Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Splenic Cell Separation
2.3. Virus Preparation and Viral Infection of Mice
2.4. Immunization
2.5. Plaque Assay
2.6. Infection-Center Assay
2.7. Antibodies and Flow Cytometry
2.8. Immunofluorescent Staining
2.9. Quantitative Reverse Transcription-Polymerase Chain Reaction (PCR)
2.10. Proliferation Assay
2.11. Enzyme-Linked Immunosorbent Assay (ELISA) for Cytokines and Anti-TMEV Antibodies
2.12. Western Blot
2.13. Autoantibody Detection
2.14. Statistical Analysis
3. Results
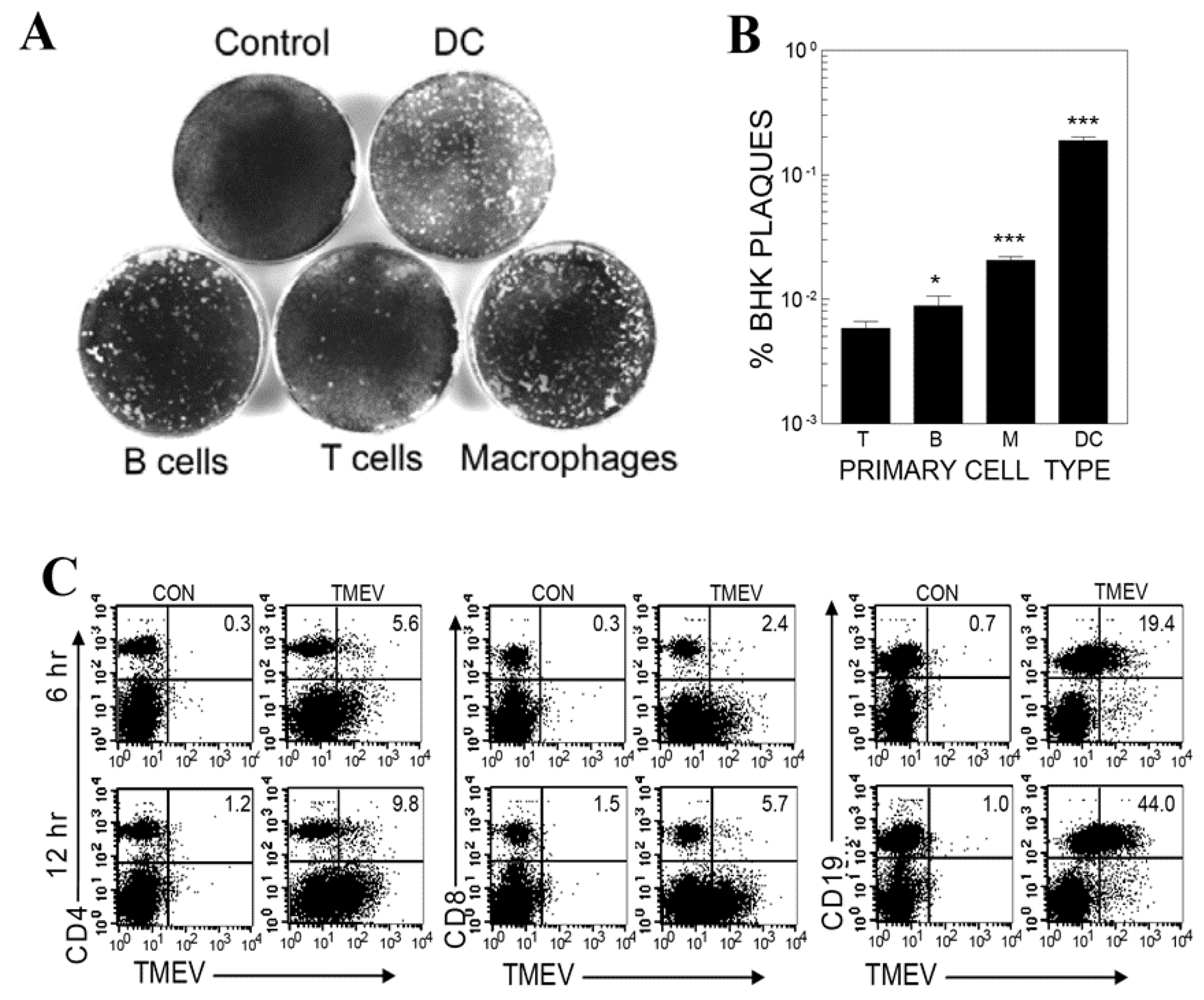
3.1. Viral Infection and Replication Levels in Different Cell Types of Splenic Cell Cultures
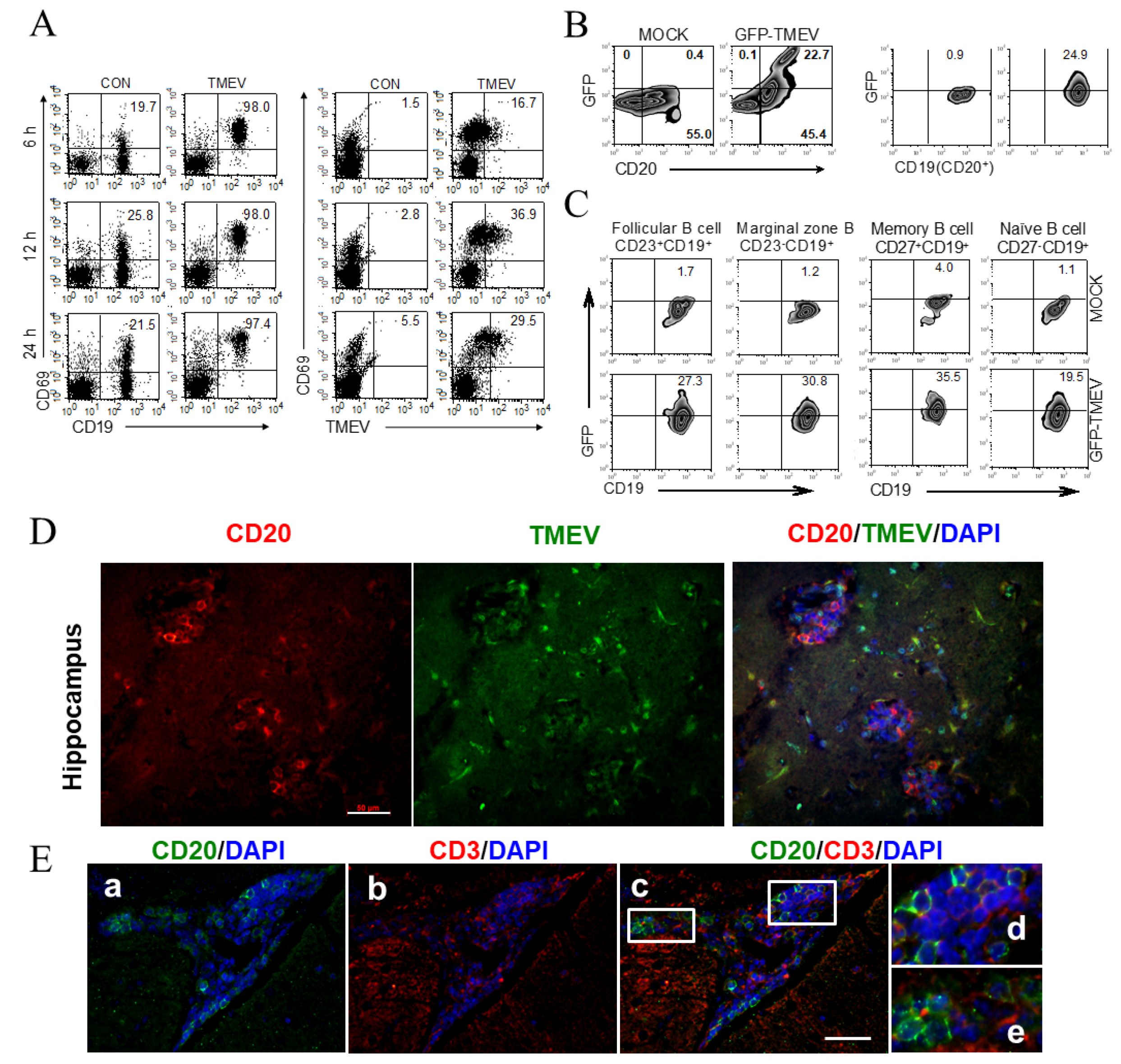
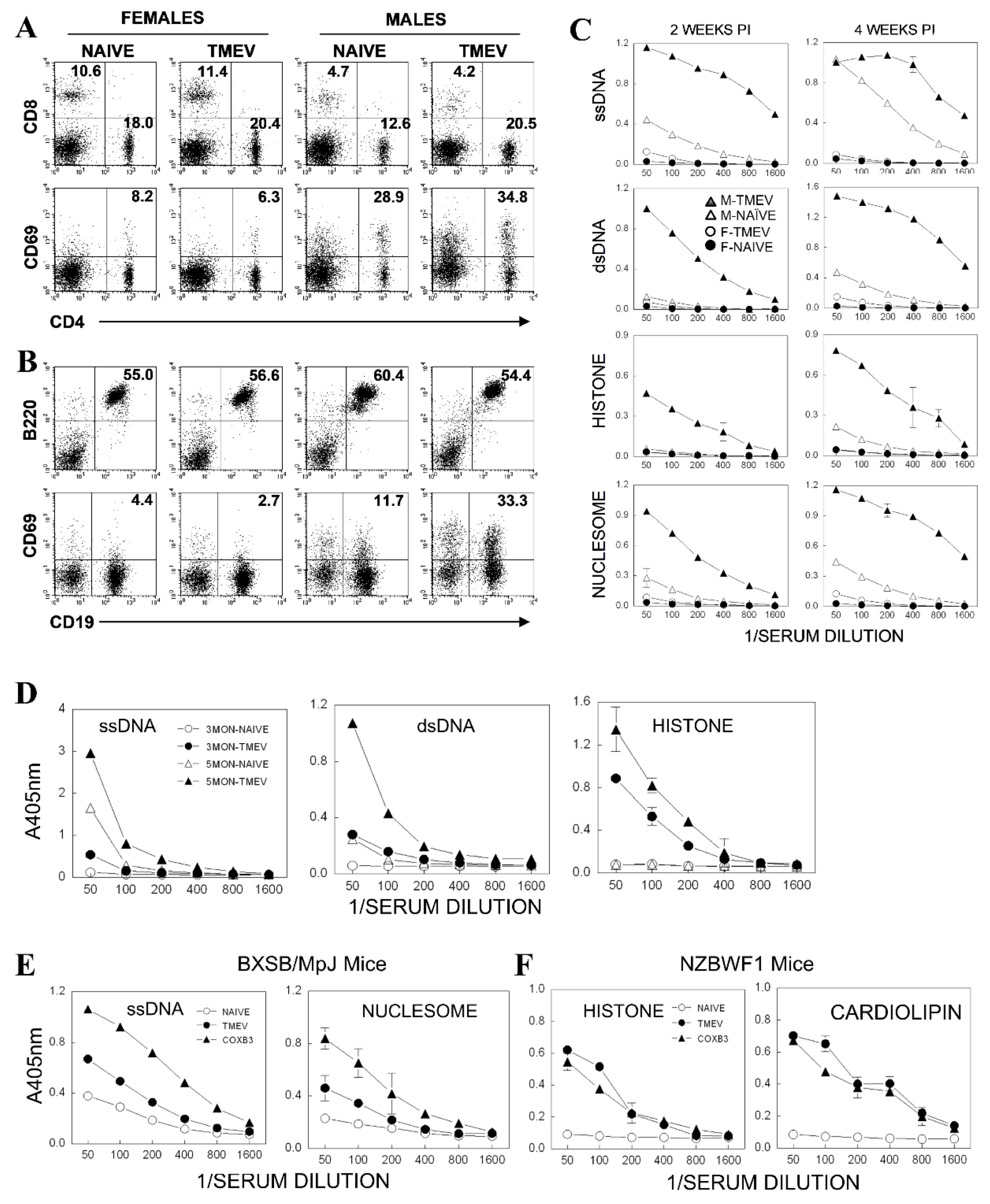
3.2. Subpopulation of B Cells Permissive to TMEV Infection
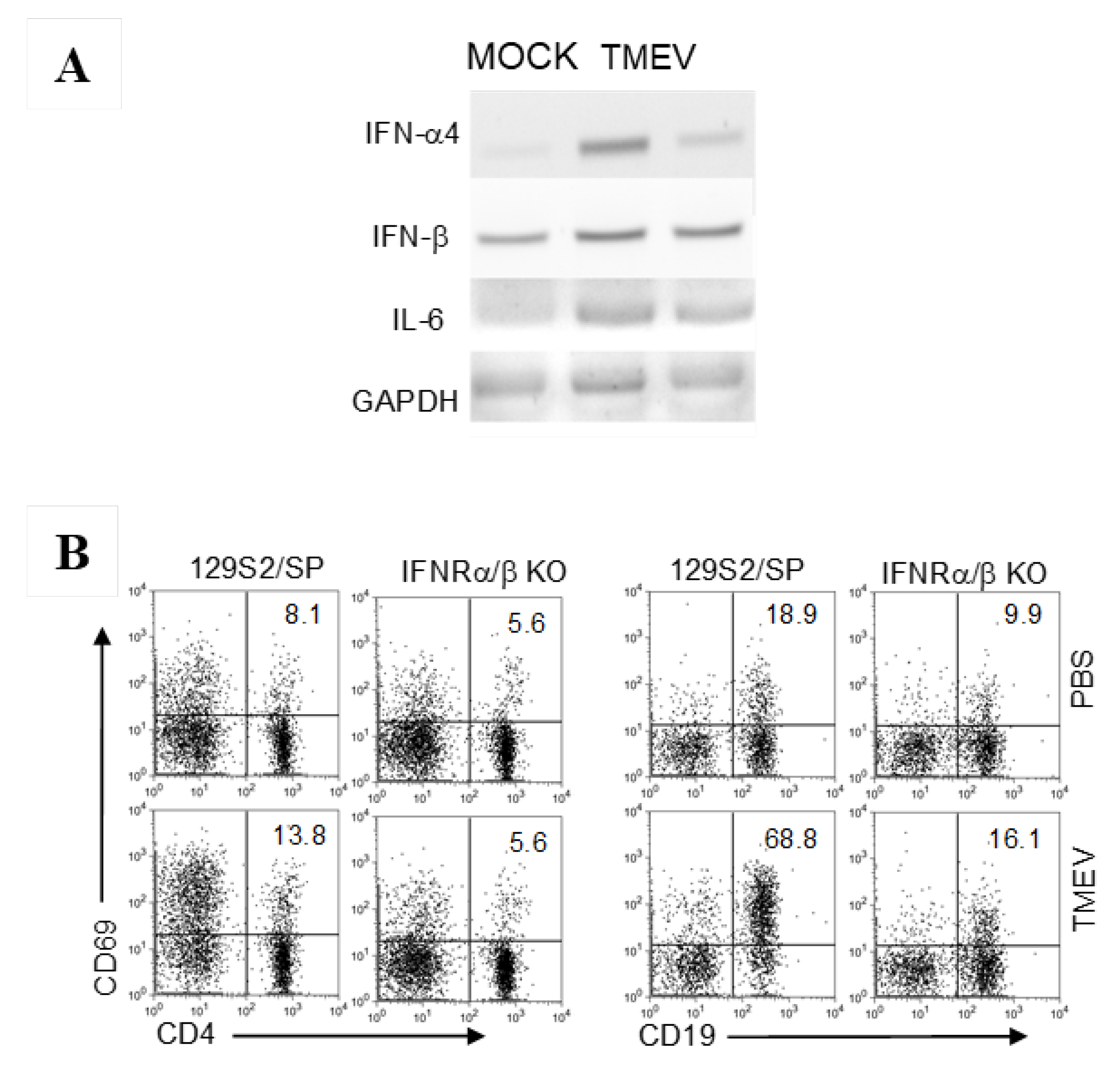
3.3. Cytokine Gene Activation in B Cells upon TMEV Infection
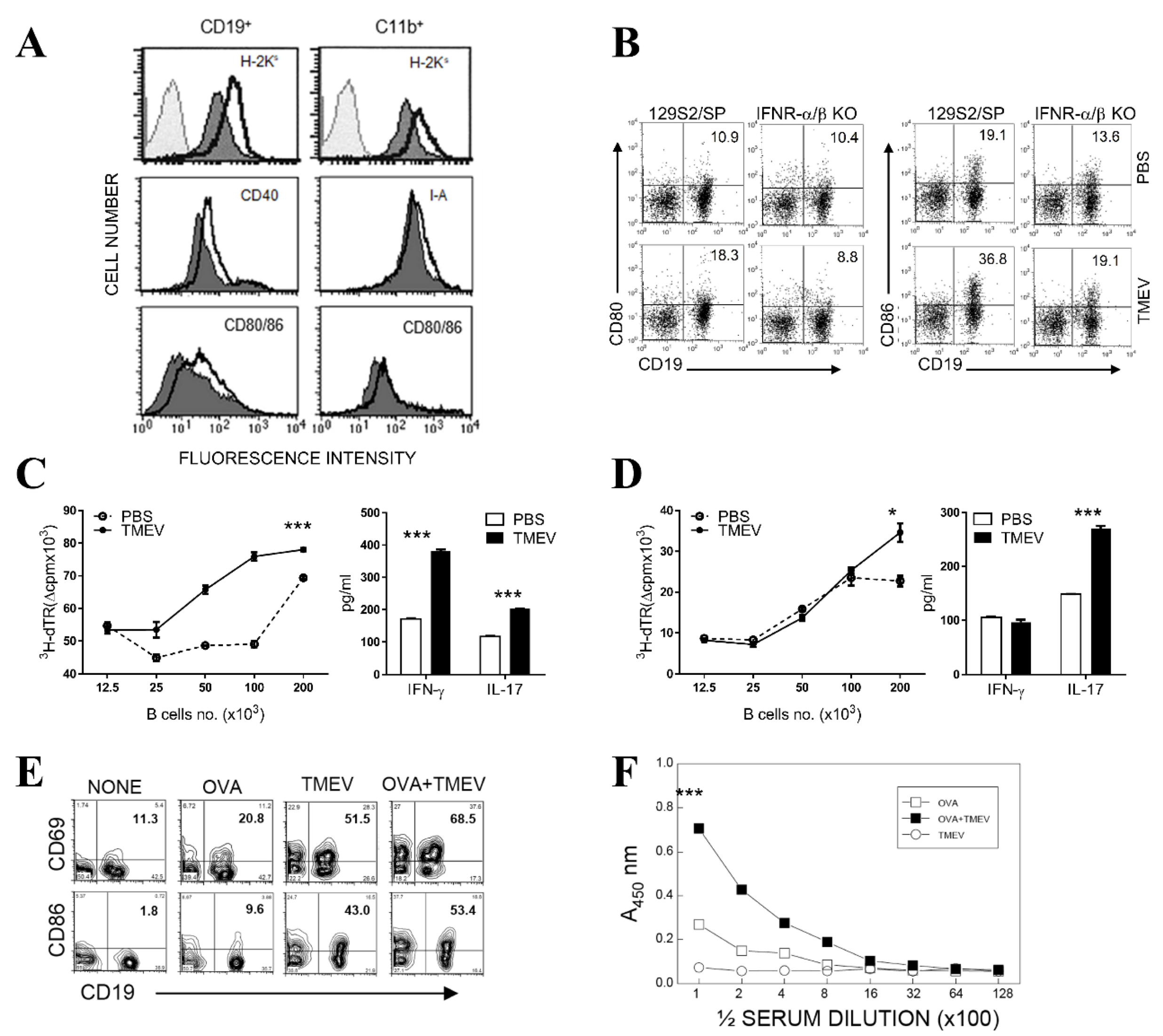
3.4. Upregulated Expression of Costimulatory Molecules on B Cells after TMEV Infection
3.5. Elevated T Cell-Activating Function of B Cells Following TMEV Infection In Vitro
3.6. Elevated Antibody Production in Mice Infected with TMEV
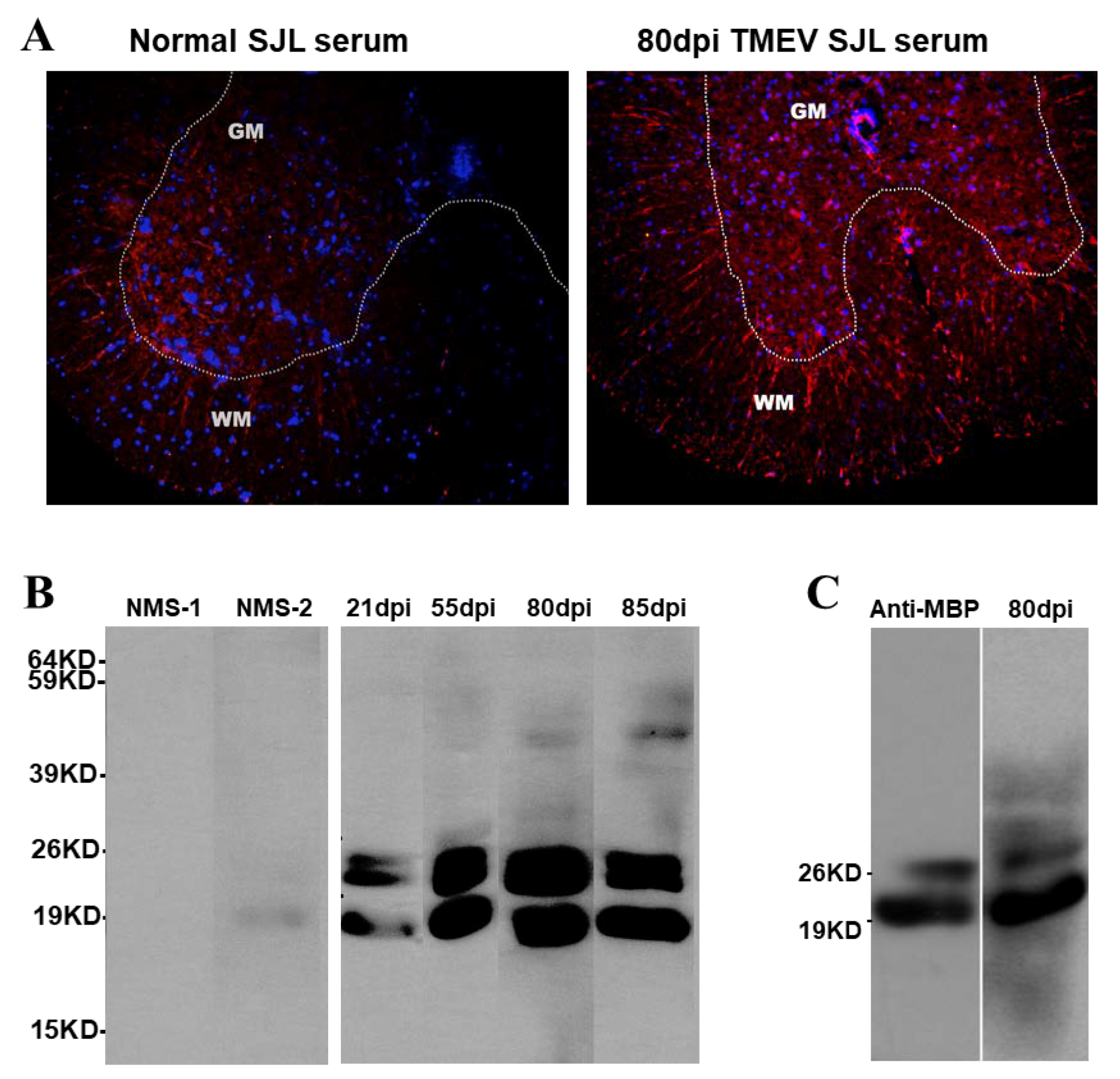
3.7. Reactivity of Serum Antibodies from TMEV-Infected Mice to Normal CNS Components
3.8. Acceleration of Autoantibody Production in Autoimmune-Prone Mice after TMEV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipton, H.L. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immune 1975, 11, 1147–1155. [Google Scholar] [CrossRef]
- Rodriguez, M.; Lafuse, W.P.; Leibowitz, J.; David, C.S. Partial suppression of Theiler’s virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology 1986, 36, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.J.; Tonks, P.; Nash, A.A.; Blakemore, W.F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J. Gen. Virol. 1987, 68, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Yauch, R.L.; Kim, B.S. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler’s virus is located within VP1(233-244). J. Immunol. 1994, 153, 4508–4519. [Google Scholar]
- Pope, J.G.; Karpus, W.J.; VanderLugt, C.; Miller, S.D. Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease. Evidence for a CD4+ T cell-mediated pathology. J. Immunol. 1996, 156, 4050–4058. [Google Scholar]
- Crane, M.A.; Yauch, R.; Dal Canto, M.C.; Kim, B.S. Effect of immunization with Theiler’s virus on the course of demyelinating disease. J. Neuroimmunol. 1993, 45, 67–73. [Google Scholar] [CrossRef]
- Mohindru, M.; Kang, B.; Kim, B.S. Initial capsid-specific CD4+ T cell responses protect against Theiler’s murine encephalomyelitisvirus-induced demyelinating disease. Eur. J. Immunol. 2006, 36, 2106–2115. [Google Scholar] [CrossRef]
- Kang, B.S.; Palma, J.P.; Lyman, M.A.; Dal Canto, M.; Kim, B.S. Antibody response is required for protection from Theiler’s virus-induced encephalitis in C57BL/6 mice in the absence of CD8+ T cells. Virology 2005, 340, 84–94. [Google Scholar] [CrossRef]
- Pullen, L.C.; Miller, S.D.; Dal Canto, M.C.; Kim, B.S. Class I-deficient resistant mice intracerebrally inoculated with Theiler’s virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 1993, 23, 2287–2293. [Google Scholar] [CrossRef]
- Myoung, J.; Kang, H.S.; Hou, W.; Meng, L.; Dal Canto, M.C.; Kim, B.S. Epitope-specific CD8+ T cells play a differential pathogenic role in the development of a viral disease model for multiple sclerosis. J. Virol. 2012, 86, 13717–13728. [Google Scholar] [CrossRef]
- Hou, W.; Kang, H.S.; Kim, B.S. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009, 206, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Jin, Y.H.; Kang, H.S.; Kim, B.S. Interleukin-6 (IL-6) and IL-17 Synergistically Promote Viral Persistence by Inhibiting Cellular Apoptosis and Cytotoxic T Cell Function. J. Virol. 2014, 88, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; So, E.Y.; Kim, B.S. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 2007, 3, e124. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Kaneyama, T.; Kang, M.H.; Kang, H.S.; Koh, C.S.; Kim, B.S. TLR3 signaling is either protective or pathogenic for the development of Theiler’s virus-induced demyelinating disease depending on the time of viral infection. J. Neuroinflamm. 2011, 8, 178. [Google Scholar] [CrossRef]
- Jin, Y.H.; Kang, H.S.; Hou, W.; Meng, L.; Kim, B.S. The level of viral infection of antigen-presenting cells correlates with the level of development of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 2015, 89, 1867–1878. [Google Scholar] [CrossRef]
- Jin, Y.H.; Mohindru, M.; Kang, M.H.; Fuller, A.C.; Kang, B.; Gallo, D.; Kim, B.S. Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler’s virus. J. Virol. 2007, 81, 11690–11702. [Google Scholar] [CrossRef]
- Aubert, C.; Chamorro, M.; Brahic, M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb. Pathog. 1987, 3, 319–326. [Google Scholar] [CrossRef]
- Zheng, L.; Calenoff, M.A.; Dal Canto, M.C. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler’s murine encephalomyelitis virus infection leading to demyelination. J. Neuroimmunol. 2001, 118, 256–267. [Google Scholar] [CrossRef]
- Lipton, H.L.; Kumar, A.S.; Trottier, M. Theiler’s virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 2005, 111, 214–223. [Google Scholar] [CrossRef]
- Rubio, N.; Capa, L. Differential IL-1 synthesis by astrocytes from Theiler’s murine encephalomyelitis virus-susceptible and -resistant strains of mice. Cell Immunol. 1993, 149, 237–247. [Google Scholar] [CrossRef]
- Palma, J.P.; Kim, B.S. Induction of selected chemokines in glial cells infected with Theiler’s virus. J. Neuroimmunol. 2001, 117, 166–170. [Google Scholar] [CrossRef]
- Kim, B.S.; Palma, J.P.; Kwon, D.; Fuller, A.C. Innate immune response induced by Theiler’s murine encephalomyelitis virus infection. Immunol. Res. 2005, 31, 1–12. [Google Scholar] [CrossRef]
- So, E.Y.; Kang, M.H.; Kim, B.S. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 2006, 53, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.A.; Williams, B.R.; Miller, S.D. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia 2007, 55, 239–252. [Google Scholar] [CrossRef] [PubMed]
- So, E.Y.; Kim, B.S. Theiler’s virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia 2009, 57, 1216–1226. [Google Scholar] [CrossRef]
- Jin, Y.H.; Kim, S.J.; So, E.Y.; Meng, L.; Colonna, M.; Kim, B.S. Melanoma differentiation-associated gene 5 is critical for protection against Theiler’s virus-induced demyelinating disease. J. Virol. 2012, 86, 1531–1543. [Google Scholar] [CrossRef]
- Kappler, J.; White, J.; Wegmann, D.; Mustain, E.; Marrack, P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc. Natl. Acad. Sci. USA 1982, 79, 3604–3607. [Google Scholar] [CrossRef]
- Chesnut, R.W.; Grey, H.M. Antigen presentation by B cells and its significance in T-B interactions. Adv. Immunol. 1986, 39, 51–94. [Google Scholar] [CrossRef]
- Pasare, C.; Medzhitov, R. Toll-like receptors and acquired immunity. Semin. Immunol. 2004, 16, 23–26. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Santiago-Raber, M.L.; Baudino, L.; Izui, S. Emerging roles of TLR7 and TLR9 in murine SLE. J. Autoimmun. 2009, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M.; Marshak-Rothstein, A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin. Immunol. 2011, 23, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011, 11, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- McCright, I.J.; Fujinami, R.S. Lack of correlation of Theiler’s virus binding to cells with infection. J. Neurovirol. 1997, 3, S68–S70. [Google Scholar]
- Dal Canto, M.C.; Melvold, R.W.; Kim, B.S.; Miller, S.D. Two models of multiple sclerosis: Experimental allergic encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus (TMEV) infection. A pathological and immunological comparison. Microsc. Res. Tech. 1995, 32, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Von Budingen, H.C.; Bar-Or, A.; Zamvil, S.S. B cells in multiple sclerosis: Connecting the dots. Curr. Opin. Immunol. 2011, 23, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Barun, B.; Bar-Or, A. Treatment of multiple sclerosis with anti-CD20 antibodies. Clin. Immunol. 2012, 142, 31–37. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Cree, B.A.C.; Hauser, S.L. Ocrelizumab and Other CD20(+) B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 835–841. [Google Scholar] [CrossRef]
- Pullen, L.C.; Park, S.H.; Miller, S.D.; Dal Canto, M.C.; Kim, B.S. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler’s virus-induced demyelinating disease. J. Immunol. 1995, 155, 4497–4503. [Google Scholar]
- Dutta, S.K.; Myrup, A.C. Infectious center assay of intracellular virus and infective virus titer for equine mononuclear cells infected in vivo and in vitro with equine herpesviruses. Can. J. Comp. Med. 1983, 47, 64–69. [Google Scholar] [PubMed]
- Kang, B.S.; Lyman, M.A.; Kim, B.S. Differences in avidity and epitope recognition of CD8(+) T cells infiltrating the central nervous systems of SJL/J mice infected with BeAn and DA strains of Theiler’s murine encephalomyelitis virus. J. Virol. 2002, 76, 11780–11784. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Adams, S.; Stanik, V.; Datta, S.K. Nucleosome: A major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993, 177, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Chiang, M.Y.; Ecklund, D.; Zhang, L.; Ramsey-Goldman, R.; Datta, S.K. Megakaryocyte progenitors are the main APCs inducing Th17 response to lupus autoantigens and foreign antigens. J. Immunol. 2012, 188, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- Jelachich, M.L.; Bandyopadhyay, P.; Blum, K.; Lipton, H.L. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology 1995, 209, 437–444. [Google Scholar] [CrossRef]
- Qi, Y.; Dal Canto, M.C. Effect of Theiler’s murine encephalomyelitis virus and cytokines on cultured oligodendrocytes and astrocytes. J. Neurosci. Res. 1996, 45, 364–374. [Google Scholar] [CrossRef]
- Kang, M.H.; So, E.Y.; Park, H.; Kim, B.S. Replication of Theiler’s virus requires NF-kappaB-activation: Higher viral replication and spreading in astrocytes from susceptible mice. Glia 2008, 56, 942–953. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Ramsdell, F.; Hjerrild, K.A.; Armitage, R.J.; Grabstein, K.H.; Hennen, K.B.; Farrah, T.; Fanslow, W.C.; Shevach, E.M.; Alderson, M.R. Molecular characterization of the early activation antigen CD69: A type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur. J. Immunol. 1993, 23, 1643–1648. [Google Scholar] [CrossRef]
- Stevenson, P.G.; Doherty, P.C. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J. Virol. 1999, 73, 1075–1079. [Google Scholar] [CrossRef]
- Uchida, J.; Lee, Y.; Hasegawa, M.; Liang, Y.; Bradney, A.; Oliver, J.A.; Bowen, K.; Steeber, D.A.; Haas, K.M.; Poe, J.C.; et al. Mouse CD20 expression and function. Int. Immunol. 2004, 16, 119–129. [Google Scholar] [CrossRef]
- Jelachich, M.L.; Bramlage, C.; Lipton, H.L. Differentiation of M1 myeloid precursor cells into macrophages results in binding and infection by Theiler’s murine encephalomyelitis virus and apoptosis. J. Virol. 1999, 73, 3227–3235. [Google Scholar] [CrossRef]
- Armerding, D.; Katz, D.H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J. Exp. Med. 1974, 139, 24–43. [Google Scholar] [CrossRef]
- Vitetta, E.; Pure, E.; Isakson, P.; Buck, L.; Uhr, J. The activation of murine B cells: The role of surface immunoglobulins. Immunol. Rev. 1980, 52, 211–231. [Google Scholar] [CrossRef]
- Satoh, J.; Paty, D.W.; Kim, S.U. Differential effects of beta and gamma interferons on expression of major histocompatibility complex antigens and intercellular adhesion molecule-1 in cultured fetal human astrocytes. Neurology 1995, 45, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.M.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; Di Pucchio, T.; Belardelli, F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Caramalho, I.; Demengeot, J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 2002, 14, 411–419. [Google Scholar] [CrossRef]
- Jin, Y.H.; Hou, W.; Kim, S.J.; Fuller, A.C.; Kang, B.; Goings, G.; Miller, S.D.; Kim, B.S. Type I interferon signals control Theiler’s virus infection site, cellular infiltration and T cell stimulation in the CNS. J. Neuroimmunol. 2010, 226, 27–37. [Google Scholar] [CrossRef]
- Rauch, H.C.; Montgomery, I.N.; Hinman, C.L.; Harb, W.; Benjamins, J.A. Chronic Theiler’s virus infection in mice: Appearance of myelin basic protein in the cerebrospinal fluid and serum antibody directed against MBP. J. Neuroimmunol. 1987, 14, 35–48. [Google Scholar] [CrossRef]
- Fujinami, R.S.; Zurbriggen, A.; Powell, H.C. Monoclonal antibody defines determinant between Theiler’s virus and lipid -like structures. J. Neuroimmunol. 1988, 20, 25–32. [Google Scholar] [CrossRef]
- Pisitkun, P.; Deane, J.A.; Difilippantonio, M.J.; Tarasenko, T.; Satterthwaite, A.B.; Bolland, S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006, 312, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Fu, Q.; Deng, Y.; Qian, X.; Zhao, J.; Kaufman, K.M.; Wu, Y.L.; Yu, C.Y.; Tang, Y.; Chen, J.Y.; et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 2010, 107, 15838–15843. [Google Scholar] [CrossRef] [PubMed]
- Talal, N.; Steinberg, A.D.; Daley, G.G. Inhibition of antigodies binding polyinosinic-polycytidylic acid in human and mouse lupus sera by viral and synthetic ribonucleic acids. J. Clin. Investig. 1971, 50, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Meeker, T.; Chan, J.H.; Guiducci, C.; Coffman, R.L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007, 37, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Vanderlugt, C.L.; Begolka, W.S.; Pao, W.; Yauch, R.L.; Neville, K.L.; Katz-Levy, Y.; Carrizosa, A.; Kim, B.S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997, 3, 1133–1136. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Antiviral signaling through pattern recognition receptors. J. Biochem. 2007, 141, 137–145. [Google Scholar] [CrossRef]
- Moore, C.B.; Ting, J.P. Regulation of mitochondrial antiviral signaling pathways. Immunity 2008, 28, 735–739. [Google Scholar] [CrossRef]
- Kim, B.S.; Jin, Y.H.; Meng, L.; Hou, W.; Kang, H.S.; Park, H.S.; Koh, C.S. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J. Neuroinflamm. 2012, 9, 217. [Google Scholar] [CrossRef]
- Kiefer, K.; Oropallo, M.A.; Cancro, M.P.; Marshak-Rothstein, A. Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol. 2012, 90, 498–504. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine--40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Kuno, K.; Matsushima, K. The IL-1 receptor signaling pathway. J. Leukoc. Biol. 1994, 56, 542–547. [Google Scholar] [CrossRef]
- Palma, J.P.; Yauch, R.L.; Lang, S.; Kim, B.S. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler’s virus-induced demyelination. J. Immunol. 1999, 162, 6543–6551. [Google Scholar] [PubMed]
- Kramer, M.; Schulte, B.M.; Toonen, L.W.; Barral, P.M.; Fisher, P.B.; Lanke, K.H.; Galama, J.M.; van Kuppeveld, F.J.; Adema, G.J. Phagocytosis of picornavirus-infected cells induces an RNA-dependent antiviral state in human dendritic cells. J. Virol. 2008, 82, 2930–2937. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Wang, C.; Jiang, B. Rotavirus and coxsackievirus infection activated different profiles of toll-like receptors and chemokines in intestinal epithelial cells. Inflamm. Res. 2009, 58, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.B.; Harley, I.T.; Guthridge, J.M.; James, J.A. The curiously suspicious: A role for Epstein-Barr virus in lupus. Lupus 2006, 15, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Kats, A.; Cavallo, M.; Shoenfeld, Y. Clinical and molecular evidence for association of SLE with parvovirus B19. Lupus 2010, 19, 783–792. [Google Scholar] [CrossRef]
- Geginat, J.; Paroni, M.; Pagani, M.; Galimberti, D.; De Francesco, R.; Scarpini, E.; Abrignani, S. The Enigmatic Role of Viruses in Multiple Sclerosis: Molecular Mimicry or Disturbed Immune Surveillance? Trends Immunol. 2017, 38, 498–512. [Google Scholar] [CrossRef]
- Burnard, S.; Lechner-Scott, J.; Scott, R.J. EBV and MS: Major cause, minor contribution or red-herring? Mult. Scler. Relat. Disord. 2017, 16, 24–30. [Google Scholar] [CrossRef][Green Version]
- Avalos, A.M.; Meyer-Wentrup, F.; Ploegh, H.L. B-cell receptor signaling in lymphoid malignancies and autoimmunity. Adv. Immunol. 2014, 123, 1–49. [Google Scholar] [CrossRef]
- Ntoufa, S.; Vilia, M.G.; Stamatopoulos, K.; Ghia, P.; Muzio, M. Toll-like receptors signaling: A complex network for NF-kappaB activation in B-cell lymphoid malignancies. Semin. Cancer Biol. 2016, 39, 15–25. [Google Scholar] [CrossRef]
- Sharifi, L.; Mirshafiey, A.; Rezaei, N.; Azizi, G.; Magaji Hamid, K.; Amirzargar, A.A.; Asgardoon, M.H.; Aghamohammadi, A. The role of toll-like receptors in B-cell development and immunopathogenesis of common variable immunodeficiency. Expert. Rev. Clin. Immunol. 2016, 12, 195–207. [Google Scholar] [CrossRef]
- Kim, S.J.; Jin, Y.H.; Kim, B.S. Prostaglandin E2 produced following infection with Theiler’s virus promotes the pathogenesis of demyelinating disease. PLoS ONE 2017, 12, e0176406. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Choe, Y.K.; Kim, B.S. Analysis of antibody responses to predominant linear epitopes of Theiler’s murine encephalomyelitis virus. J. Virol. 1994, 68, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Gilli, F.; Li, L.; Campbell, S.J.; Anthony, D.C.; Pachner, A.R. The effect of B-cell depletion in the Theiler’s model of multiple sclerosis. J. Neurol. Sci. 2015, 359, 40–47. [Google Scholar] [CrossRef] [PubMed]
- McClain, M.T.; Harley, J.B.; James, J.A. The role of Epstein-Barr virus in systemic lupus erythematosus. Front. Biosci. 2001, 6, E137–E147. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Shoenfeld, Y. Infections and SLE. Autoimmunity 2005, 38, 473–485. [Google Scholar] [CrossRef]
- Nelson, P.; Rylance, P.; Roden, D.; Trela, M.; Tugnet, N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus 2014, 23, 596–605. [Google Scholar] [CrossRef]
- Gilden, D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 2005, 4, 195–202. [Google Scholar] [CrossRef]
- ’t Hart, B.A.; Kap, Y.S. An essential role of virus-infected B cells in the marmoset experimental autoimmune encephalomyelitis model. Mult. Scler. J. Exp. Transl. Clin. 2017, 3. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-H.; Kim, C.X.; Huang, J.; Kim, B.S. Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis. Cells 2020, 9, 1787. https://doi.org/10.3390/cells9081787
Jin Y-H, Kim CX, Huang J, Kim BS. Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis. Cells. 2020; 9(8):1787. https://doi.org/10.3390/cells9081787
Chicago/Turabian StyleJin, Young-Hee, Charles X. Kim, Jocelin Huang, and Byung S. Kim. 2020. "Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis" Cells 9, no. 8: 1787. https://doi.org/10.3390/cells9081787
APA StyleJin, Y.-H., Kim, C. X., Huang, J., & Kim, B. S. (2020). Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis. Cells, 9(8), 1787. https://doi.org/10.3390/cells9081787




