R.; Dieguez, C. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011, 60, 1177–1185. [Google Scholar] [CrossRef]
- Bartke, A. Pleiotropic effects of growth hormone signaling in aging. Trends Endocrinol. Metab. 2011, 22, 437–442. [Google Scholar] [CrossRef]
- Hayashi, A.A.; Proud, C.G. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1647–E1655. [Google Scholar] [CrossRef]
- Gorshtein, A.; Rubinfeld, H.; Kendler, E.; Theodoropoulou, M.; Cerovac, V.; Stalla, G.K.; Cohen, Z.R.; Hadani, M.; Shimon, I. Mammalian target of rapamycin inhibitors rapamycin and RAD001 (everolimus) induce anti-proliferative effects in GH-secreting pituitary tumor cells in vitro. Endocr. Relat. Cancer 2009, 16, 1017–1027. [Google Scholar] [CrossRef]
- Tulipano, G.; Faggi, L.; Cacciamali, A.; Spinello, M.; Cocchi, D.; Giustina, A. Role of AMP-activated protein kinase activators in antiproliferative multi-drug pituitary tumour therapies: Effects of combined treatments with compounds affecting the mTOR-p70S6 kinase axis in cultured pituitary tumour cells. J. Neuroendocrinol. 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef]




| Compound | Target/Action | Commercial Source | Dose (Ref No) |
|---|---|---|---|
| compound C (CC) | AMPK inhibition | P5499; Sigma Aldrich (St Louis, MO, USA) | 10 μg [4,18] |
| etomoxir (ETOM) | CPT1 inhibition | E1905; Sigma Aldrich (St Louis, MO, USA) | 10 μg [13,18] |
| rapamycin (RAPA) | mTOR inhibition | 553210; Calbiochem (San Diego, CA, USA) | 50 μg [15,20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, M.J.; Novelle, M.G.; Rodríguez-Pacheco, F.; Lage, R.; Varela, L.; López, M.; Pinilla, L.; Tena-Sempere, M.; Diéguez, C. AMPK-Dependent Mechanisms but Not Hypothalamic Lipid Signaling Mediates GH-Secretory Responses to GHRH and Ghrelin. Cells 2020, 9, 1940. https://doi.org/10.3390/cells9091940
Vázquez MJ, Novelle MG, Rodríguez-Pacheco F, Lage R, Varela L, López M, Pinilla L, Tena-Sempere M, Diéguez C. AMPK-Dependent Mechanisms but Not Hypothalamic Lipid Signaling Mediates GH-Secretory Responses to GHRH and Ghrelin. Cells. 2020; 9(9):1940. https://doi.org/10.3390/cells9091940
Chicago/Turabian StyleVázquez, María J., Marta G. Novelle, Francisca Rodríguez-Pacheco, Ricardo Lage, Luis Varela, Miguel López, Leonor Pinilla, Manuel Tena-Sempere, and Carlos Diéguez. 2020. "AMPK-Dependent Mechanisms but Not Hypothalamic Lipid Signaling Mediates GH-Secretory Responses to GHRH and Ghrelin" Cells 9, no. 9: 1940. https://doi.org/10.3390/cells9091940
APA StyleVázquez, M. J., Novelle, M. G., Rodríguez-Pacheco, F., Lage, R., Varela, L., López, M., Pinilla, L., Tena-Sempere, M., & Diéguez, C. (2020). AMPK-Dependent Mechanisms but Not Hypothalamic Lipid Signaling Mediates GH-Secretory Responses to GHRH and Ghrelin. Cells, 9(9), 1940. https://doi.org/10.3390/cells9091940









