Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells
Abstract
1. Introduction
2. Methods
2.1. Cell Cultures
2.2. Preparation of Saffron Crudes, Compounds and DCLK1 Inhibitors
2.3. RNA Extraction and Quantitative PCR
2.4. Co-Immunoprecipitation (Co-IP)
2.5. Protein Extraction and Western Blot
2.6. MTT Assay
2.7. Flow Cytometry
2.8. Migration Assay
2.9. Live Cell Imaging
2.10. Kinase Assay
2.11. FRET Measurement
2.12. Liquid Chromatography
2.13. Statistical Analysis
3. Results
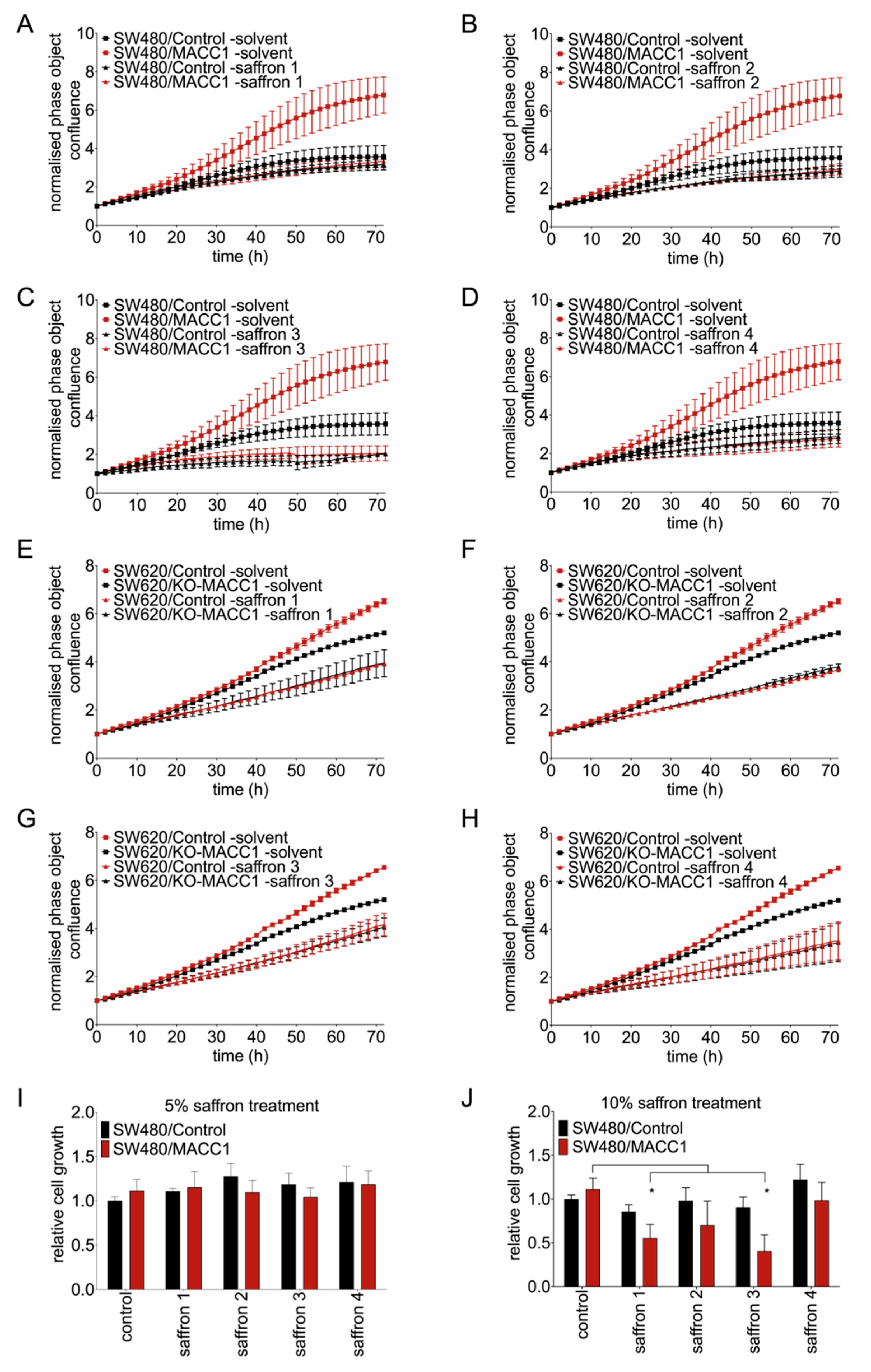
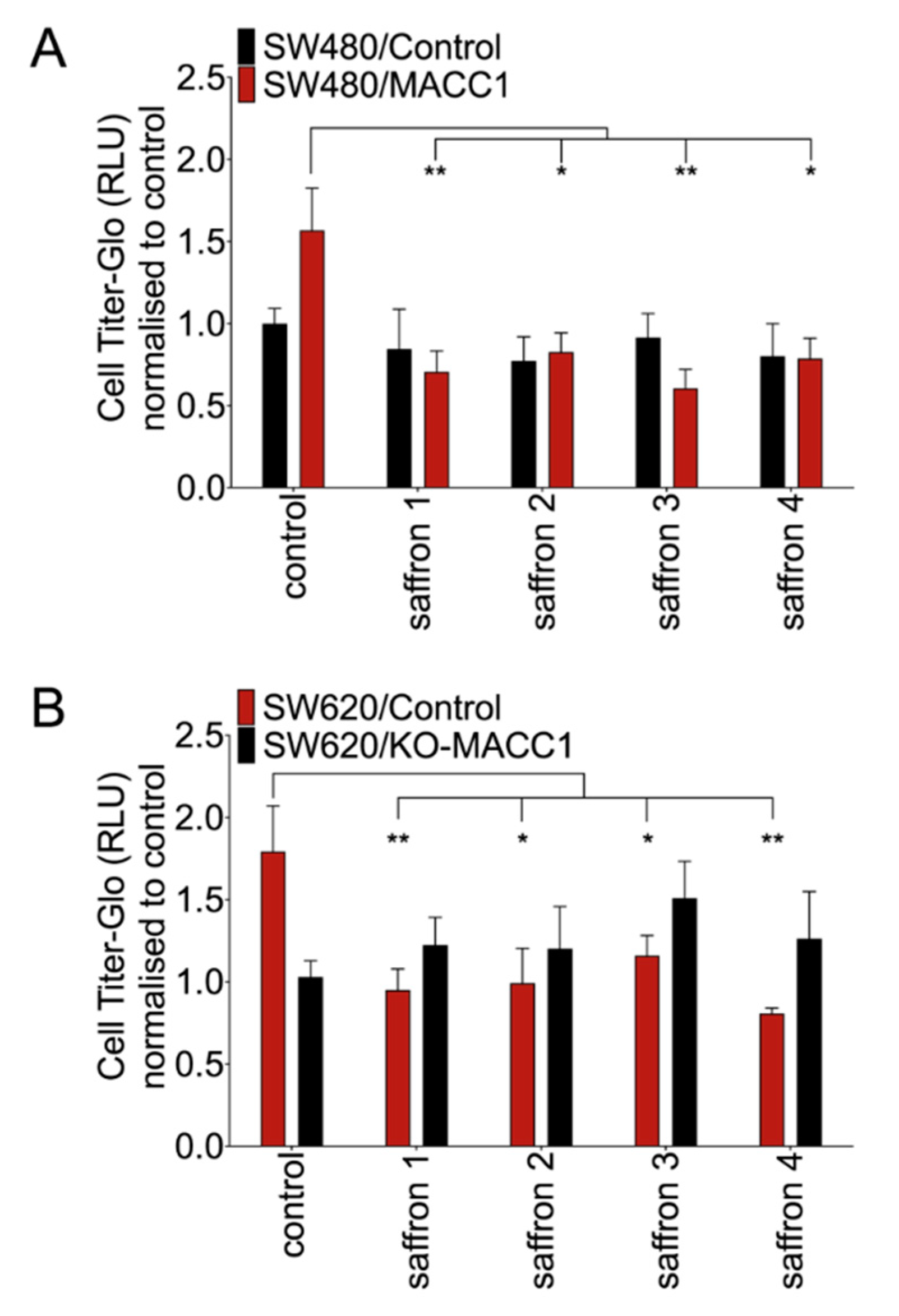
3.1. Saffron Restricts Proliferation and Viability of CRC Cells with High MACC1 Expression
3.2. Saffron Reduces the Migration Rate of MACC1-Expressing CRC Cell Lines
3.3. Saffron Restricts Proliferation of CRC Cells with High MACC1 Expression through Cell Cycle Arrest
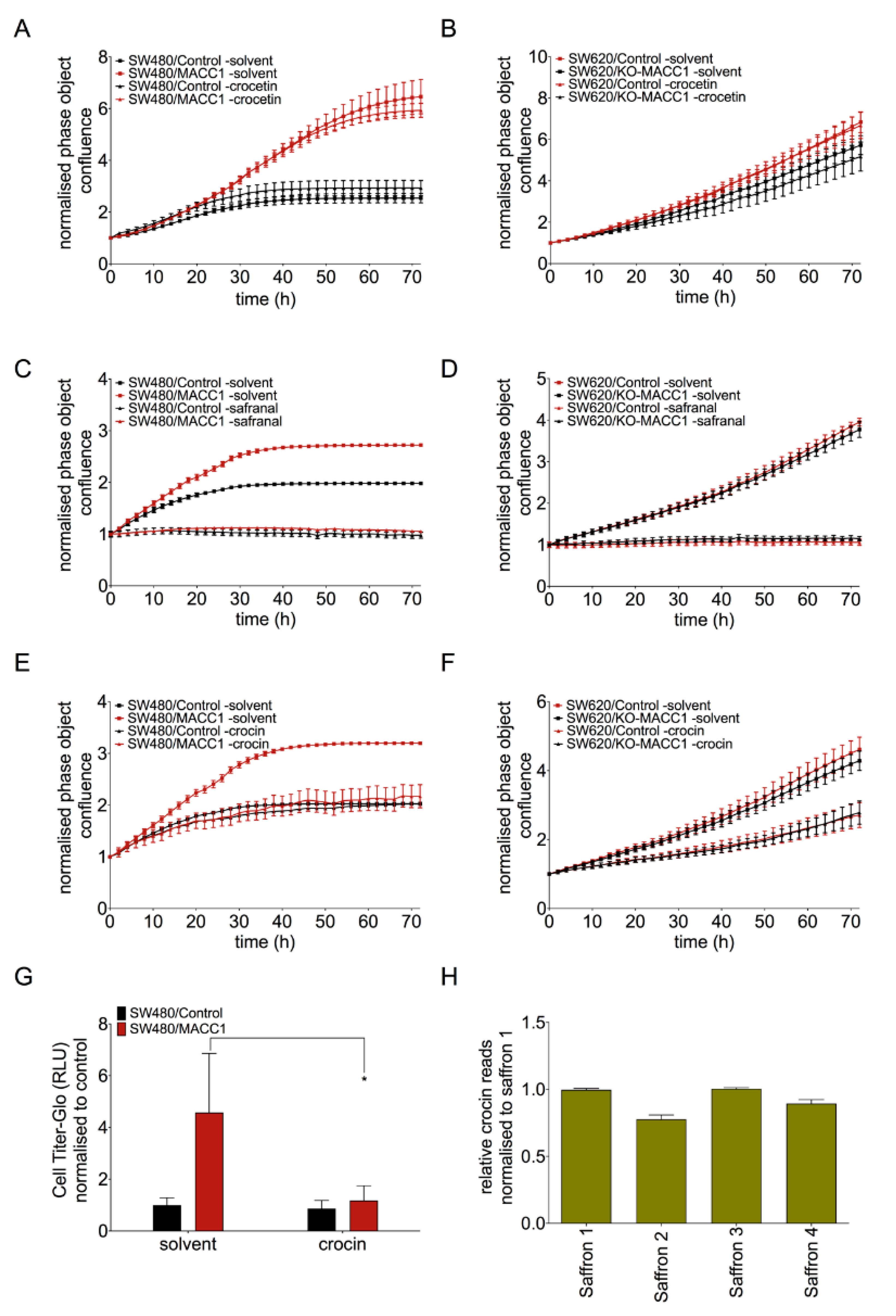
3.4. Crocin Is the Main Compound Responsible for MACC1-Dependent Anti-Proliferative Effect of Saffron
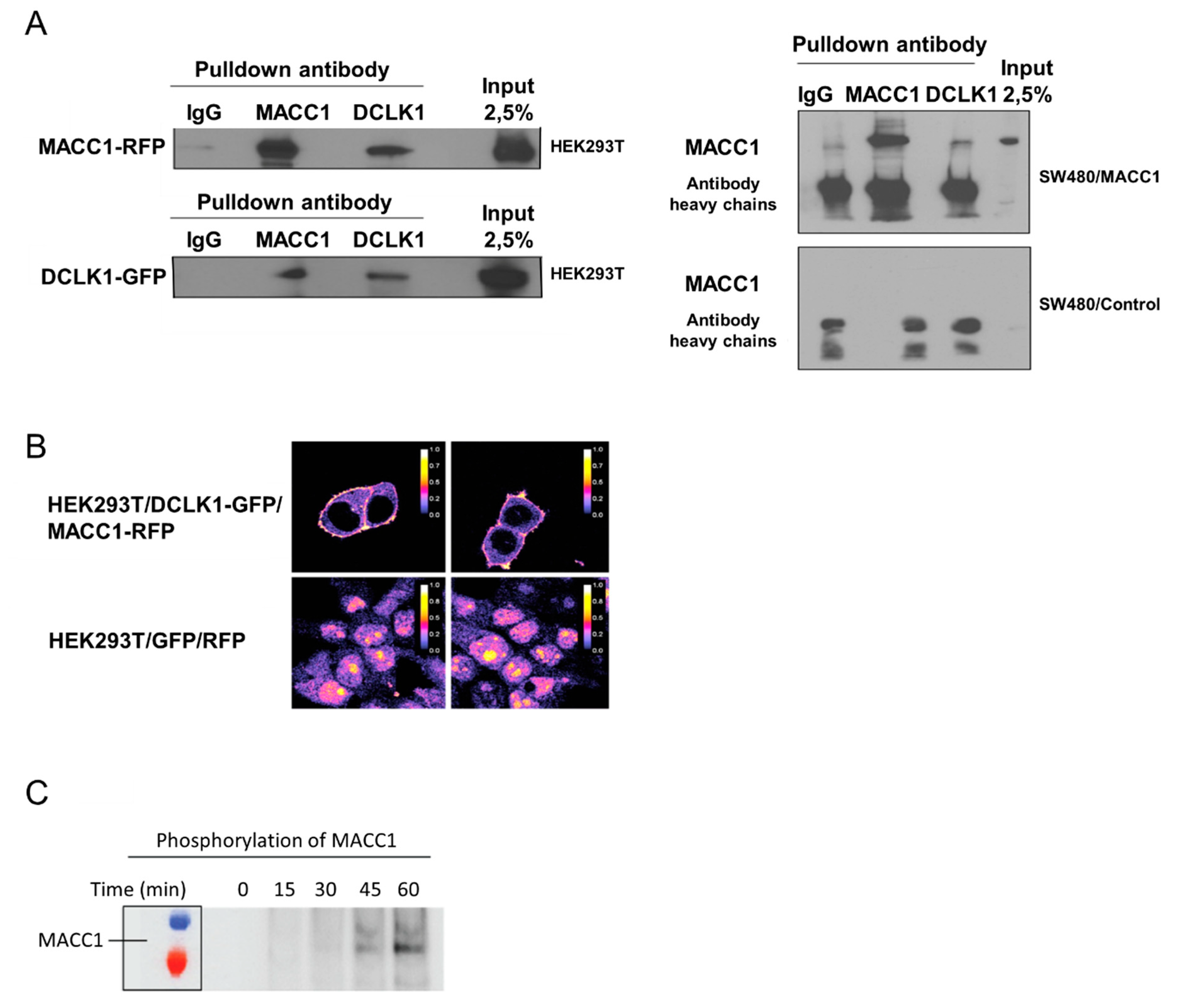
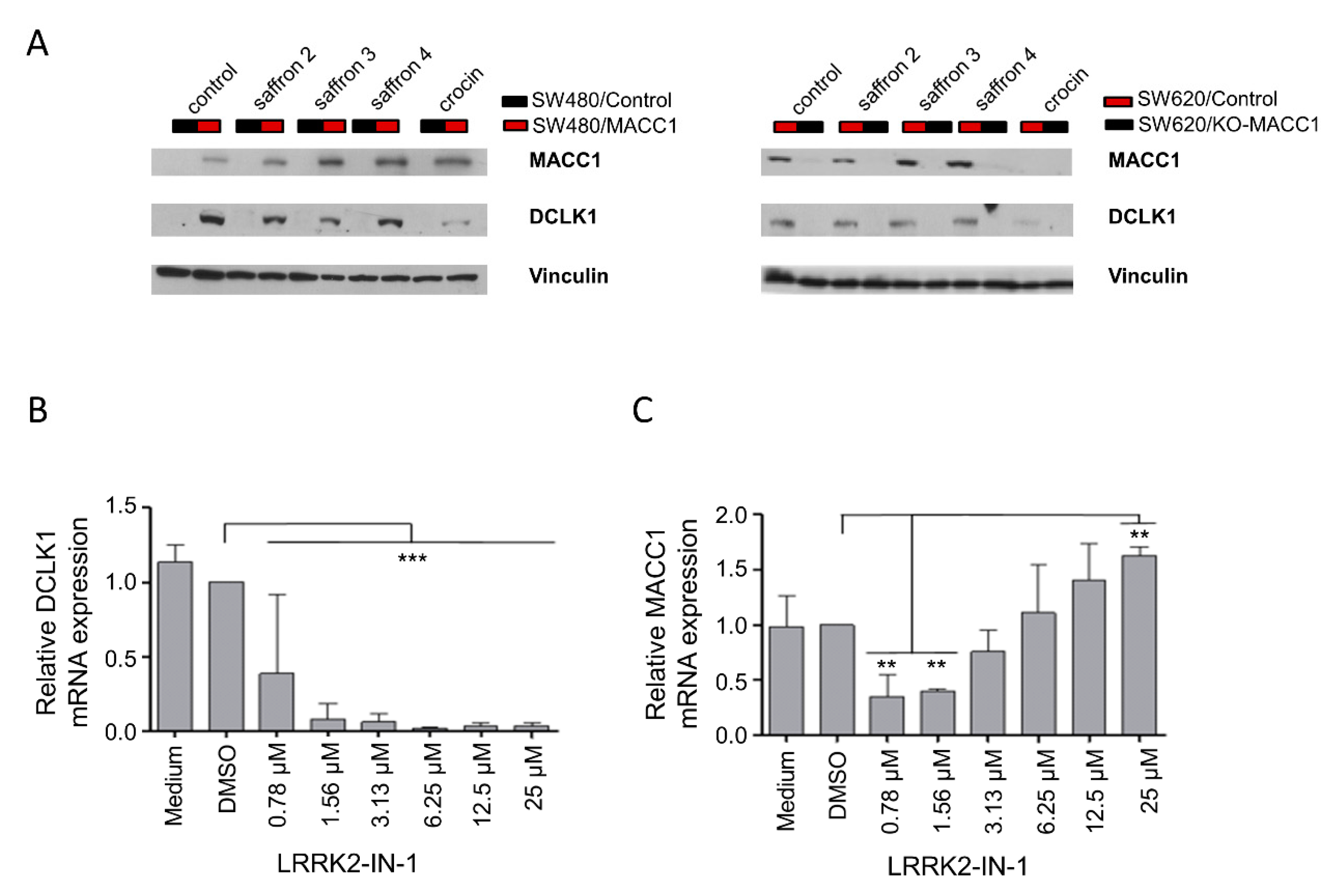
3.5. Saffron Reduces DCLK1 Levels in CRC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel:, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. 394 CA: A Cancer Journal for Clinicians Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Kadla, S.A.; Rasool, V.; Ganai, B.A. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol. 2013, 34, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2009, 15, 59–67. [Google Scholar] [CrossRef]
- Radhakrishnan, H.; Walther, W.; Zincke, F.; Kobelt, D.; Imbastari, F.; Erdem, M.; Kortüm, B.; Dahlmann, M.; Stein, U. MACC1—The first decade of a key metastasis molecule from gene discovery to clinical translation. Cancer Metastasis Rev. 2018, 37, 805–820. [Google Scholar] [CrossRef]
- Rohr, U.-P.; Herrmann, P.; Ilm, K.; Zhang, H.; Lohmann, S.; Reiser, A.; Muranyi, A.; Smith, J.; Burock, S.; Osterland, M.; et al. Prognostic value of MACC1 and proficient mismatch repair status for recurrence risk prediction in stage II colon cancer patients: The BIOGRID studies. Ann. Oncol. 2017, 28, 1869–1875. [Google Scholar] [CrossRef]
- Prguda-Mujic, J.; Milde-Langosch, K.; Mueller, V.; Suljagic, M.; Coric, J.; Ler, D. The predictive significance of Metastasis-Associated in Colon Cancer-1 (MACC1) in primary breast cancer. Ann. Clin. Lab. Sci. 2018, 48, 191–196. [Google Scholar]
- Jabir, N.R.; Firoz, C.K.; Bhushan, A.; Tabrez, S.; Kamal, M.A. The use of azoles containing natural products in cancer prevention and treatment: An overview. Anti Cancer Agents Med. Chem. 2018, 18, 6–14. [Google Scholar] [CrossRef]
- Aboelsoud, N.H. Herbal medicine in ancient Egypt. J. Med. Plants Res. 2010, 4, 82–86. [Google Scholar]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amr, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondré-Lewis, M.C.; et al. Saffron: The golden spice with therapeutic properties on digestive diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sarwat, M.; Khan, T.H. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): The molecular perspective. Crit Rev. Oncol. Hematol. 2017, 115, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Huang, W.-F.; Wang, Q.-L.; Wang, F.; Cai, E.; Hu, B.; Du, J.-C.; Wang, J.; Chen, R.; Cai, X.-J.; et al. Crocetin induces cytotoxicity in colon cancer cells via p53-independent mechanisms. Asian Pac. J. Cancer Prev. 2017, 13, 3757–3761. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta Rev. Cancer 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Kim, B.; Park, B. Saffron carotenoids inhibit STAT3 activation and promote apoptotic progression in IL-6-stimulated liver cancer cells. Oncol Rep. 2018, 39, 1883–1891. [Google Scholar] [CrossRef]
- Dhar, A.; Mehta, S.; Dhar, G.; Dhar, K.; Banerjee, S.; Van Veldhuizen, P.; Campbell, D.R.; Banerjee, S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009, 8, 315–323. [Google Scholar] [CrossRef]
- Chryssanthi, D.; Dedes, P.; Karamanos, N.; Cordopatis, P.; Lamari, F. Crocetin inhibits invasiveness of mda-mb-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011, 77, 146–151. [Google Scholar] [CrossRef]
- Suyama, K.; Shapiro, I.; Guttman, M.; Hazan, R.B. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2002, 2, 301–314. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Christou, N.; Perraud, A.; Blondy, S.; Jauberteau, M.-O.; Battu, S.; Mathonnet, M. E-cadherin: A potential biomarker of colorectal cancer prognosis. Oncol. Lett. 2017, 13, 4571–4576. [Google Scholar] [CrossRef] [PubMed]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Subramaniam, D.; Paul, S.; Kwatra, D.; Palaniyandi, K.; Islam, S.; Harihar, S.; Ramalinagam, S.; Gutheil, W.; Putty, S.; et al. Crocetinic acid inhibits hedgehog signaling to inhibit pancreatic cancer stem cells. Oncotarget 2015, 6, 27661–27673. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Geographical differentiation of saffron by GC–MS/FID and chemometrics. Eur. Food Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Samarghandian, S.; Boskabady, M.H.; Davoodi, S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn. Mag. 2010, 6, 309–314. [Google Scholar] [CrossRef]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Weygant, N.; Qu, D.; May, R.; Tierney, R.M.; Berry, W.L.; Zhao, L.; Agarwal, S.; Chandrakesan, P.; Chinthalapally, H.R.; Murphy, N.T.; et al. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget 2010, 6, 2193–2205. [Google Scholar] [CrossRef]
- Mirzaei, A.; Tavoosidana, G.; Modarressi, M.H.; Rad, A.A.; Fazeli, M.S.; Shirkoohi, R.; Tavakoli-Yaraki, M.; Madjd, Z. Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients. Tumor Biol. 2015, 36, 4801–4810. [Google Scholar] [CrossRef]
- Vedeld, H.M.; Skotheim, R.I.; Lothe, R.A.; Lind, G.E. The recently suggested intestinal cancer stem cell marker DCLK1 is an epigenetic biomarker for colorectal cancer. Epigenetics 2014, 9, 346–350. [Google Scholar] [CrossRef]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017, 153, 910–923. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon cancer survival rates with the new American Joint Committee on cancer sixth edition staging. JNCI J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, H.; Ilm, K.; Walther, W.; Shirasawa, S.; Sasazuki, T.; Daniel, P.T.; Gillissen, B.; Stein, U. MACC1 regulates Fas mediated apoptosis through STAT1/3-Mcl-1 signaling in solid cancers. Cancer Lett 2017, 403, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, M.S. Saffron chemicals and medicine usage. J. Med. Plants Res. 2010, 4, 427–430. [Google Scholar]
- Arzi, L.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R.; Jafarzadeh, N. A Comparative study on anti-invasion, antimigration, and antiadhesion effects of the bioactive carotenoids of saffron on 4t1 breast cancer cells through their effects on wnt/β-catenin pathway genes. DNA Cell Biol. 2018, 37, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed. Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Lozano, P.; Castellar, M.; Simancas, M.; Iborra, J. A quantitative high-performance liquid chromatographic method to analyse commercial saffron (Crocus sativus L.) products. J. Chromatogr. A 1999, 830, 477–483. [Google Scholar] [CrossRef]
- Esmaeilian, Y.; Galavi, M.; Ramroudi, M.; Mashhadi, M.; Boojar, A. Diurnal variability of stigma compounds of saffron (Crocus sativus L.) at different harvest times. Ann. Biol Res. 2012, 3, 1562–1568. [Google Scholar]
- Li, S.; Qu, Y.; Shen, X.-Y.; Ouyang, T.; Fu, W.-B.; Luo, T.; Wang, H.-Q. Multiple signal pathways involved in crocetin-induced apoptosis in KYSE-150 Cells. Pharmacology 2019, 103, 263–272. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Z.; Xie, Q.; Zhang, Z.; Zhang, H.; Ma, J. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 1–13. [Google Scholar] [CrossRef]
- Al-Hrout, A.; Chaiboonchoe, A.; Khraiwesh, B.; Murali, C.; Baig, B.; El-Awady, R.; Tarazi, H.; Alzahmi, A.; Nelson, D.R.; Greish, Y.E.; et al. Safranal induces DNA double-strand breakage and ER-stress-mediated cell death in hepatocellular carcinoma cells. Sci Rep. 2018, 8, 16951. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Q.; Shang, J.; Lu, L.; Chen, G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J. Cell Physiol. 2019, 117, 17876–17885. [Google Scholar] [CrossRef] [PubMed]
- Arzi, L.; Farahi, A.; Jafarzadeh, N.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R. Inhibitory effect of crocin on metastasis of triple-negative breast cancer by interfering with Wnt/β-Catenin pathway in murine model. DNA Cell Biol. 2018, 37, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S.; Javid, F.; Rashan, L. Dietary crocin reverses melanoma metastasis. J. Biomed. Res. 2017, 32, 39. [Google Scholar]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anti-cancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017, 69, 1419–1427. [Google Scholar] [CrossRef]
- Gao, T.; Wang, M.; Xu, L.; Wen, T.; Liu, J.; An, G. DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 2131–2140. [Google Scholar] [CrossRef]
- Liu, H.; Wen, T.; Zhou, Y.; Fan, X.; Du, T.; Gao, T.; Li, L.; Liu, J.; Yang, L.; Yao, J.; et al. DCLK1 plays a metastatic-promoting role in human breast cancer cells. Biomed. Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Ito, H.; Tanaka, S.; Akiyama, Y.; Shimada, S.; Adikrisna, R.; Matsumura, S.; Aihara, A.; Mitsunori, Y.; Ban, D.; Ochiai, T.; et al. Dominant expression of DCLK1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoS ONE 2016, 11, e0146564. [Google Scholar] [CrossRef]
- Shu, T.; Tseng, H.C.; Sapir, T.; Stern, P.; Zhou, Y.; Sanada, K.; Fischer, A.; Coquelle, F.M.; Reiner, O.; Tsai, L.H. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 2006, 49, 25–39. [Google Scholar] [CrossRef]
- Lipka, J.; Kapitein, L.C.; Jaworski, J.; Hoogenraad, C.C. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 2016, 35, 302–318. [Google Scholar] [CrossRef]
- Chandrakesan, P.; Weygant, N.; May, R.; Qu, D.; Chinthalapally, H.R.; Sureban, S.M.; Ali, N.; Lightfoot, S.A.; Umar, S.; Houchen, C.W.; et al. DCLK1 facilitates intestinal tumor growth via enhancing pluripotency and epithelial mesenchymal transition. Oncotarget 2014, 5, 9269–9280. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, A.; Tanaka, T.; Kazama, S.; Nagata, H.; Kawai, K.; Hata, K.; Otani, K.; Nishikawa, T.; Sasaki, K.; Kaneko, M.; et al. DCLK1 expression in colorectal polyps increases with the severity of dysplasia. In Vivo 2018, 32, 365–371. [Google Scholar] [PubMed]
- Mohammadi, Y.; Tavangar, S.M.; Saidijam, M.; Amini, R.; Etemadi, K.; Karimi Dermanim, F.; Najafi, R. DCLK1 plays an important role in colorectal cancer tumorgenesis through the regulation of miR-200c. Biomed. Pharmacother. 2018, 103, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Quante, M.; Wang, T.C. Functional implication of Dclk1 and Dclk1-expressing cells in cancer. Small GTPases 2017, 8, 164–171. [Google Scholar] [CrossRef]
- Konoshima, T.; Takasaki, M.; Tokuda, H.; Morimoto, S.; Tanaka, H.; Kawata, E.; Xuan, L.J.; Saito, H.; Sugiura, M.; Molnar, J.; et al. Crocin and crocetin derivatives inhibit skin tumour promotion in mice. Phyther Res. 1998, 12, 400–404. [Google Scholar] [CrossRef]
- Kawamura, D.; Takemoto, Y.; Nishimoto, A.; Ueno, K.; Hosoyama, T.; Shirasawa, B.; Tanaka, T.; Kugimiya, N.; Harada, E.; Hamano, K. Enhancement of cytotoxic effects of gemcitabine by Dclk1 inhibition through suppression of Chk1 phosphorylation in human pancreatic cancer cells. Oncol. Rep. 2017, 38, 3238–3244. [Google Scholar] [CrossRef]
- Suehiro, Y.; Takemoto, Y.; Nishimoto, A.; Ueno, K.; Shirasawa, B.; Tanaka, T.; Kugimiya, N.; Suga, A.; Harada, E.; Hamano, K. Dclk1 inhibition cancels 5-FU-induced cell-cycle arrest and decreases cell survival in colorectal cancer. Anti Cancer Res. 2018, 38, 6225–6230. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Weygant, N.; Qu, D.; Berry, W.L.; May, R.; Chandrakesan, P.; Owen, D.B.; Sureban, S.M.; Ali, N.; Janknecht, R.; Houchen, C.W. Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol. Cancer 2014, 13, 1–14. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güllü, N.; Kobelt, D.; Brim, H.; Rahman, S.; Timm, L.; Smith, J.; Soleimani, A.; Di Marco, S.; Bisti, S.; Ashktorab, H.; et al. Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells. Cells 2020, 9, 1829. https://doi.org/10.3390/cells9081829
Güllü N, Kobelt D, Brim H, Rahman S, Timm L, Smith J, Soleimani A, Di Marco S, Bisti S, Ashktorab H, et al. Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells. Cells. 2020; 9(8):1829. https://doi.org/10.3390/cells9081829
Chicago/Turabian StyleGüllü, Nazli, Dennis Kobelt, Hassan Brim, Shaman Rahman, Lena Timm, Janice Smith, Akbar Soleimani, Stefano Di Marco, Silvia Bisti, Hassan Ashktorab, and et al. 2020. "Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells" Cells 9, no. 8: 1829. https://doi.org/10.3390/cells9081829
APA StyleGüllü, N., Kobelt, D., Brim, H., Rahman, S., Timm, L., Smith, J., Soleimani, A., Di Marco, S., Bisti, S., Ashktorab, H., & Stein, U. (2020). Saffron Crudes and Compounds Restrict MACC1-Dependent Cell Proliferation and Migration of Colorectal Cancer Cells. Cells, 9(8), 1829. https://doi.org/10.3390/cells9081829








