PKHhigh/CD133+/CD24− Renal Stem-Like Cells Isolated from Human Nephrospheres Exhibit In Vitro Multipotency
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues
2.2. Nephrosphere Cultures
2.3. Immunofluorescence and FACS Analysis
2.4. FACS Sorting
2.5. RSC Cultured on Decellularized Extracellular Matrix (ECM) Kidney Scaffolds and Three-Dimensional (3D) Staining
2.6. Histologic Characterization
2.7. In Vitro Single Cell Differentiation
2.8. 3D Culture on Matrigel and Staining
2.9. Statistical Analysis.
3. Results
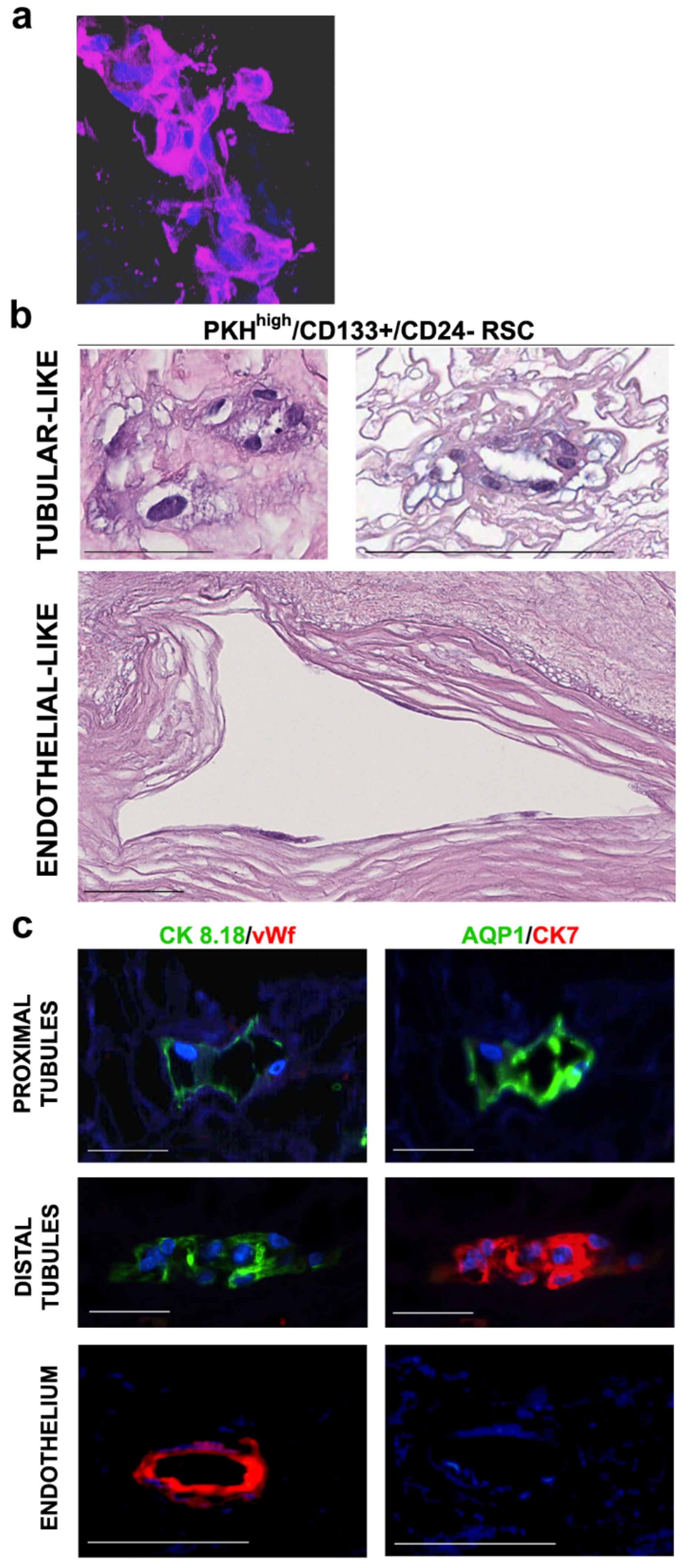
3.1. Human RSC Cultured on Human Decellularized Scaffolds
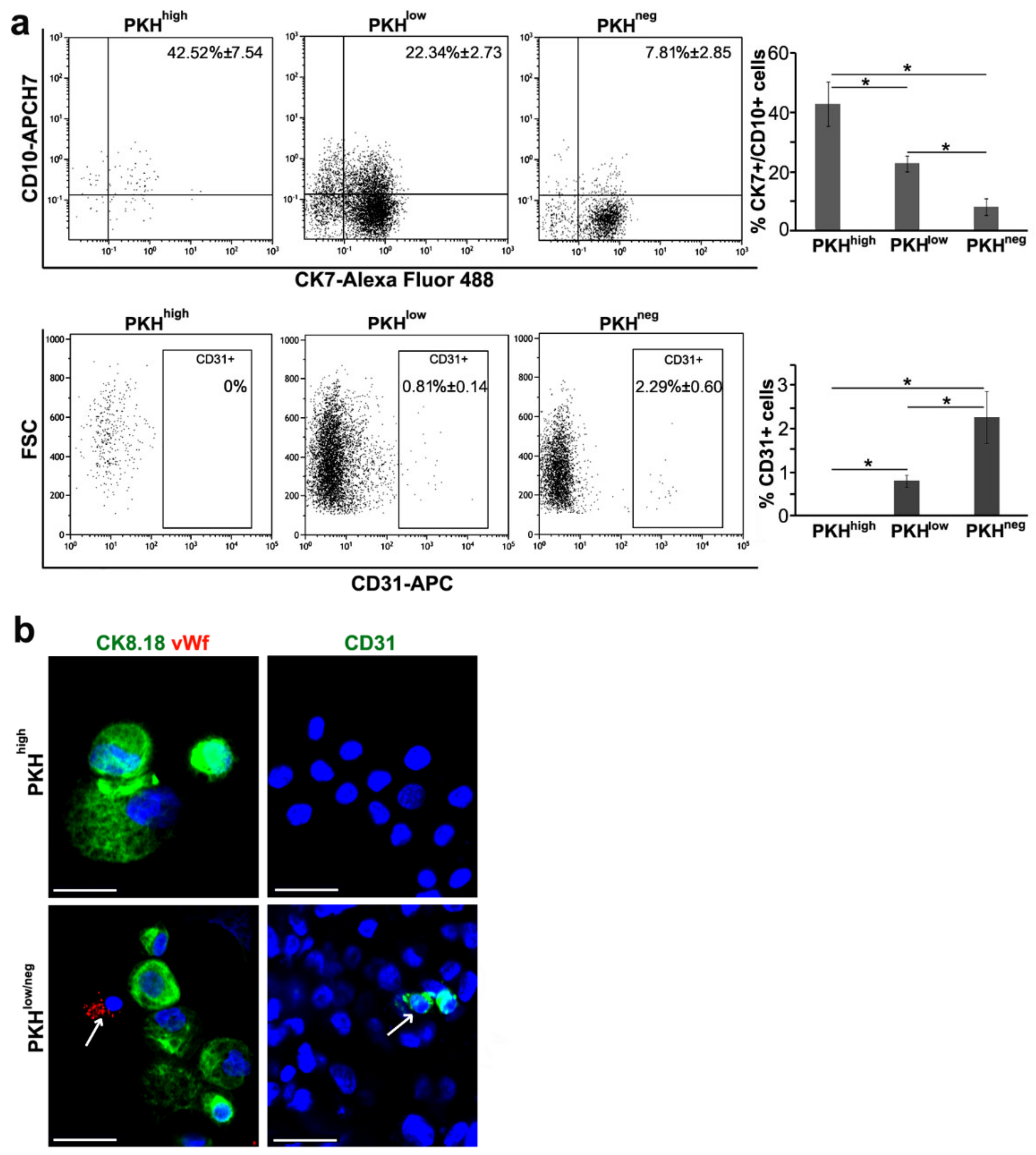
3.2. Cell Commitment Toward Epithelium and Endothelium
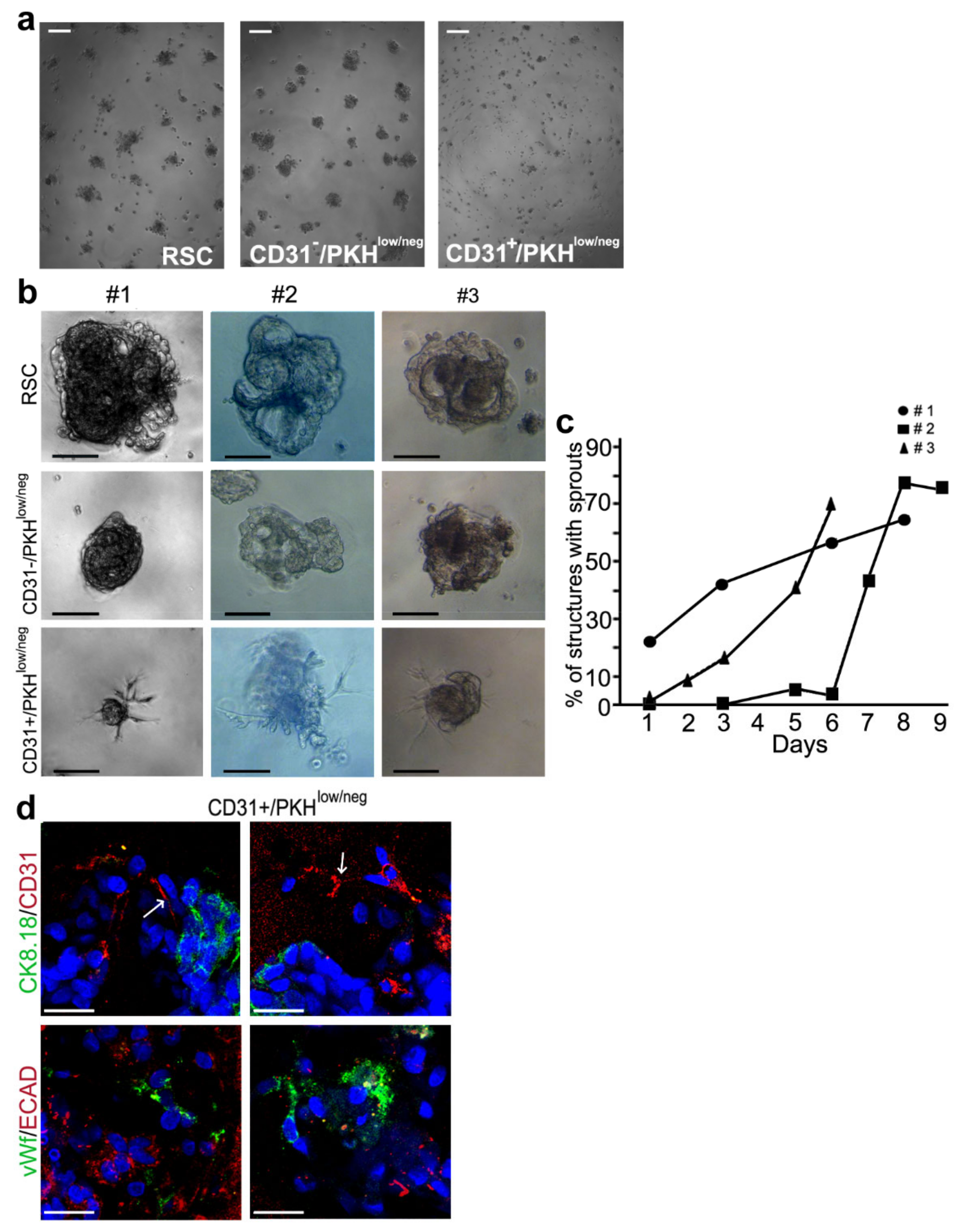
3.3. Morphogenesis 3D Assay
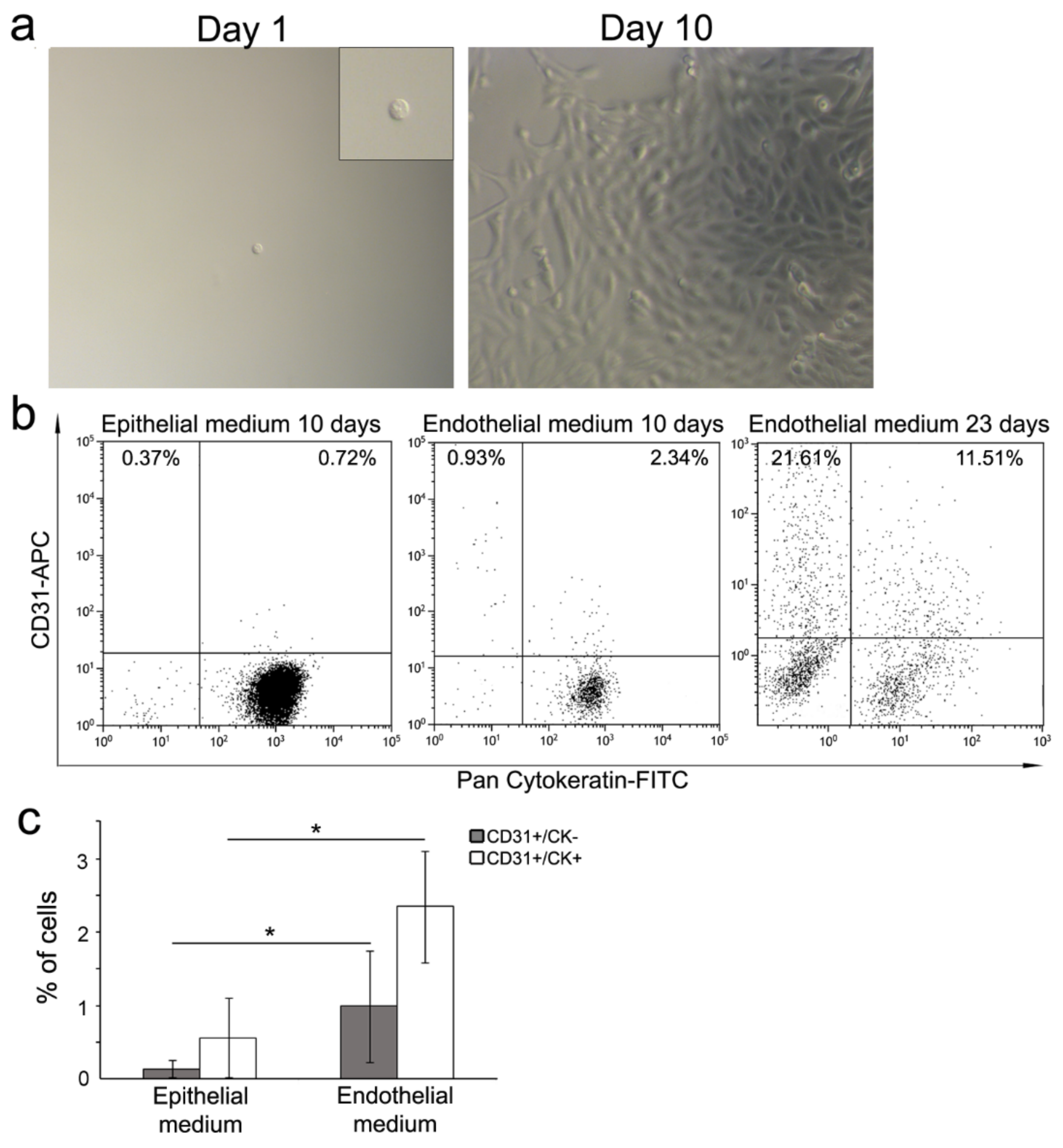
3.4. Single Cell Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prescott, L.F. The normal urinary excretion rates of renal tubular cells, leucocytes and red blood cells. Clin. Sci. 1966, 31, 425–435. [Google Scholar]
- Lameire, N.H.; Bagga, A.; Cruz, D.; de Maesneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; van Biesen, W.; et al. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Montoro, D.T.; Contreras-Trujillo, H.; Harari-Steinberg, O.; Newman, A.M.; Tsai, J.M.; Lim, X.; Van-Amerongen, R.; Bowman, A.; Januszyk, M.; et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014, 7, 1270–1283. [Google Scholar] [CrossRef]
- Johnson, H.A.; Vera-Roman, J.M. Compensatory renal enlargement. Hypertrophy versus hyperplasia. Am. J. Pathol. 1996, 49, 1–13. [Google Scholar]
- Lombardi, D.; Becherucci, F.; Romagnani, P. How much can the tubule regenerate and who does it? An open question. Nephrol. Dial. Transplant. 2016, 31, 1243–1250. [Google Scholar] [CrossRef]
- Pleniceanu, O.; Omer, D.; Harari-Steinberg, O.; Dekel, B. Renal lineage cells as a source for renal regeneration. Pediatr. Res. 2018, 83, 267–274. [Google Scholar] [CrossRef]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.A.; Nardi, S.; et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 2018, 9, 1344–1362. [Google Scholar] [CrossRef]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef]
- Bussolati, B.; Moggio, A.; Collino, F.; Aghemo, G.; D’Armento, G.; Grange, C.; Camussi, G. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am. J. Physiol. Renal. Physiol. 2012, 302, F116–F128. [Google Scholar] [CrossRef]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosoli, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Angelotti, M.L.; Ronconi, E.; Ballerini, L.; Peired, A.; Mazzinghi, B.; Sagrinati, C.; Parente, E.; Gacci, M.; Carini, M.; Rotondi, M.; et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012, 30, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Camussi, G. Therapeutic use of human renal progenitor cells for kidney regeneration. Nat. Rev. Nephrol. 2015, 11, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, S.; Zipeto, M.A.; Torsello, B.; Bovo, G.; di Stefano, V.; Bugarin, C.; Zordan, P.; Viganò, P.; Cattoretti, G.; Strada, G.; et al. PKHhigh cells within clonal human nephrospheres provide a purified adult renal stem cell population. Stem Cell Res. 2013, 11, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, O.H.; Omer, D.; Gnatek, Y.; Pleniceanu, O.; Goldberg, S.; Cohen-Zontag, O.; Pri-Chen, S.; Kanter, I.; Ben-Haim, N.; Becker, E.; et al. Ex Vivo Expanded 3D Human Kidney Spheres Engraft Long Term and Repair Chronic Renal Injury in Mice. Cell. Rep. 2020, 30, 852–869. [Google Scholar] [CrossRef]
- Bombelli, S.; Meregalli, C.; Scalia, C.; Bovo, G.; Torsello, B.; de Marco, S.; Cadamuro, M.; Viganò, P.; Strada, G.; Cattoretti, G.; et al. Nephrosphere-derived cells are induced to multilineage differentiation when cultured on human decellularized kidney scaffolds. Am. J. Pathol. 2018, 188, 184–195. [Google Scholar] [CrossRef]
- Bianchi, C.; Bombelli, S.; Raimondo, F.; Torsello, B.; Angeloni, V.; Ferrero, S.; di Stefano, V.; Chinello, C.; Cifola, I.; Invernizzi, L.; et al. Primary cell cultures from human renal cortex and renal-cell carcinoma evidence a differential expression of two spliced isoforms of Annexin A3. Am. J. Pathol. 2010, 176, 1660–1670. [Google Scholar] [CrossRef]
- Torsello, B.; Bianchi, C.; Meregalli, C.; di Stefano, V.; Invernizzi, L.; de Marco, S.; Bovo, G.; Brivio, R.; Strada, G.; Bombelli, S.; et al. Arg tyrosine kinase modulates TGF-β1 production in human renal tubular cells under high-glucose conditions. J. Cell Sci. 2016, 129, 2925–2936. [Google Scholar] [CrossRef]
- di Stefano, V.; Torsello, B.; Bianchi, C.; Cifola, I.; Mangano, E.; Bovo, G.; Cassina, V.; de Marco, S.; Corti, R.; Meregalli, C.; et al. Major Action of Endogenous Lysyl Oxidase in Clear Cell Renal Cell Carcinoma Progression and Collagen Stiffness Revealed by Primary Cell Cultures. Am. J. Pathol. 2016, 186, 2473–2485. [Google Scholar] [CrossRef]
- Bolognesi, M.M.; Manzoni, M.; Scalia, C.R.; Zannella, S.; Bosisio, F.M.; Farretta, M.; Cattoretti, G. Multiplex Staining by Sequential Immunostaining and Antibody Removal on Routine Tissue Sections. J. Histochem. Cytochem. 2017, 65, 431–444. [Google Scholar] [CrossRef]
- Bruno, S.; Bassani, B.; D’Urso, D.G.; Pitaku, I.; Cassinotti, E.; Pelosi, G.; Boni, L.; Dominioni, L.; Noonan, D.M.; Mortara, L.; et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. 2018, 32, 5365–5377. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Peloso, A.; Ferrario, J.; Maiga, B.; Benzoni, I.; Bianco, C.; Citro, A.; Currao, M.; Malara, A.; Gaspari, A.; Balduini, A.; et al. Creation and implantation of acellular rat renal ECM-based scaffolds. Organogenesis 2015, 11, 58–74. [Google Scholar] [CrossRef]
- Caralt, M.; Uzarski, J.S.; Iacob, S.; Obergfell, K.P.; Berg, N.; Bijonowski, B.M.; Kiefer, K.M.; Ward, H.H.; Wandinger-Ness, A.; Miller, W.M.; et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. 2015, 15, 64–75. [Google Scholar] [CrossRef]
- Sciancalepore, A.G.; Portone, A.; Moffa, M.; Persano, L.; de Luca, M.; Paiano, A.; Sallustio, F.; Schena, F.P.; Bucci, C.; Pisignano, D. Micropatterning control of tubular commitment in human adult renal stem cells. Biomaterials 2016, 94, 57–69. [Google Scholar] [CrossRef]
- Remuzzi, A.; Figliuzzi, M.; Bonandrini, B.; Silvani, S.; Azzollini, N.; Nossa, R.; Benigni, A.; Remuzzi, G. Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization. Sci. Rep. 2017, 7, 43502. [Google Scholar] [CrossRef]
- Pavenstädt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef]
- Heiss, M.; Hellström, M.; Kalén, M.; May, T.; Weber, H.; Hecker, M.; Augustin, H.G.; Korff, T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015, 29, 3076–3084. [Google Scholar] [CrossRef]
- Pece, S.; Tosoni, D.; Confalonieri, S.; Mazzarol, G.; Vecchi, M.; Ronzoni, S.; Bernard, L.; Viale, G.; Pelicci, P.G.; di Fiore, P.P. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010, 140, 62–73. [Google Scholar] [CrossRef]
- Little, M.H.; McMahon, A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012, 4, a00830. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva-de-Sousa-Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Gupta, N.; Kroll, K.Y.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.F. Evidence of renal angiomyolipoma neoplastic stem cells arising from renal epithelial cells. Nat. Commun. 2017, 8, 1466–1482. [Google Scholar] [CrossRef] [PubMed]
- Becherucci, F.; Romagnani, P. Angiomyolipoma: A link between stemness and tumorigenesis in the kidney. Nat. Rev. Nephrol. 2018, 14, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombelli, S.; Meregalli, C.; Grasselli, C.; Bolognesi, M.M.; Bruno, A.; Eriani, S.; Torsello, B.; De Marco, S.; Bernasconi, D.P.; Zucchini, N.; et al. PKHhigh/CD133+/CD24− Renal Stem-Like Cells Isolated from Human Nephrospheres Exhibit In Vitro Multipotency. Cells 2020, 9, 1805. https://doi.org/10.3390/cells9081805
Bombelli S, Meregalli C, Grasselli C, Bolognesi MM, Bruno A, Eriani S, Torsello B, De Marco S, Bernasconi DP, Zucchini N, et al. PKHhigh/CD133+/CD24− Renal Stem-Like Cells Isolated from Human Nephrospheres Exhibit In Vitro Multipotency. Cells. 2020; 9(8):1805. https://doi.org/10.3390/cells9081805
Chicago/Turabian StyleBombelli, Silvia, Chiara Meregalli, Chiara Grasselli, Maddalena M. Bolognesi, Antonino Bruno, Stefano Eriani, Barbara Torsello, Sofia De Marco, Davide P. Bernasconi, Nicola Zucchini, and et al. 2020. "PKHhigh/CD133+/CD24− Renal Stem-Like Cells Isolated from Human Nephrospheres Exhibit In Vitro Multipotency" Cells 9, no. 8: 1805. https://doi.org/10.3390/cells9081805
APA StyleBombelli, S., Meregalli, C., Grasselli, C., Bolognesi, M. M., Bruno, A., Eriani, S., Torsello, B., De Marco, S., Bernasconi, D. P., Zucchini, N., Mazzola, P., Bianchi, C., Grasso, M., Albini, A., Cattoretti, G., & Perego, R. A. (2020). PKHhigh/CD133+/CD24− Renal Stem-Like Cells Isolated from Human Nephrospheres Exhibit In Vitro Multipotency. Cells, 9(8), 1805. https://doi.org/10.3390/cells9081805





