Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. FLS and Cell Culture
2.3. Reverse Transcription Quantitative PCR (RT-qPCR)
2.4. ELISA
2.5. Cell Proliferation Assays
2.6. Inhibition of TLR-4
2.7. Statistical Analysis
3. Results
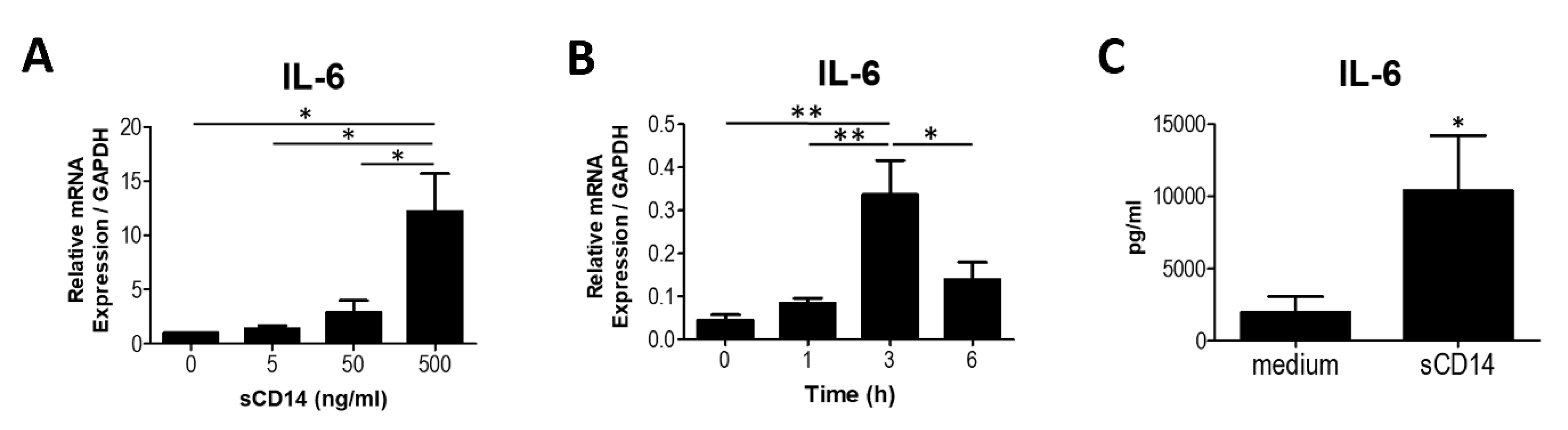
3.1. Soluble CD14 Induces the Synthesis of IL-6 in RA-FLS
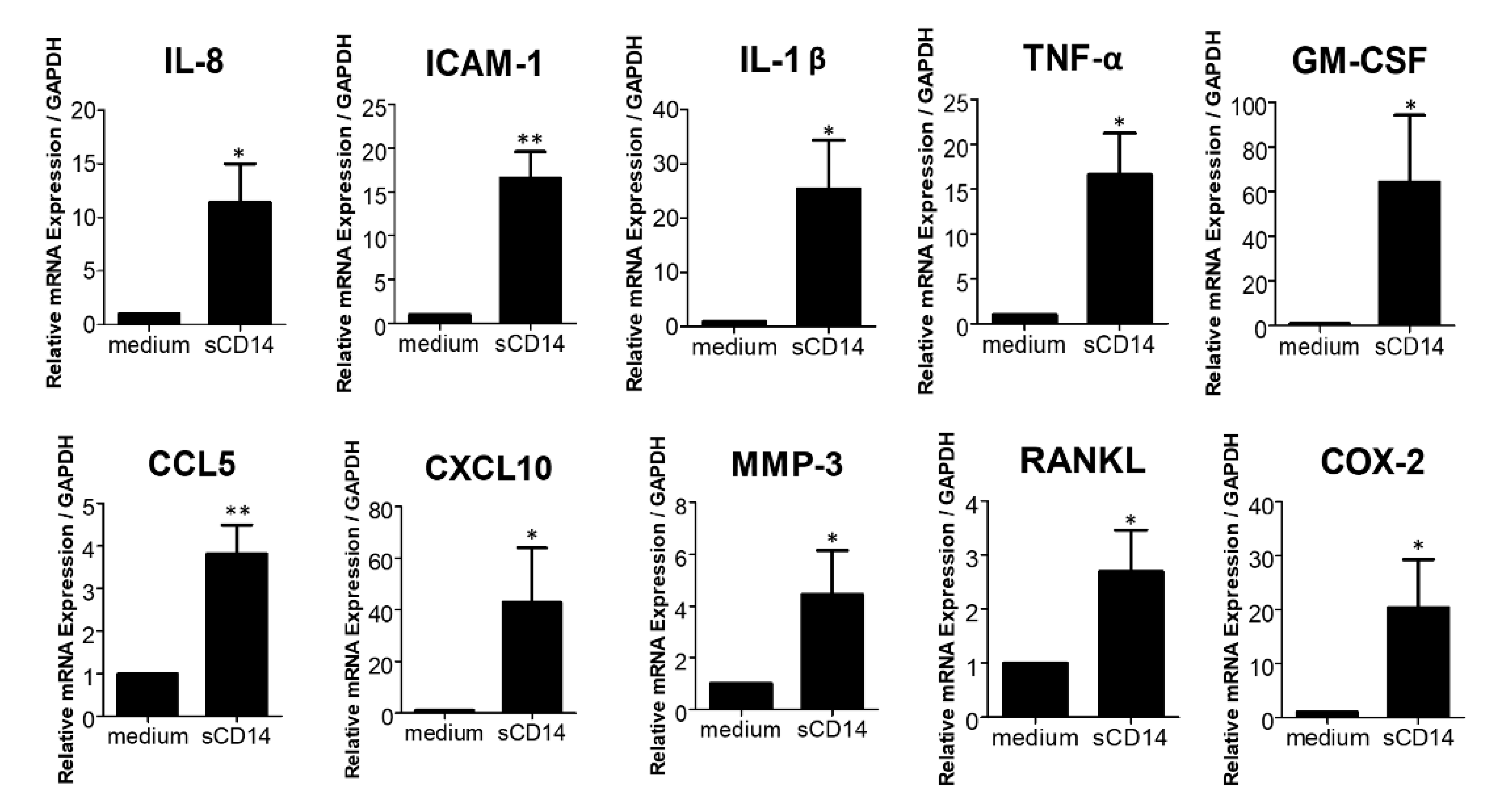
3.2. Soluble CD14 Induces the Expression of Pro-Inflammatory Cytokines, Chemokines, and Mediators by RA-FLS
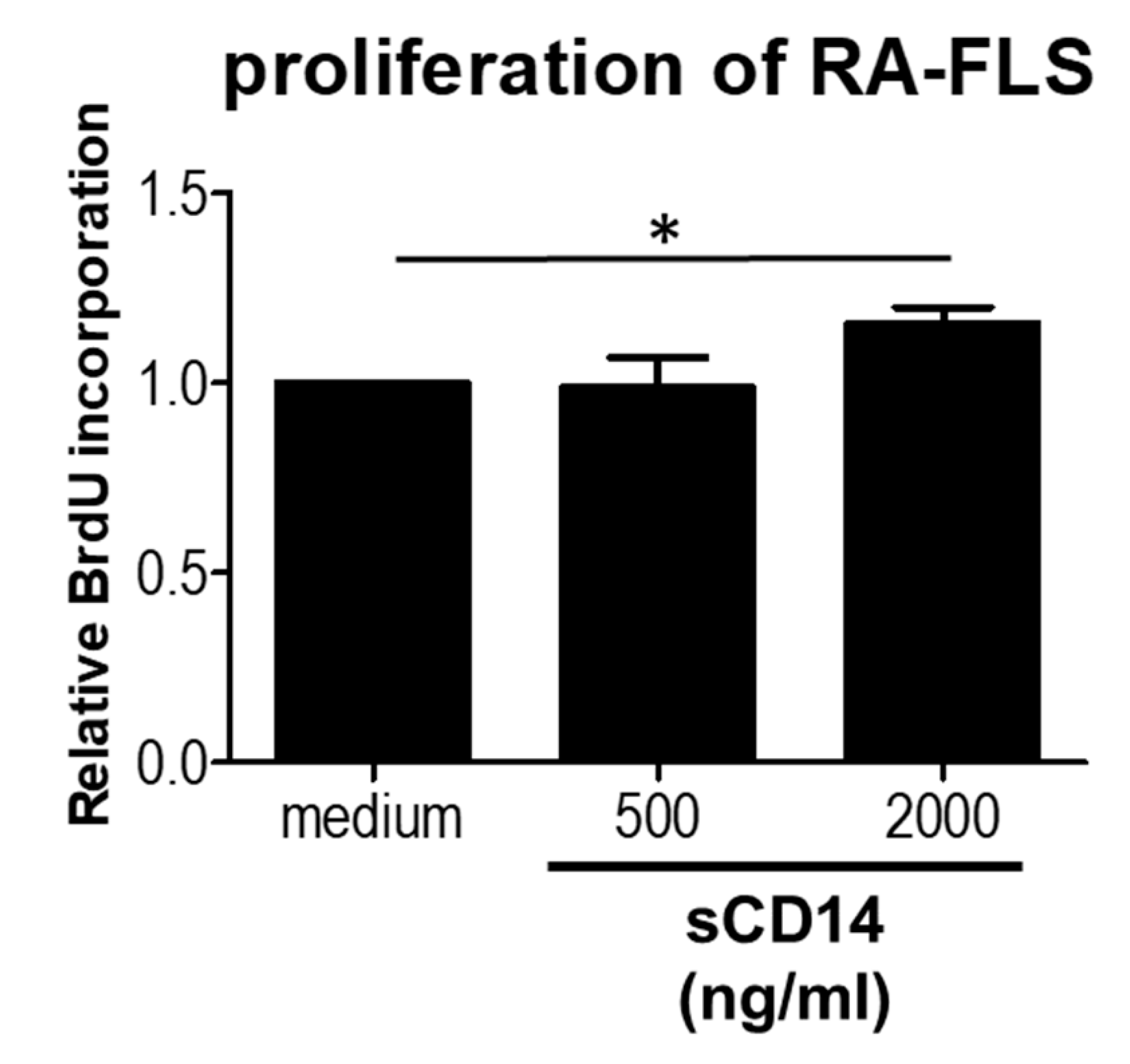
3.3. High Concentrations of sCD14 Promote the Proliferation of RA-FLS
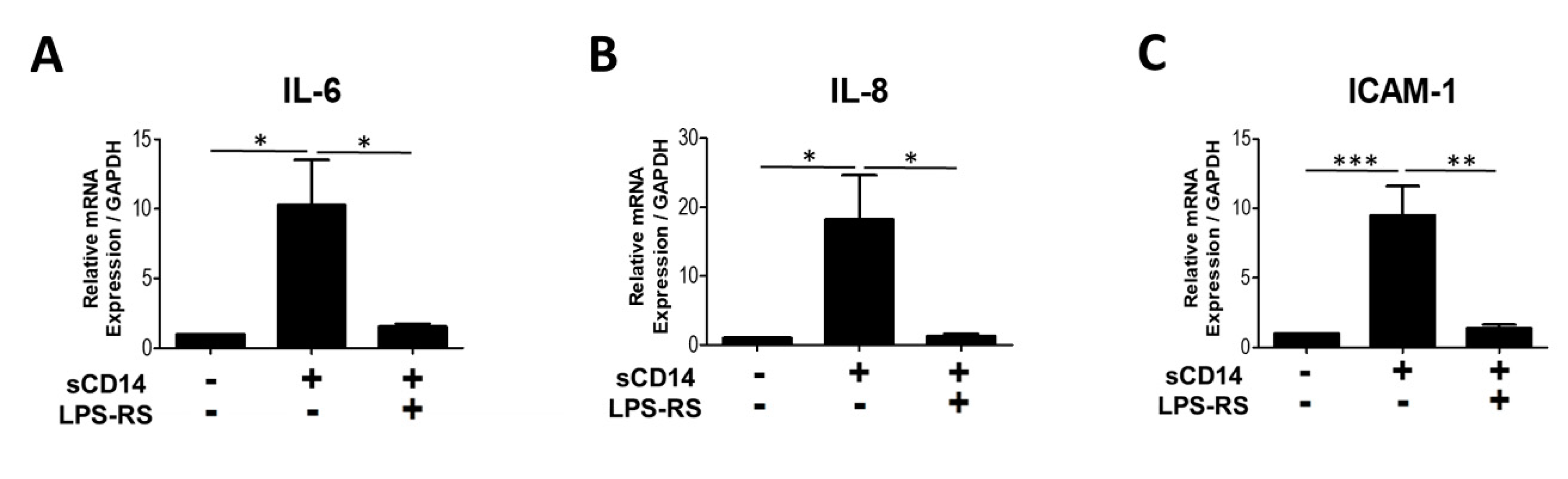
3.4. The TLR-4 Antagonist LPS-RS Abolishes sCD14-Induced IL-6, IL-8, and ICAM-1 Expression by RA-FLS
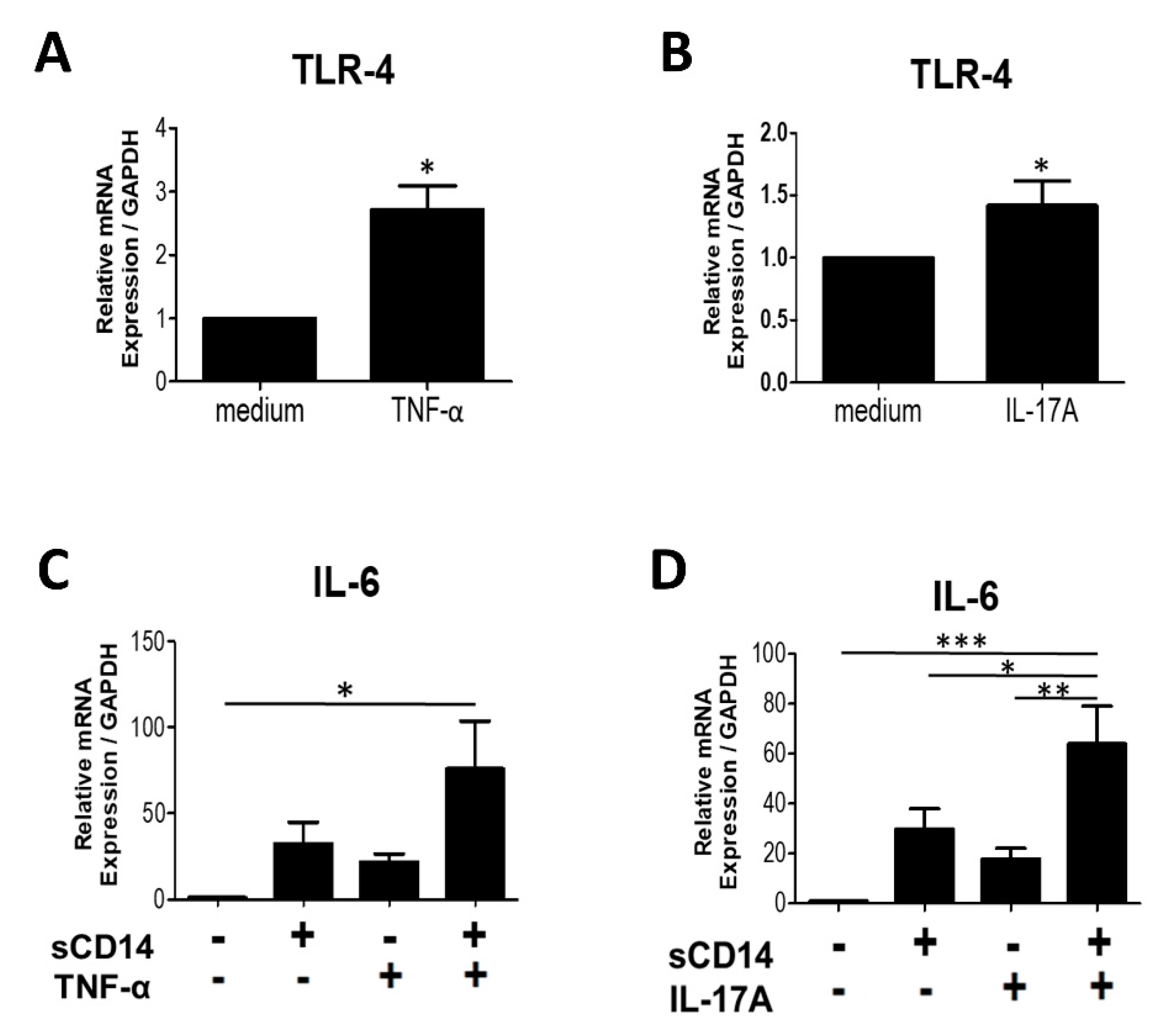
3.5. Addition of TNF-α or IL-17A Augments the Response of RA-FLS to sCD14
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Haziot, A.; Chen, S.; Ferrero, E.; Low, M.G.; Silber, R.; Goyert, S.M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 1988, 141, 547–552. [Google Scholar] [PubMed]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, S.J.; Rah, S.-H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.-O.; Park, B.S.; Yoon, T.-Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T.L.; Blecha, F. CD14 and other recognition molecules for lipopolysaccharide: A review. Immunopharmacology 1995, 29, 187–205. [Google Scholar] [CrossRef]
- Yu, S.; Nakashima, N.; Xu, B.H.; Matsuda, T.; Izumihara, A.; Sunahara, N.; Nakamura, T.; Tsukano, M.; Matsuyama, T. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: In the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumatol. Int. 1998, 17, 237–243. [Google Scholar] [CrossRef]
- Kang, M.J.; Park, Y.-J.; You, S.; Yoo, S.-A.; Choi, S.; Kim, D.-H.; Cho, C.-S.; Yi, E.C.; Hwang, D.; Kim, W.-U. Urinary proteome profile predictive of disease activity in rheumatoid arthritis. J. Proteome Res. 2014, 13, 5206–5217. [Google Scholar] [CrossRef]
- Bas, S.; Gauthier, B.R.; Spenato, U.; Stingelin, S.; Gabay, C. CD14 is an acute-phase protein. J. Immunol. 2004, 172, 4470–4479. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Wang, B.; Liu, J.; Yoshiya, S.; Kurosaka, M.; Kumagai, S. Trichostatin A cooperates with Fas-mediated signal to induce apoptosis in rheumatoid arthritis synovial fibroblasts. J. Rheumatol. 2006, 33, 1052–1060. [Google Scholar] [PubMed]
- Yoshida, Y.; Tanaka, T. Interleukin 6 and rheumatoid arthritis. Biomed. Res. Int. 2014, 2014, 698313. [Google Scholar] [CrossRef]
- Zvaifler, N.J.; Firestein, G.S. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994, 37, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Hayakawa, N.; Mihara, M. IL-6 trans-signaling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-α and IL-17. Rheumatology 2008, 47, 1635–1640. [Google Scholar] [CrossRef]
- Gutiérrez-Cañas, I.; Juarranz, Y.; Santiago, B.; Arranz, A.; Martinez, C.; Galindo, M.; Payá, M.; Gomariz, R.P.; Pablos, J.L. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology 2006, 45, 527–532. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Yoon, B.-Y.; Kim, J.I.; Heo, Y.-M.; Woo, Y.-J.; Park, S.H.; Kim, H.Y.; Kim, S.-I.; Cho, M.-L. Interleukin-17 increases the expression of Toll-like receptor 3 via the STAT3 pathway in rheumatoid arthritis fibroblast-like synoviocytes. Immunology 2014, 141, 353–361. [Google Scholar] [CrossRef]
- Qu, Z.; Garcia, C.H.; O’Rourke, L.M.; Planck, S.R.; Kohli, M.; Rosenbaum, J.T. Local proliferation of fibroblast—like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994, 37, 212–220. [Google Scholar] [CrossRef]
- Lévêque, M.; Jeune, K.S.-L.; Jouneau, S.; Moulis, S.; Desrues, B.; Belleguic, C.; Brinchault, G.; le Trionnaire, S.; Gangneux, J.-P.; Dimanche-Boitrel, M.-T. Soluble CD14 acts as a DAMP in human macrophages: Origin and involvement in inflammatory cytokine/chemokine production. FASEB J. 2017, 31, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Shive, C.L.; Jiang, W.; Anthony, D.D.; Lederman, M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015, 29, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Pierer, M.; Wagner, U.; Rossol, M.; Ibrahim, S. Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen-induced arthritis in DBA1J mice. PLoS ONE 2011, 6, e23539. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Kanda, V.; Bush-Joseph, C.; Verma, N.; Chubinskaya, S.; Mikecz, K.; Glant, T.T.; Malfait, A.-M.; Crow, M.K.; Spear, G.T. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012, 64, 2268–2277. [Google Scholar] [CrossRef]
- Feghali, K.; Tanabe, S.; Grenier, D. Soluble CD14 Induces Cytokine Release by Human Oral Epithelial Cells. J. Periodontal Res. 2011, 46, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, G.; Verweyen, E.; Pretzer, C.; Kessel, K.; Hirono, K.; Ichida, F.; Okabe, M.; Cabral, D.A.; Foell, D.; Brown, K.L.; et al. Monocyte-Derived Interleukin-1β As the Driver of S100A12-Induced Sterile Inflammatory Activation of Human Coronary Artery Endothelial Cells: Implications for the Pathogenesis of Kawasaki Disease. Arthritis Rheumatol. 2019, 71, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Diah, S.; Desiana, R.; Bagus, S.; Aulanni’Am, A. The Expression of TLR-2 and TLR-4 Protein in the Epithelial Cells of the Oral Mucosal Patients with Recurrent Apthous Stomatitis (RAS). IJCPCR 2016, 8, 1659–1662. [Google Scholar]
- Alonso-Pérez, A.; Franco-Trepat, E.; Guillán-Fresco, M.; Jorge-Mora, A.; López, V.; Pino, J.; Gualillo, O.; Gómez, R. Role of Toll-Like Receptor 4 on Osteoblast Metabolism and Function. Front. Physiol. 2018, 9, 504. [Google Scholar] [CrossRef]
- Dunzendorfer, S.; Lee, H.K.; Soldau, K.; Tobias, P.S. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: Roles of LBP and sCD14 in mediating LPS responses. FASEB J. 2004, 18, 1117–1119. [Google Scholar] [CrossRef]
- Zeuke, S.; Ulmer, A.J.; Kusumoto, S.; Katus, H.A.; Heine, H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002, 56, 126–134. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Gao, R.; Su, Y.; Umehara, H.; Dong, L.; Gong, F. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology 2013, 52, 1739–1747. [Google Scholar] [CrossRef][Green Version]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011, 63, 53–62. [Google Scholar] [CrossRef]
- Lee, D.G.; Woo, J.W.; Kwok, S.K.; Cho, M.L.; Park, S.H. MRP8 promotes Th17 differentiation via upregulation of IL-6 production by fibroblast-like synoviocytes in rheumatoid arthritis. Exp. Mol. Med. 2013, 45, e20. [Google Scholar] [CrossRef]
- Moreland, L. Unmet needs in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, 1–7. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Monnet, E.; Choy, E.H.; McInnes, I.; Kobakhidze, T.; de Graaf, K.; Jacqmin, P.; Lapeyre, G.; de Min, C. Efficacy and safety of NI-0101, an anti-toll-like receptor 4 monoclonal antibody, in patients with rheumatoid arthritis after inadequate response to methotrexate: A phase II study. Ann. Rheum. Dis. 2020, 79, 316–323. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| IL-1β | AAACAGATGAAGTGCTCCTTCCAGG | TGGAGAACACCACTTGTTGCTCCA |
| IL-8 | ATGACTTCCAAGCTGGCCGTGGCT | TCTCAGCCCTCTTCAAAAACTTCTC |
| ICAM-1 | ATGCCCAGACATCTGTGTCC | GGGGTCTCTATGCCCAACAA |
| GM-CSF | CACTGCTGCTGAGATGAATGAAA | GTCTGTAGGCAGGTCGGCTC |
| CCL5 | TGC CTC CCA TAT TCC TCG G | CTA GCT CAT CTC CAA AGA |
| CXCL10 | GAAATTATTCCTGCAAGCCAATTT | TCACCCTTCTTTTTCATTGTAGCA |
| MMP-3 | TGGCATTCAGTCCCTCTATGG | AGGACAAAGCAGGATCACAGTT |
| COX-2 | ATTGACCAGAGCAGGCAGAT | CAGGATACAGCTCCACAGCA |
| TLR-4 | CAGAGTTGCTTTCAATGGCATC | AGACTGTAATCAAGAACCTGGAGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichise, Y.; Saegusa, J.; Tanaka-Natsui, S.; Naka, I.; Hayashi, S.; Kuroda, R.; Morinobu, A. Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4. Cells 2020, 9, 1689. https://doi.org/10.3390/cells9071689
Ichise Y, Saegusa J, Tanaka-Natsui S, Naka I, Hayashi S, Kuroda R, Morinobu A. Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4. Cells. 2020; 9(7):1689. https://doi.org/10.3390/cells9071689
Chicago/Turabian StyleIchise, Yoshihide, Jun Saegusa, Shino Tanaka-Natsui, Ikuko Naka, Shinya Hayashi, Ryosuke Kuroda, and Akio Morinobu. 2020. "Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4" Cells 9, no. 7: 1689. https://doi.org/10.3390/cells9071689
APA StyleIchise, Y., Saegusa, J., Tanaka-Natsui, S., Naka, I., Hayashi, S., Kuroda, R., & Morinobu, A. (2020). Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4. Cells, 9(7), 1689. https://doi.org/10.3390/cells9071689






