MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Stimulations
2.2. Isolation of Human Peripheral Blood Mononuclear Cells (PBMC), Differentiation, and Stimulation of Primary Macrophages
2.3. siRNA-Mediated Genetic Suppression of TLR4 and IRF3
2.4. TLR4 Neutralization
2.5. Chemical Inhibition of TLR4-Mediated Signaling
2.6. Trafficking Inhibition
2.7. Induction of Oxidative Stress, ROS Measurement, and Cell Treatments with Anti-Oxidants/ROS Scavengers
2.8. Real-Time qRT-PCR
2.9. ELISA
2.10. Flow Cytometry
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Increased MIP-1α Expression in THP-1 Cells and Primary Human Macrophages Co-Stimulated with Palmitate and TNF-α
3.2. MIP-1α Co-Induction by Palmitate and TNF-α Involves the TLR4-Mediated Signaling and Clathrin-Mediated Endocytosis (CME)
3.3. MIP-1α Co-Induced by Palmitate and TNF-α Involves the IRF3 Pathway
3.4. MIP-1α Co-Induced by Palmitate and TNF-α Involves Signaling Via c-Jun and NF-κB
3.5. MIP-1α Expression Induced by Palmitate and/or TNF-α, in Presence or Absence of Oxidative Stress
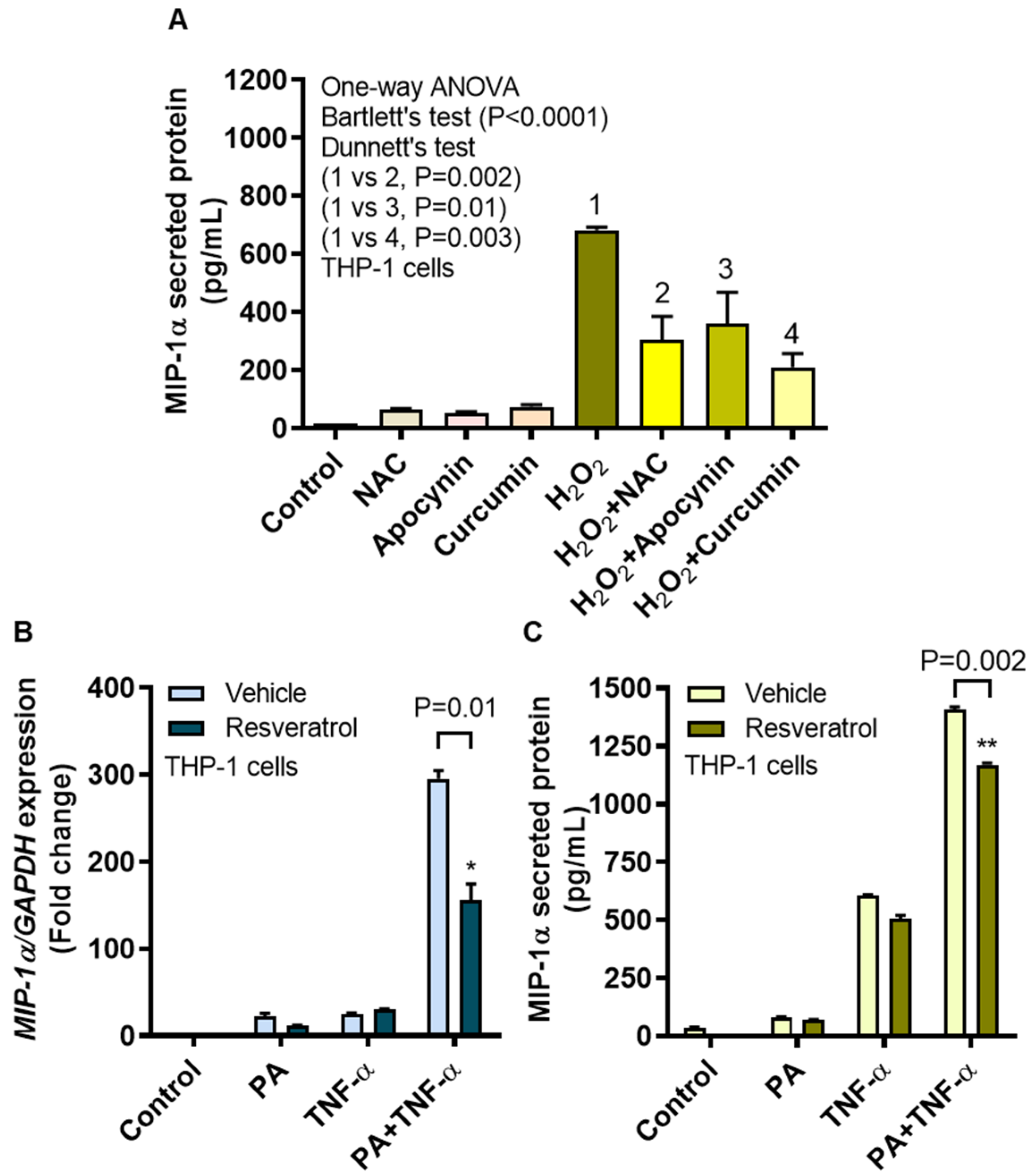
3.6. MIP-1α Induction by Oxidative Stress Is Counteracted by ROS/NF-κB Inhibitors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CME | Clathrin-mediated endocytosis |
| CPZ | Chlorpromazine |
| DCFH-DA | Dichloro-dihydro-fluorescein diacetate |
| ELISA | Enzyme-linked immunosorbent assay |
| FFAs | Free fatty acids |
| GSH | Reduced glutathione |
| H2O2 | Hydrogen peroxide |
| IRF3 | Interferon regulatory factor-3 |
| LPS | Lipopolysaccharide |
| MFI | Mean fluorescence intensity |
| MIP-1α | Macrophage inflammatory protein-1 |
| MIPs | Macrophage inflammatory proteins |
| MyD88 | Myeloid differentiation-88 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIK | NF-κB-inducing kinase |
| NOX | NADPH oxidase |
| O2–• | Superoxide anion |
| OD | Optical density |
| OxPAPC | Oxidized phospholipids such as 1-palmitoyl-2-arachidonyl-snglycero-3-phosphorylcholine |
| PBMC | Peripheral blood mononuclear cells |
| qRT-PCR | Quantitative reverse transcription, polymerase chain reaction |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SI | Staining index |
| TLR4 | Toll-like receptor |
| TLRs | Toll-like receptors |
| TNFR | TNF receptor |
| TNF-α | Tumor necrosis factor-α |
| TRAM | TRIF related adaptor molecule |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
References
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. (Rio J.) 2007, 83, S192–S203. [Google Scholar] [CrossRef]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar] [CrossRef]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [Google Scholar] [CrossRef]
- Johnson, E.A.; Dao, T.L.; Guignet, M.A.; Geddes, C.E.; Koemeter-Cox, A.I.; Kan, R.K. Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J. Neuroinflamm. 2011, 8, 41. [Google Scholar] [CrossRef]
- Honey, K. CCL3 and CCL4 actively recruit CD8+ T cells. Nat. Rev. Immunol. 2006, 6, 427. [Google Scholar] [CrossRef]
- White, G.E.; Iqbal, A.J.; Greaves, D.R. CC chemokine receptors and chronic inflammation--therapeutic opportunities and pharmacological challenges. Pharmacol. Rev. 2013, 65, 47–89. [Google Scholar] [CrossRef]
- Jordan, L.A.; Fenner, B.F.; Davies, R.; Harvey, A.K.; Choy, E.H.; Errington, R.; Bokarewa, M.; Williams, A.S. 02.31 Targeted inhibition of macrophage inflammatory protein 1-alpha (ccl3) prevents pit formation by human osteoclasts and potently attenuates the erosion of bone in collagen-induced arthritis. Ann. Rheumat. Dis. 2017, 76, A21. [Google Scholar] [CrossRef]
- Amft, N.; Bowman, S.J. Chemokines and cell trafficking in Sjogren’s syndrome. Scand. J. Immunol. 2001, 54, 62–69. [Google Scholar] [CrossRef]
- Ping, D.; Jones, P.L.; Boss, J.M. TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity 1996, 4, 455–469. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Maeshima, N.; Fernandez, R.C. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front. Cell Infect. Microbiol. 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Roub, A.; Kochumon, S.; Akther, N.; Thomas, R.; Kumari, M.; Koshy, M.S.; Tiss, A.; Hannun, Y.A.; Tuomilehto, J.; et al. The Synergy between Palmitate and TNF-alpha for CCL2 Production Is Dependent on the TRIF/IRF3 Pathway: Implications for Metabolic Inflammation. J. Immunol. 2018, 200, 3599–3611. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- O’Dea, E.; Hoffmann, A. The regulatory logic of the NF-kappaB signaling system. Cold Spring Harb. Perspect. Biol. 2010, 2, a000216. [Google Scholar] [CrossRef]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef]
- Zandi, E.; Chen, Y.; Karin, M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: Discrimination between free and NF-kappaB-bound substrate. Science 1998, 281, 1360–1363. [Google Scholar] [CrossRef]
- Lyons, C.L.; Kennedy, E.B.; Roche, H.M. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients 2016, 8, 247. [Google Scholar] [CrossRef]
- Ahmad, R.; Akhter, N.; Al-Roub, A.; Kochumon, S.; Wilson, A.; Thomas, R.; Ali, S.; Tuomilehto, J.; Sindhu, S. MIP-1alpha Induction by Palmitate in the Human Monocytic Cells Implicates TLR4 Signaling Mechanism. Cell Physiol. Biochem. 2019, 52, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Vazquez, I.; Fernandez-Veledo, S.; Kramer, D.K.; Vila-Bedmar, R.; Garcia-Guerra, L.; Lorenzo, M. Insulin resistance associated to obesity: The link TNF-alpha. Arch. Physiol. Biochem. 2008, 114, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.T.; Ahmad, R.; Morisset, R.; Ahmad, A.; Menezes, J. Peripheral blood cytotoxic gammadelta T lymphocytes from patients with human immunodeficiency virus type 1 infection and AIDS lyse uninfected CD4+ T cells, and their cytocidal potential correlates with viral load. J. Virol. 2003, 77, 1848–1855. [Google Scholar] [CrossRef]
- Erridge, C.; Kennedy, S.; Spickett, C.M.; Webb, D.J. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: Roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 2008, 283, 24748–24759. [Google Scholar] [CrossRef]
- Inoue, Y.; Tanaka, N.; Tanaka, Y.; Inoue, S.; Morita, K.; Zhuang, M.; Hattori, T.; Sugamura, K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007, 81, 8722–8729. [Google Scholar] [CrossRef]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Canto, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Deng, X.; Tamai, R. Mouse macrophages primed with alendronate down-regulate monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha (MIP-1alpha) production in response to Toll-like receptor (TLR) 2 and TLR4 agonist via Smad3 activation. Int. Immunopharmacol. 2009, 9, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Wang, X.; Lantier, L.; Lyubetskaya, A.; Eguchi, J.; Kang, S.; Tenen, D.; Roh, H.C.; Kong, X.; Kazak, L.; et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Investig. 2016, 126, 2839–2854. [Google Scholar] [CrossRef] [PubMed]
- Haridas, V.; Shrivastava, A.; Su, J.; Yu, G.L.; Ni, J.; Liu, D.; Chen, S.F.; Ni, Y.; Ruben, S.M.; Gentz, R.; et al. VEGI, a new member of the TNF family activates nuclear factor-kappa B and c-Jun N-terminal kinase and modulates cell growth. Oncogene 1999, 18, 6496–6504. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Pillon, N.J.; Azizi, P.M.; Li, Y.E.; Liu, J.; Wang, C.; Chan, K.L.; Hopperton, K.E.; Bazinet, R.P.; Heit, B.; Bilan, P.J.; et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E35–E44. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol.Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-alpha and Palmitate Requires MyD88 and Involves MAPK/NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef]
- Takei, K.; Haucke, V. Clathrin-mediated endocytosis: Membrane factors pull the trigger. Trends Cell Biol. 2001, 11, 385–391. [Google Scholar] [CrossRef]
- Tatematsu, M.; Yoshida, R.; Morioka, Y.; Ishii, N.; Funami, K.; Watanabe, A.; Saeki, K.; Seya, T.; Matsumoto, M. Raftlin Controls Lipopolysaccharide-Induced TLR4 Internalization and TICAM-1 Signaling in a Cell Type-Specific Manner. J. Immunol. 2016, 196, 3865–3876. [Google Scholar] [CrossRef] [PubMed]
- Husebye, H.; Halaas, O.; Stenmark, H.; Tunheim, G.; Sandanger, O.; Bogen, B.; Brech, A.; Latz, E.; Espevik, T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006, 25, 683–692. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.; Beck, F.X.; Neuhofer, W. Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 2012, 302, F38–F46. [Google Scholar] [CrossRef]
- Akhter, N.; Hasan, A.; Shenouda, S.; Wilson, A.; Kochumon, S.; Ali, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. TLR4/MyD88 -mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. J. Diabetes Metab. Disord. 2018, 17, 77–84. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Frasnelli, S.C.; Bastos Ade, S.; Orrico, S.R.; Rossa, C., Jr. Modulation of cell proliferation, survival and gene expression by RAGE and TLR signaling in cells of the innate and adaptive immune response: Role of p38 MAPK and NF-KB. J. Appl. Oral Sci. 2014, 22, 185–193. [Google Scholar] [CrossRef]
- Abate, C.; Patel, L.; Rauscher, F.J., 3rd; Curran, T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 1990, 249, 1157–1161. [Google Scholar] [CrossRef]
- Jaramillo, M.; Olivier, M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase- and cyclic adenosine 5’-monophosphate (cAMP)-dependent pathways: Involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. J. Immunol. 2002, 169, 7026–7038. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Delgado-Ramos, L.; Ayala, G.; Pelechano, V.; Medina, D.A.; Carrasco, F.; Gonzalez, R.; Andres-Leon, E.; Steinmetz, L.; Warringer, J.; et al. The cellular growth rate controls overall mRNA turnover, and modulates either transcription or degradation rates of particular gene regulons. Nucleic Acids Res. 2016, 44, 3643–3658. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Simpson, L.; Carter, J.; Hassenbein, D.; Leikauf, G.D. Ozone inhalation stimulates expression of a neutrophil chemotactic protein, macrophage inflammatory protein 2. Toxicol. Appl. Pharmacol. 1993, 119, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, L.H.; Parlakpinar, H.; Ozhan, O.; Ermis, N.; Polat, A.; Vardi, N.; Tanbek, K.; Yildiz, A.; Acet, A. Inhibition of NADPH oxidase by apocynin promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress in rats. Free Radic. Res. 2017, 51, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef] [PubMed]
- Odewumi, C.O.; Latinwo, L.M.; Ruden, M.L.; Badisa, V.L.; Fils-Aime, S.; Badisa, R.B. Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. Environ. Toxicol. 2016, 31, 1612–1619. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Vanaudenaerde, B.M.; Dupont, L.J.; Demedts, M.G.; Verleden, G.M. N-acetylcysteine reduces chemokine release via inhibition of p38 MAPK in human airway smooth muscle cells. Eur. Respir. J. 2003, 22, 43–49. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindhu, S.; Akhter, N.; Wilson, A.; Thomas, R.; Arefanian, H.; Al Madhoun, A.; Al-Mulla, F.; Ahmad, R. MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells 2020, 9, 1799. https://doi.org/10.3390/cells9081799
Sindhu S, Akhter N, Wilson A, Thomas R, Arefanian H, Al Madhoun A, Al-Mulla F, Ahmad R. MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells. 2020; 9(8):1799. https://doi.org/10.3390/cells9081799
Chicago/Turabian StyleSindhu, Sardar, Nadeem Akhter, Ajit Wilson, Reeby Thomas, Hossein Arefanian, Ashraf Al Madhoun, Fahd Al-Mulla, and Rasheed Ahmad. 2020. "MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress" Cells 9, no. 8: 1799. https://doi.org/10.3390/cells9081799
APA StyleSindhu, S., Akhter, N., Wilson, A., Thomas, R., Arefanian, H., Al Madhoun, A., Al-Mulla, F., & Ahmad, R. (2020). MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells, 9(8), 1799. https://doi.org/10.3390/cells9081799








