Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production
Abstract
:1. Introduction
2. Regulators of IGF Levels Clinically
3. Molecular Regulation of IGF-I Production
4. GRP94
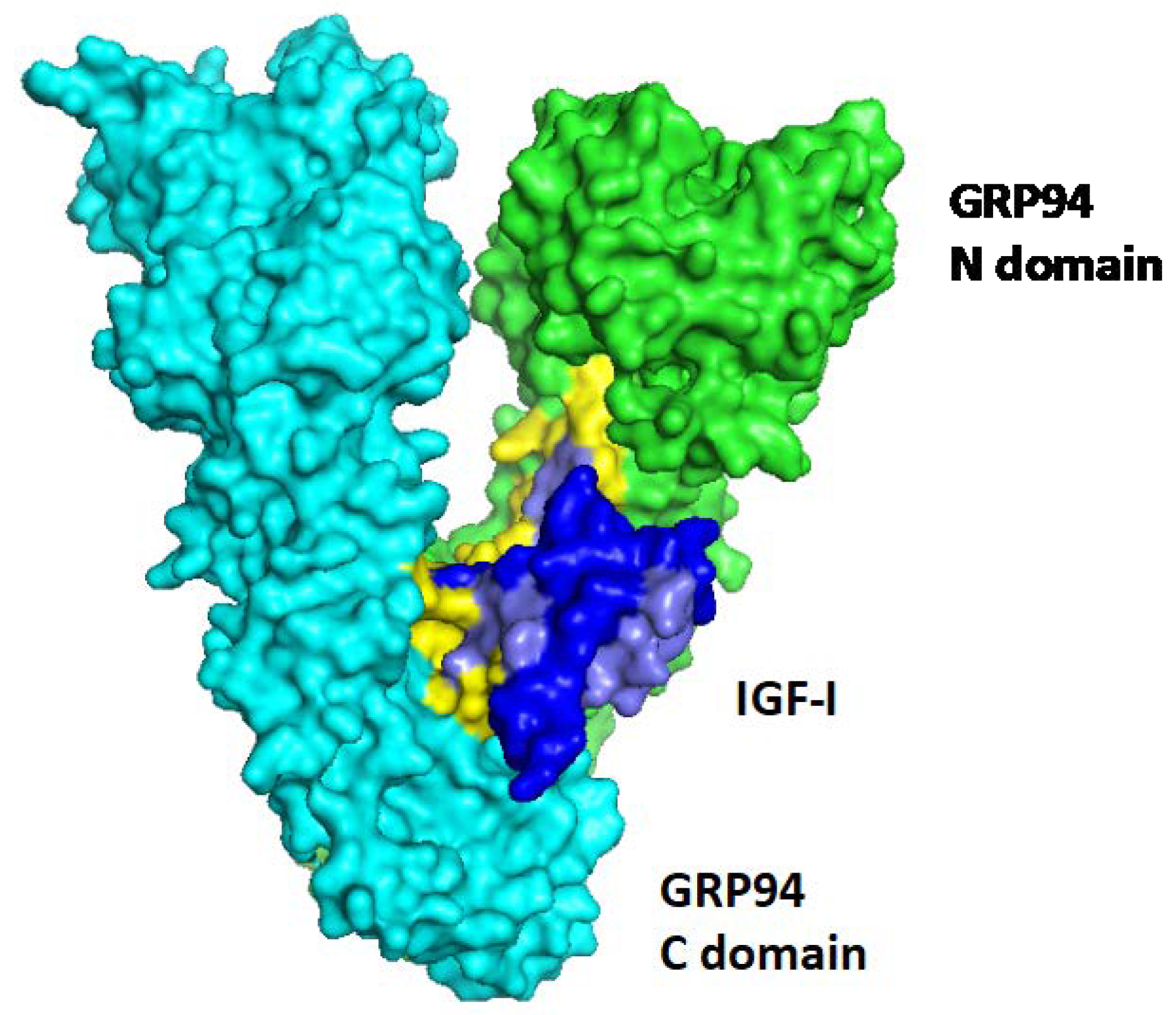
5. GRP94 as an Obligate Chaperone for IGF-I and IGF-II Production
6. Conclusions
6.1. Implications for Novel Mechanisms of Idiopathic Short Stature
6.2. Implications for Cancer Treatment
Funding
Conflicts of Interest
References
- Le, R.D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar]
- Grimberg, A.; Divall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. On Behalf Of The Drug And Therapeutics Committee And Ethics Committee Of The Pediatric Endocrine Society Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collett-Solberg, P.F.; Ambler, G.; Backeljauw, P.F.; Bidlingmaier, M.; Biller, B.M.; Boguszewski, M.C.; Cheung, P.T.; Choong, C.S.Y.; Cohen, L.E.; Cohen, P.; et al. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm. Res. Paediatr. 2019, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.F.; Albertsson-Wikland, K.; Rosberg, S.; Ranke, M.B. Serum levels of IGF-I and IGF binding protein 3 reflect spontaneous growth hormone secretion. J. Clin. Endocrinol. Metab. 1993, 76, 1610–1616. [Google Scholar] [PubMed]
- Allen, D.B.; Backeljauw, P.; Bidlingmaier, M.; Biller, B.M.K.; Boguszewski, M.; Burman, P.; Butler, G.; Chihara, K.; Christiansen, J.S.; Cianfarani, S.; et al. GH safety workshop position paper: A critical appraisal of recombinant human GH therapy in children and adults. Eur. J. Endocrinol. 2015, 174, P1–P9. [Google Scholar] [CrossRef]
- Katznelson, L.; Laws, E.R.; Melmed, S.; Molitch, M.; Murad, M.H.; Utz, A.; Wass, J.A.H. Acromegaly: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Qiao, N.; He, M.; Shen, M.; Zhang, Q.; Zhang, Z.; Shou, X.; Wang, Y.; Zhao, Y.; Tritos, N.A. Comparative Efficacy Of Medical Treatment For Acromegaly: A Systemic Review And Network Meta-Analysis Of Integrated Randomized Trials And Observational Studies. Endocr. Pr. 2020, 26, 454–462. [Google Scholar] [CrossRef]
- Hawkes, C.P.; Grimberg, A. Insulin-Like Growth Factor-I is a Marker for the Nutritional State. Pediatr. Endocrinol. Rev. 2015, 13, 499–511. [Google Scholar]
- Misra, M.; Klibanski, A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014, 2, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2016, 13, 174–186. [Google Scholar] [CrossRef]
- Schneider, H.J.; Saller, B.; Klotsche, J.; Erwa, W.; Wittchen, H.-U.; März, W.; Stalla, G.K. Opposite associations of age-dependent insulin-like growth factor-I standard deviation scores with nutritional state in normal weight and obese subjects. Eur. J. Endocrinol. 2006, 154, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Klaauw, A.; Biermasz, N.R.; Zelissen, P.M.J.; Pereira, A.M.; Lentjes, E.G.W.M.; Smit, J.W.; Van Thiel, S.W.; Romijn, J.A.; Roelfsema, F. Administration route-dependent effects of estrogens on IGF-I levels during fixed GH replacement in women with hypopituitarism. Eur. J. Endocrinol. 2007, 157, 709–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofqvist, C.; Andersson, E.; Gelander, L.; Rosberg, S.; Blum, W.F.; Wikland, A.K. Reference values for IGF-I throughout childhood and adolescence: A model that accounts simultaneously for the effect of gender, age, and puberty. J. Clin. Endocrinol. Metab. 2001, 86, 5870–5876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Sohmiya, M.; Oka, N.; Kato, Y. Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults. Eur. J. Endocrinol. 1991, 124, 497–500. [Google Scholar] [CrossRef]
- Chanson, P.; Arnoux, A.; Mavromati, M.; Brailly-Tabard, S.; Massart, C.; Young, J.; Piketty, M.-L.; Souberbielle, J.-C. VARIETE Investigators Reference Values for IGF-I Serum Concentrations: Comparison of Six Immunoassays. J. Clin. Endocrinol. Metab. 2016, 101, 3450–3458. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, D.R.; Participants, O.B.O.T.C. Consensus Statement on the Standardization and Evaluation of Growth Hormone and Insulin-like Growth Factor Assays. Clin. Chem. 2011, 57, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2013, 59, 240–249. [Google Scholar] [CrossRef]
- Wakeling, E.L.; Brioude, F.; Lokulo-Sodipe, O.; O’Connell, S.M.; Salem, J.; Bliek, J.; Canton, A.P.M.; Chrzanowska, K.H.; Davies, J.H.; Dias, R.P.; et al. Diagnosis and management of Silver–Russell syndrome: First international consensus statement. Nat. Rev. Endocrinol. 2016, 13, 105–124. [Google Scholar] [CrossRef]
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Yearb. Paediatr. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef]
- Camacho-Hubner, C. Normal Physiology of Growth Hormone and Insulin-Like Growth Factors in Childhood. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK279164/ (accessed on 6 August 2020).
- Yakar, S.; Werner, H.; Rosen, C.J.; Rosen, C. 40 YEARS OF IGF1: Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef]
- Teglund, S.; McKay, C.; Schuetz, E.; Van Deursen, J.M.; Stravopodis, D.J.; Wang, D.; Brown, M.; Bodner, S.; Grosveld, G.; Ihle, J.N. Stat5a and Stat5b Proteins Have Essential and Nonessential, or Redundant, Roles in Cytokine Responses. Cell 1998, 93, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, A.A.; Proud, C.G. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am. J. Physiol. Metab. 2007, 292, E1647–E1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-J.; Elsasser, T.; Kahl, S. AKT/eNOS signaling module functions as a potential feedback loop in the growth hormone signaling pathway. J. Mol. Signal. 2009, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Lanning, N.J.; Carter-Su, C. JAK2, But Not Src Family Kinases, Is Required for STAT, ERK, and Akt Signaling in Response to Growth Hormone in Preadipocytes and Hepatoma Cells. Mol. Endocrinol. 2008, 22, 1825–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwa, V. STAT5B deficiency: Impacts on human growth and immunity. Growth Horm. IGF Res. 2016, 28, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofoed, E.M.; Hwa, V.; Little, B.; Woods, K.A.; Buckway, C.K.; Tsubaki, J.; Pratt, K.L.; Bezrodnik, L.; Jasper, H.; Tepper, A.; et al. Growth Hormone Insensitivity Associated with aSTAT5bMutation. N. Engl. J. Med. 2003, 349, 1139–1147. [Google Scholar] [CrossRef]
- Rinderknecht, E.; Humbel, R.E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Boil. Chem. 1978, 253, 2769–2776. [Google Scholar]
- Tricoli, J.V.; Rall, L.B.; Scott, J.; Bell, G.I.; Shows, T.B. Localization of insulin-like growth factor genes to human chromosomes 11 and 12. Nature 1984, 310, 784–786. [Google Scholar] [CrossRef]
- Hwa, V.; Oh, Y.; Rosenfeld, G.R. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef]
- Dauber, A.; Muñoz-Calvo, M.T.; Barrios, V.; Domené, H.M.; Kloverpris, S.; Serra-Juhé, C.; Desikan, V.; Pozo, J.; Muzumdar, R.; Martos-Moreno, G.Á.; et al. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF -I availability. EMBO Mol. Med. 2016, 8, 363–374. [Google Scholar] [CrossRef]
- Li, J.; Choi, E.; Yu, H.; Bai, X.-C. Structural basis of the activation of type 1 insulin-like growth factor receptor. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Slaaby, R.; Schäffer, L.; Lautrup-Larsen, I.; Andersen, A.S.; Shaw, A.C.; Mathiasen, I.S.; Brandt, J. Hybrid Receptors Formed by Insulin Receptor (IR) and Insulin-like Growth Factor I Receptor (IGF-IR) Have Low Insulin and High IGF-1 Affinity Irrespective of the IR Splice Variant. J. Boil. Chem. 2006, 281, 25869–25874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foti, M.; Moukil, M.A.; Dudognon, P.; Carpentier, J.-L. Insulin and IGF-1 receptor trafficking and signalling. Novartis Found. Symp. 2004, 262, 125–147. [Google Scholar] [CrossRef]
- Maile, L.A.; Clemmons, D.R. The αVβ3 Integrin Regulates Insulin-Like Growth Factor I (IGF-I) Receptor Phosphorylation by Altering the Rate of Recruitment of the Src-Homology 2-Containing Phosphotyrosine Phosphatase-2 to the Activated IGF-I Receptor. Endocrinology 2002, 143, 4259–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.; Jones, E.Y.; Forbes, B.E.; Jones, E.Y. Keeping IGF-II under control: Lessons from the IGF-II–IGF2R crystal structure. Trends Biochem. Sci. 2009, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.; Varewijck, A.J.; Brugts, M.P. The insulin-like growth factor-I receptor stimulating activity (IRSA) in health and disease. Growth Horm. IGF Res. 2019, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Ostrovsky, O.; Ahmed, N.T.; Argon, Y. The Chaperone Activity of GRP94 Toward Insulin-like Growth Factor II Is Necessary for the Stress Response to Serum Deprivation. Mol. Boil. Cell 2009, 20, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.L.; Macer, D.R.; Wooding, F.B. Endoplasmin is a reticuloplasmin. J. Cell Sci. 1988, 90, 485–491. [Google Scholar]
- Soldano, K.L.; Jivan, A.; Nicchitta, C.V.; Gewirth, D.T. Structure of the N-terminal Domain of GRP94. J. Boil. Chem. 2003, 278, 48330–48338. [Google Scholar] [CrossRef] [Green Version]
- Jahn, M.; Tych, K.; Girstmair, H.; Steinmaßl, M.; Hugel, T.; Buchner, J.; Rief, M. Folding and Domain Interactions of Three Orthologs of Hsp90 Studied by Single-Molecule Force Spectroscopy. Structure 2018, 26, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, C.; Sriram, U.; Ciric, B.; Ostrovsky, O.; Gallucci, S.; Argon, Y. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen-presenting cells. Int. Immunol. 2006, 18, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Blachere, N.E.; Srivastava, P.K. Heat shock protein-based cancer vaccines and related thoughts on immunogenicity of human tumors. Semin. Cancer Boil. 1995, 6, 349–355. [Google Scholar] [CrossRef]
- Ostrovsky, O.; Makarewich, C.A.; Snapp, E.L.; Argon, Y. An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc. Natl. Acad. Sci. USA 2009, 106, 11600–11605. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Hong, F.; Gewirth, D.; Guo, B.; Liu, B.; Li, H.Z. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J. Biol. Chem. 2012, 287, 6735–6742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansa-Addo, E.A.; Thaxton, J.; Hong, F.; Wu, X.B.; Zhang, Y.L.; Fufle, W.C.; Metelli, A.; Riesenberg, B.; Williams, K.; Gewirth, T.D.; et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr. Top. Med. Chem. 2016, 16, 2765–2778. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Liu, B.; Chiosis, G.; Gewirth, D.T.; Li, Z. α7 Helix Region of αI Domain Is Crucial for Integrin Binding to Endoplasmic Reticulum Chaperone gp96. J. Boil. Chem. 2013, 288, 18243–18248. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yang, Y.; Qiu, Z.; Staron, M.; Hong, F.; Li, Y.; Wu, S.; Li, Y.; Hao, B.; Bona, R.; et al. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat. Commun. 2010, 1, 79. [Google Scholar] [CrossRef] [Green Version]
- Melnick, J.; Dul, J.L.; Argon, Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature 1994, 370, 373–375. [Google Scholar] [CrossRef]
- Gidalevitz, T.; Simen, B.B.; Ostrovsky, O.; Vogen, S.M.; Dul, J.L.; Argon, Y. GRP94 activity is necessary for completion of immunoglobulin light chain folding. In Revision. 2011. [Google Scholar]
- Liu, B.; Li, Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood 2008, 112, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Staron, M.; Yang, Y.; Liu, B.; Li, J.; Shen, Y.; Zuniga-Pflucker, J.C.; Aguila, H.L.; Goldschneider, I.; Li, Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood 2010, 115, 2380–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weekes, M.P.; Antrobus, R.; Talbot, S.; Hör, S.; Simecek, N.; Smith, D.L.; Bloor, S.; Randow, F.; Lehner, P.J. Proteomic Plasma Membrane Profiling Reveals an Essential Role for gp96 in the Cell Surface Expression of LDLR Family Members, Including the LDL Receptor and LRP6. J. Proteome Res. 2012, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D.; et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staron, M.; Wu, S.; Hong, F.; Stojanovic, A.; Du, P.X.; Bona, R.; Liu, B.; Li, L.Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood 2011, 117, 7136–7144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, E.R.; Park, S.; James, J.K.; Makarewich, C.A.; Philippou, A.; Eletto, D.; Lei, H.; Brisson, B.; Ostrovsky, O.; Li, Z.; et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012, 26, 3691–3702. [Google Scholar] [CrossRef] [Green Version]
- Khilji, M.S.; Bresson, S.E.; Verstappen, D.; Pihl, C.; Andersen, P.A.K.; Agergaard, J.B.; Dahlby, T.; Bryde, T.H.; Klindt, K.; Nielsen, C.K.; et al. The inducible beta5i proteasome subunit contributes to proinsulin degradation in GRP94-deficient beta-cells and is overexpressed in type 2 diabetes pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E892–E900. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Chen, L.B.; Nigam, S.K. Several endoplasmic reticulum stress proteins, including ERp72, interact with thyroglobulin during its maturation. J. Boil. Chem. 1994, 269, 22990–22995. [Google Scholar]
- Di Jeso, B.; Park, Y.-N.; Ulianich, L.; Treglia, A.S.; Urbanas, M.L.; High, S.; Arvan, P. Mixed-Disulfide Folding Intermediates between Thyroglobulin and Endoplasmic Reticulum Resident Oxidoreductases ERp57 and Protein Disulfide Isomerase. Mol. Cell. Boil. 2005, 25, 9793–9805. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, B.X.; Metelli, A.; Thaxton, J.E.; Hong, F.; Rachidi, S.; Ansa-Addo, E.; Sun, S.; Vasu, C.; Yang, Y.; et al. GP96 is a GARP chaperone and controls regulatory T cell functions. J. Clin. Investig. 2015, 125, 859–869. [Google Scholar] [CrossRef]
- Vajdos, F.F.; Ultsch, M.; Schaffer, M.L.; Deshayes, K.D.; Liu, J.; Skelton, N.J.; De Vos, A.M. Crystal structure of human insulin-like growth factor-1: Detergent binding inhibits binding protein interactions. Biochemistry 2001, 40, 11022–11029. [Google Scholar] [CrossRef]
- Dollins, D.E.; Warren, J.J.; Immormino, R.M.; Gewirth, D.T. Structures of GRP94-Nucleotide Complexes Reveal Mechanistic Differences between the hsp90 Chaperones. Mol. Cell 2007, 28, 41–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, B.G.; Hourai, Y.; Weng, Z. Accelerating Protein Docking in ZDOCK Using an Advanced 3D Convolution Library. PLoS ONE 2011, 6, e24657. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Johnson, J.L. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 2010, 93, 211–217. [Google Scholar] [CrossRef] [PubMed]
- King, F.W.; Wawrzynów, A.; Höhfeld, J.; Zylicz, M. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 2001, 20, 6297–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, G.; Nordenson, C.; Still, M.; Rönnlund, A.; Tuck, S.; Naredi, P.L.J. ASNA-1 Positively Regulates Insulin Secretion in C. elegans and Mammalian Cells. Cell 2007, 128, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 Chaperone Machinery. J. Boil. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef] [Green Version]
- Hoter, A.; El-Sabban, M.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 2012, 1823, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 Is Essential for Mesoderm Induction and Muscle Development Because It Regulates Insulin-like Growth Factor Secretion. Mol. Boil. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef]
- Li, W.; Kennedy, S.; Ruvkun, G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003, 17, 844–858. [Google Scholar] [CrossRef] [Green Version]
- Ostrovsky, O.; Eletto, D.; Makarewich, C.; Barton, E.R.; Argon, Y. Glucose regulated protein 94 is required for muscle differentiation through its control of the autocrine production of insulin-like growth factors. Biochim. Biophys. Acta 2010, 1803, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzec, M.; Hawkes, C.P.; Eletto, D.; Boyle, S.; Rosenfeld, R.; Hwa, V.; Wit, J.-M.; Van Duyvenvoorde, H.A.; Oostdijk, W.; Losekoot, M.; et al. A Human Variant of Glucose-Regulated Protein 94 That Inefficiently Supports IGF Production. Endocrinology 2016, 157, 1914–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Hodish, I.; Rhodes, C.J.; Arvan, P. Proinsulin maturation, misfolding, and proteotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 15841–15846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Hua, Q.-X.; Hu, S.-Q.; Jia, W.; Yang, Y.; Saith, S.E.; Whittaker, J.; Arvan, P.; Weiss, M.A. Deciphering the Hidden Informational Content of Protein Sequences. J. Boil. Chem. 2010, 285, 30989–31001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hober, S.; Ljung, J.L.; Uhlén, M.; Nilsson, B. Insulin-like growth factors I and II are unable to form and maintain their native disulfides under in vivo redox conditions. FEBS Lett. 1999, 443, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Sohma, Y.; Hua, Q.X.; Liu, M.; Philips, N.B.; Hu, S.-Q.; Whittaker, J.; Whittaker, L.J.; Ng, A.; Roberts, C.T., Jr.; Arvan, P.; et al. Contribution of residue B5 to the folding and function of insulin and IGF-I: Constraints and fine-tuning in the evolution of a protein family. J. Biol. Chem. 2010, 285, 5040–5055. [Google Scholar] [CrossRef] [Green Version]
- Eletto, D.; Maganty, A.; Eletto, D.; Dersh, D.; Makarewich, C.; Biswas, C.; Paton, J.C.; Paton, A.W.; Doroudgar, S.; Glembotski, C.C.; et al. Limitation of individual folding resources in the ER leads to outcomes distinct from the unfolded protein response. J. Cell Sci. 2012, 125, 4865–4875. [Google Scholar] [CrossRef] [Green Version]
- Meunier, L.; Usherwood, Y.-K.; Chung, K.T.; Hendershot, L.M. A Subset of Chaperones and Folding Enzymes Form Multiprotein Complexes in Endoplasmic Reticulum to Bind Nascent Proteins. Mol. Boil. Cell 2002, 13, 4456–4469. [Google Scholar] [CrossRef]
- Yakar, S.; Liu, J.-L.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; Leroith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.X.; Hong, F.; Zhang, Y.-L.; Ansa-Addo, E.; Li, Z. GRP94/gp96 in Cancer: Biology, Structure, Immunology, and Drug Development. Adv. Cancer Res. 2016, 129, 165–190. [Google Scholar]
- Kim, Y.K.; Lee, A.S. Transcriptional activation of the glucose-regulated protein genes and their heterologous fusion genes by beta-mercaptoethanol. Mol. Cell. Boil. 1987, 7, 2974–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, R.K.; Dubeau, L.; Kleiner, H.; Parr, T.; Nichols, P.; Ko, B.; Dong, D.; Ko, H.; Mao, C.; DiGiovanni, J.; et al. Cancer-inducible transgene expression by the Grp94 promoter: Spontaneous activation in tumors of various origins and cancer-associated macrophages. Cancer Res. 2002, 62, 7207–7212. [Google Scholar] [PubMed]
- Samani, A.A.; Yakar, S.; Leroith, D.; Brodt, P. The Role of the IGF System in Cancer Growth and Metastasis: Overview and Recent Insights. Endocr. Rev. 2007, 28, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Dejeans, N.; Glorieux, C.; Guenin, S.; Beck, R.; Sid, B.; Rousseau, R.; Bisig, B.; Delvenne, P.; Calderon, P.B.; Verrax, J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free. Radic. Boil. Med. 2012, 52, 993–1002. [Google Scholar] [CrossRef]
- Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 2000, 183, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yakar, S.; Rosen, C.J.; Bouxsein, M.L.; Sun, H.; Mejia, W.; Kawashima, Y.; Wu, Y.; Emerton, K.; Williams, V.; Jepsen, K.; et al. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J. 2008, 23, 709–719. [Google Scholar] [CrossRef]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, G.L.; Wu, Y.-P.; Liu, J.-L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef]
- Bunn, R.C.; Fowlkes, J.L. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol. Metab. 2003, 14, 176–181. [Google Scholar] [CrossRef]
| Protein Substrate | Refs | Major Expression | Notable Structural Features |
|---|---|---|---|
| Immunoglobulin L chain H chain | [50,51] | B lineage cells | Immunoglobulin fold Non-glycosylated secreted Glycosylated secreted or membrane-spanning |
| Toll-like receptor | [52,53] | Ubiquitous, predominantly leukocytes | Leucine-rich repeats; Membrane-spanning proteins |
| Integrins | [48] | Ubiquitous | Immunoglobulin superfamily membrane-spanning heterodimers |
| LRP6 | [54,55] | EGF-like repeats β-propeller motifs Interacts indirectly via MesD | |
| Glycoprotein Ib-IX-V complex | [56] | Platelets | |
| Insulin-like proteins IGF-I IGF-II Insulin | [38,57,58] | Ubiquitous Pancreatic β cells | |
| Thyroglobulin | [59,60] | Thyrocytes | Large disulfide-bonded protease-type repeats |
| GARP | [61] | Treg cells; Platelets | Membrane-spanning leucine-rich repeats domains Tregs and platelets |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argon, Y.; Bresson, S.E.; Marzec, M.T.; Grimberg, A. Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production. Cells 2020, 9, 1844. https://doi.org/10.3390/cells9081844
Argon Y, Bresson SE, Marzec MT, Grimberg A. Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production. Cells. 2020; 9(8):1844. https://doi.org/10.3390/cells9081844
Chicago/Turabian StyleArgon, Yair, Sophie E. Bresson, Michal T. Marzec, and Adda Grimberg. 2020. "Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production" Cells 9, no. 8: 1844. https://doi.org/10.3390/cells9081844
APA StyleArgon, Y., Bresson, S. E., Marzec, M. T., & Grimberg, A. (2020). Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production. Cells, 9(8), 1844. https://doi.org/10.3390/cells9081844






