Human and Rodent Skeletal Muscles Express Angiotensin II Type 1 Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Approval for Experiments
2.2. Human Diaphragm Biopsies
2.3. Animal Muscle Dissections
2.4. Experimental Approach
2.5. Immunoblotting
2.6. Identification of AT1R Using a Fluorescence-Based Binding Assay
2.7. AT1R mRNA
2.8. Statistical Analysis
3. Results
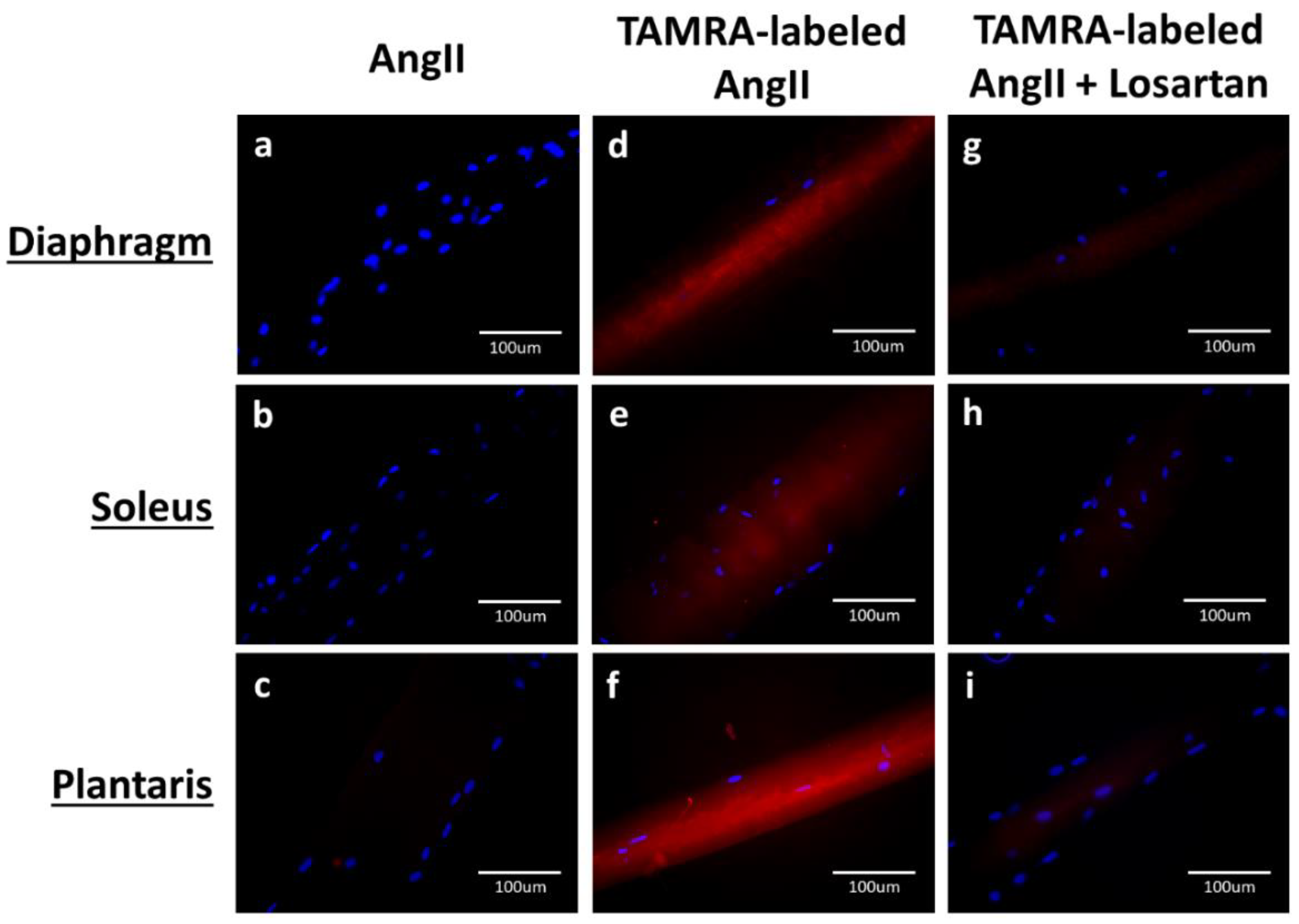
3.1. AT1R Abundance in Rat Skeletal Muscles Determined by AngII-Binding Assay
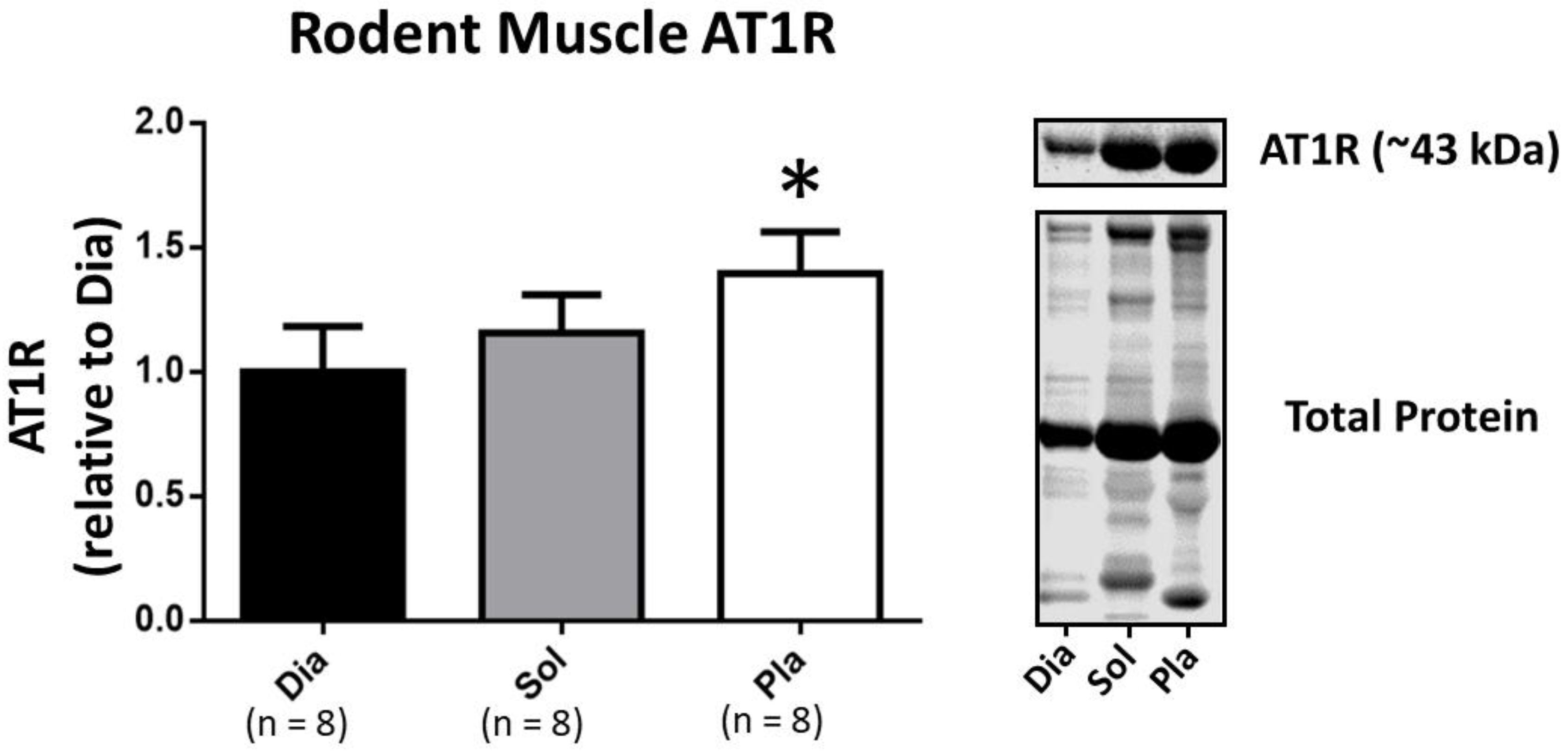
3.2. Western Blotting to Determine AT1R Abundance in Rat Skeletal Muscles
3.3. AT1R mRNA in Rat Skeletal Muscles
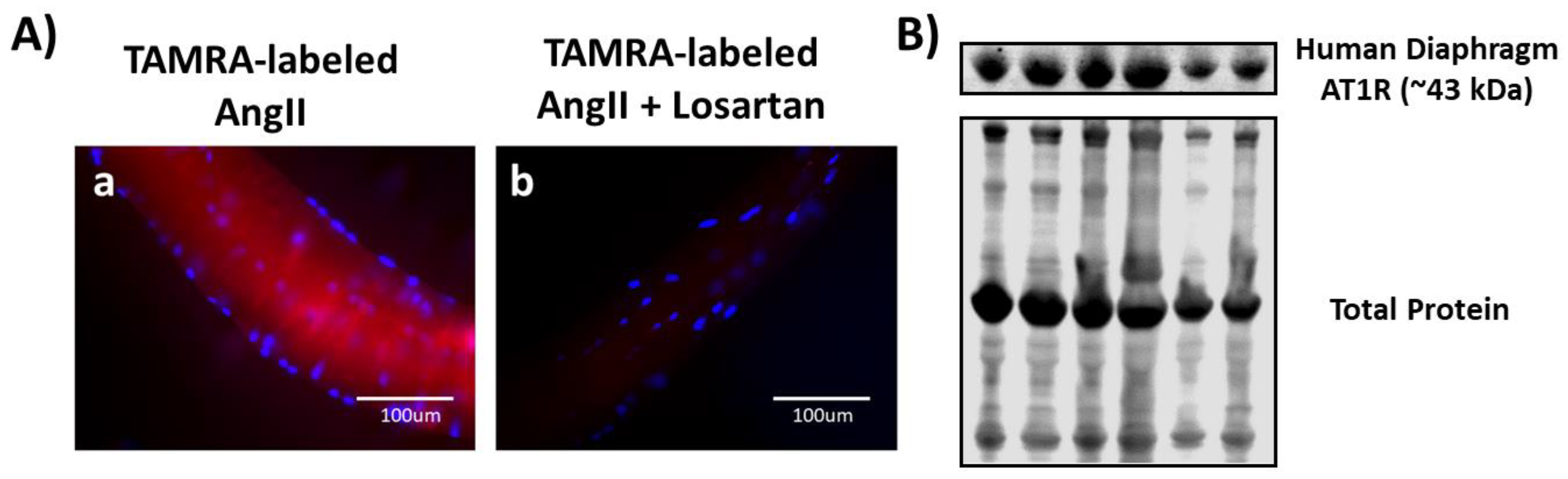
3.4. Both Ligand-Binding and Western Blotting Confirm the Presence of AT1R in Human Diaphragm
4. Discussion
4.1. Overview of Major Findings
4.2. Critique of Experimental Approach
4.3. Physiological and Clinical Implications of AT1Rs in Skeletal Muscle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabello-Verrugio, C.; Cordova, G.; Salas, J.D. Angiotensin II: Role in skeletal muscle atrophy. Curr. Protein Pept. Sci. 2012, 13, 560–569. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Morales, M.G.; Rivera, J.C.; Cabrera, D.; Simon, F. Renin-angiotensin system. An old player with novel functions in skeletal muscle. Med. Res. Rev. 2015, 35, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Morton, A.B.; Hyatt, H.; Hinkley, M.J. The Renin-Angiotensin System and Skeletal Muscle. Exerc. Sport Sci. Rev. 2018, 46, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tabony, A.M.; Galvez, S.; Mitch, W.E.; Higashi, Y.; Sukhanov, S.; Delafontaine, P. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: Potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2322–2332. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Haruna, Y.; Bonde-Petersen, F.; Takenaka, K.; Suzuki, Y.; Kawakubo, K.; Gunji, A. Effects of the renin-angiotensin-aldosterone system on the cardiovascular system during 20-days bed rest. J. Gravit. Physiol. 1997, 4, S62–S68. [Google Scholar] [PubMed]
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Van de Wal, R.M.; Plokker, H.W.; Lok, D.J.; Boomsma, F.; van der Horst, F.A.; van Veldhuisen, D.J.; van Gilst, W.H.; Voors, A.A. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int. J. Cardiol. 2006, 106, 367–372. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef]
- Schakman, O.; Kalista, S.; Barbe, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ozdemir, M.; Hyatt, H. Redox Control of Proteolysis during Inactivity-Induced Skeletal Muscle Atrophy. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Paxton, W.G.; Runge, M.; Horaist, C.; Cohen, C.; Alexander, R.W.; Bernstein, K.E. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am. J. Physiol. 1993, 264, F989–F995. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Acuna, M.J.; Morales, M.G.; Becerra, A.; Simon, F.; Brandan, E. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2011, 410, 665–670. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Morales, M.G.; Cabrera, D.; Vio, C.P.; Brandan, E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J. Cell. Mol. Med. 2012, 16, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.G.; Vazquez, Y.; Acuna, M.J.; Rivera, J.C.; Simon, F.; Salas, J.D.; Alvarez Ruf, J.; Brandan, E.; Cabello-Verrugio, C. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int. J. Biochem. Cell Biol. 2012, 44, 1993–2002. [Google Scholar] [CrossRef]
- Russell, S.T.; Sanders, P.M.; Tisdale, M.J. Angiotensin II directly inhibits protein synthesis in murine myotubes. Cancer Lett. 2006, 231, 290–294. [Google Scholar] [CrossRef]
- Sanders, P.M.; Russell, S.T.; Tisdale, M.J. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br. J. Cancer 2005, 93, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Haginoya, K.; Dai, H.; Chiba, Y.; Uematsu, M.; Hino-Fukuyo, N.; Onuma, A.; Iinuma, K.; Tsuchiya, S. Intramuscular renin-angiotensin system is activated in human muscular dystrophy. J. Neurol. Sci. 2009, 280, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Benicky, J.; Hafko, R.; Sanchez-Lemus, E.; Aguilera, G.; Saavedra, J.M. Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell. Mol. Neurobiol. 2012, 32, 1353–1365. [Google Scholar] [CrossRef]
- Herrera, M.; Sparks, M.A.; Alfonso-Pecchio, A.R.; Harrison-Bernard, L.M.; Coffman, T.M. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013, 61, 253–258. [Google Scholar] [CrossRef]
- Kwon, O.S.; Smuder, A.J.; Wiggs, M.P.; Hall, S.E.; Sollanek, K.J.; Morton, A.B.; Talbert, E.E.; Toklu, H.Z.; Tumer, N.; Powers, S.K. AT1 receptor blocker losartan protects against mechanical ventilation-induced diaphragmatic dysfunction. J. Appl. Physiol. 2015, 119, 1033–1041. [Google Scholar] [CrossRef]
- Zambelli, V.; Sigurta, A.; Rizzi, L.; Zucca, L.; Delvecchio, P.; Bresciani, E.; Torsello, A.; Bellani, G. Angiotensin-(1-7) exerts a protective action in a rat model of ventilator-induced diaphragmatic dysfunction. Intensive Care Med. Exp. 2019, 7, 8. [Google Scholar] [CrossRef]
- Delp, M.D.; Duan, C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 1996, 80, 261–270. [Google Scholar] [CrossRef]
- Murphy, K.T.; Hossain, M.I.; Swiderski, K.; Chee, A.; Naim, T.; Trieu, J.; Haynes, V.; Read, S.J.; Stapleton, D.I.; Judge, S.M.; et al. Mas Receptor Activation Slows Tumor Growth and Attenuates Muscle Wasting in Cancer. Cancer Res. 2019, 79, 706–719. [Google Scholar] [CrossRef]
- Kirshner, Z.Z.; Gibbs, R.B. Use of the REVERT((R)) total protein stain as a loading control demonstrates significant benefits over the use of housekeeping proteins when analyzing brain homogenates by Western blot: An analysis of samples representing different gonadal hormone states. Mol. Cell. Endocrinol. 2018, 473, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Bragina, M.E.; Stergiopulos, N.; Fraga-Silva, R. Fluorescence-based binding assay for screening ligands of angiotensin receptors. In The Renin-Angiotensin-Aldosterone System: Methods and Protocols, Methods in Molecular Biology; Thacher, S., Ed.; Human Press: New York, NY, USA, 2017; Volume 1614, pp. 165–174. [Google Scholar]
- Falcon, B.L.; Stewart, J.M.; Bourassa, E.; Katovich, M.J.; Walter, G.; Speth, R.C.; Sumners, C.; Raizada, M.K. Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol. Genom. 2004, 19, 255–261. [Google Scholar] [CrossRef]
- Phansalkar, N.A.S.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Calicut, India, 10–12 February 2012; pp. 218–220. [Google Scholar] [CrossRef]
- Yoshida, T.; Huq, T.S.; Delafontaine, P. Angiotensin type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J. Biol. Chem. 2014, 289, 26239–26248. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Wang, F. Radioligand saturation binding for quantitative analysis of ligand-receptor interactions. Biophys. Rep. 2015, 1, 148–155. [Google Scholar] [CrossRef]
- Breen, C.J.; Raverdeau, M.; Voorheis, H.P. Development of a quantitative fluorescence-based ligand-binding assay. Sci. Rep. 2016, 6, 25769. [Google Scholar] [CrossRef]
- Martin, R.P.; Filippelli-Silva, R.; Rodrigues, E.S.; Nakaie, C.R.; Shimuta, S.I. A fluorimetric binding assay for angiotensin II and kinin receptors. J. Pharmacol. Toxicol. Methods 2016, 79, 55–59. [Google Scholar] [CrossRef] [PubMed]
- De Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharm. Rev. 2000, 52, 415–472. [Google Scholar] [PubMed]
- Tikunov, B.A.; Mancini, D.; Levine, S. Changes in myofibrillar protein composition of human diaphragm elicited by congestive heart failure. J. Mol. Cell. Cardiol. 1996, 28, 2537–2541. [Google Scholar] [CrossRef]
- Semprun-Prieto, L.C.; Sukhanov, S.; Yoshida, T.; Rezk, B.M.; Gonzalez-Villalobos, R.A.; Vaughn, C.; Michael Tabony, A.; Delafontaine, P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem. Biophys. Res. Commun. 2011, 409, 217–221. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Sollanek, K.J.; Smuder, A.J. Ventilator-induced diaphragm dysfunction: Cause and effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R464–R477. [Google Scholar] [CrossRef]
- Yasuda, N.; Miura, S.; Akazawa, H.; Tanaka, T.; Qin, Y.; Kiya, Y.; Imaizumi, S.; Fujino, M.; Ito, K.; Zou, Y.; et al. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhry, I.; Reid, M.B.; Boriek, A.M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J. Biol. Chem. 2002, 277, 46493–46503. [Google Scholar] [CrossRef]
- Newman, S.; Road, J.; Bellemare, F.; Clozel, J.P.; Lavigne, C.M.; Grassino, A. Respiratory muscle length measured by sonomicrometry. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 753–764. [Google Scholar] [CrossRef]
- Boriek, A.M.; Hwang, W.; Trinh, L.; Rodarte, J.R. Shape and tension distribution of the active canine diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1021–R1027. [Google Scholar] [CrossRef]
- Boriek, A.M.; Rodarte, J.R.; Reid, M.B. Shape and tension distribution of the passive rat diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R33–R41. [Google Scholar] [CrossRef]
- Hussain, S.N.; Mofarrahi, M.; Sigala, I.; Kim, H.C.; Vassilakopoulos, T.; Maltais, F.; Bellenis, I.; Chaturvedi, R.; Gottfried, S.B.; Metrakos, P.; et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am. J. Respir. Crit. Care Med. 2010, 182, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Sellares, J.; Ferrer, M.; Cano, E.; Loureiro, H.; Valencia, M.; Torres, A. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med. 2011, 37, 775–784. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Castan, E.P.; Coelho, C.A.; Lopes, F.S.; Almeida, F.L.; Michelin, A.; de Souza, R.W.; Araujo, J.P., Jr.; Cicogna, A.C.; Dal Pai-Silva, M. Heart failure increases atrogin-1 and MuRF1 gene expression in skeletal muscle with fiber type-specific atrophy. J. Mol. Histol. 2010, 41, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Flisinski, M.; Brymora, A.; Elminowska-Wenda, G.; Bogucka, J.; Walasik, K.; Stefanska, A.; Strozecki, P.; Manitius, J. Morphometric analysis of muscle fibre types in rat locomotor and postural skeletal muscles in different stages of chronic kidney disease. J. Physiol. Pharm. 2014, 65, 567–576. [Google Scholar]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bechara, L.R.; Moreira, J.B.; Jannig, P.R.; Voltarelli, V.A.; Dourado, P.M.; Vasconcelos, A.R.; Scavone, C.; Ramires, P.R.; Brum, P.C. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int. J. Cardiol. 2014, 175, 499–507. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deminice, R.; Hyatt, H.; Yoshihara, T.; Ozdemir, M.; Nguyen, B.; Levine, S.; Powers, S. Human and Rodent Skeletal Muscles Express Angiotensin II Type 1 Receptors. Cells 2020, 9, 1688. https://doi.org/10.3390/cells9071688
Deminice R, Hyatt H, Yoshihara T, Ozdemir M, Nguyen B, Levine S, Powers S. Human and Rodent Skeletal Muscles Express Angiotensin II Type 1 Receptors. Cells. 2020; 9(7):1688. https://doi.org/10.3390/cells9071688
Chicago/Turabian StyleDeminice, Rafael, Hayden Hyatt, Toshinori Yoshihara, Mustafa Ozdemir, Branden Nguyen, Sanford Levine, and Scott Powers. 2020. "Human and Rodent Skeletal Muscles Express Angiotensin II Type 1 Receptors" Cells 9, no. 7: 1688. https://doi.org/10.3390/cells9071688
APA StyleDeminice, R., Hyatt, H., Yoshihara, T., Ozdemir, M., Nguyen, B., Levine, S., & Powers, S. (2020). Human and Rodent Skeletal Muscles Express Angiotensin II Type 1 Receptors. Cells, 9(7), 1688. https://doi.org/10.3390/cells9071688





