Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney
Abstract
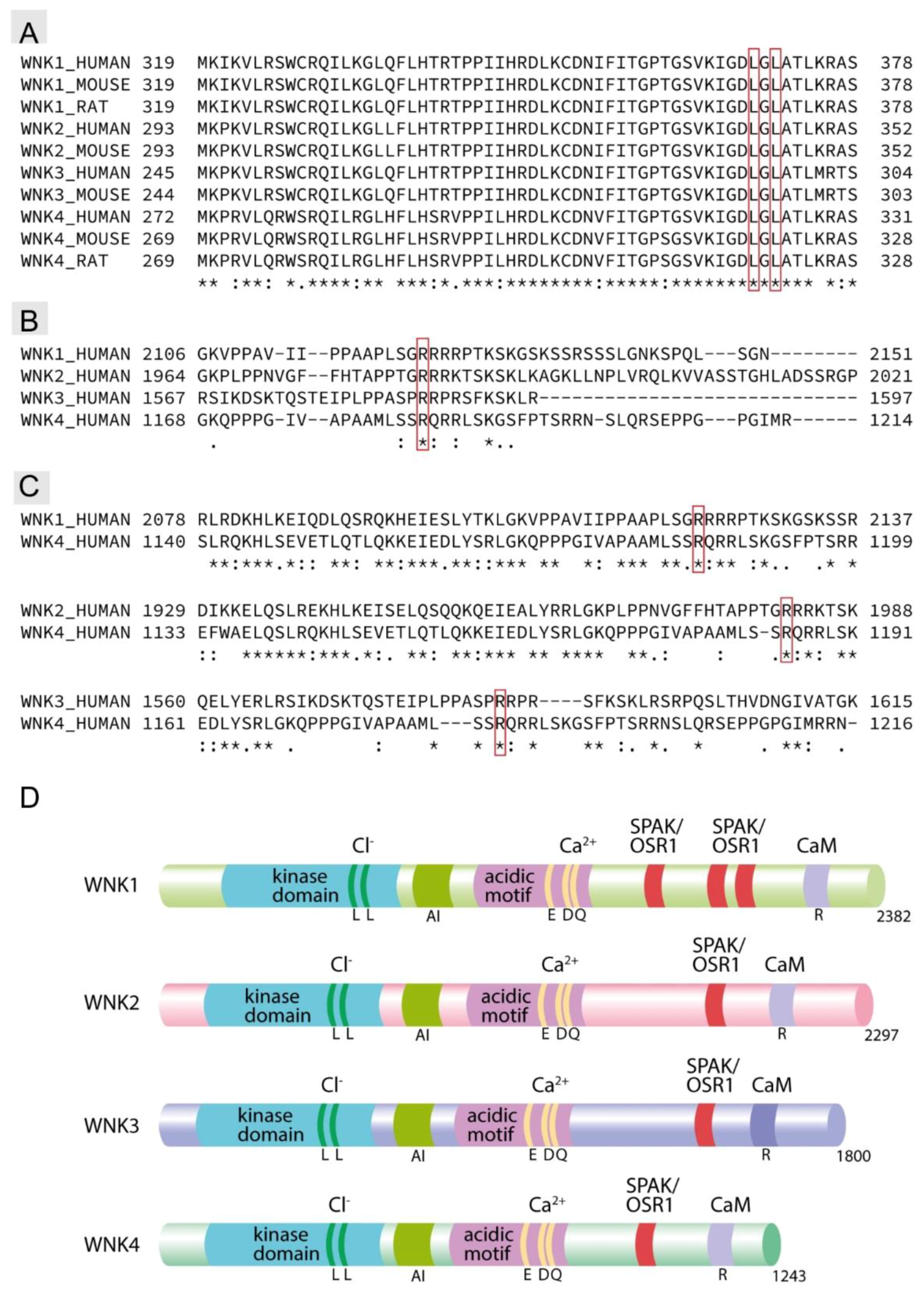
1. Introduction
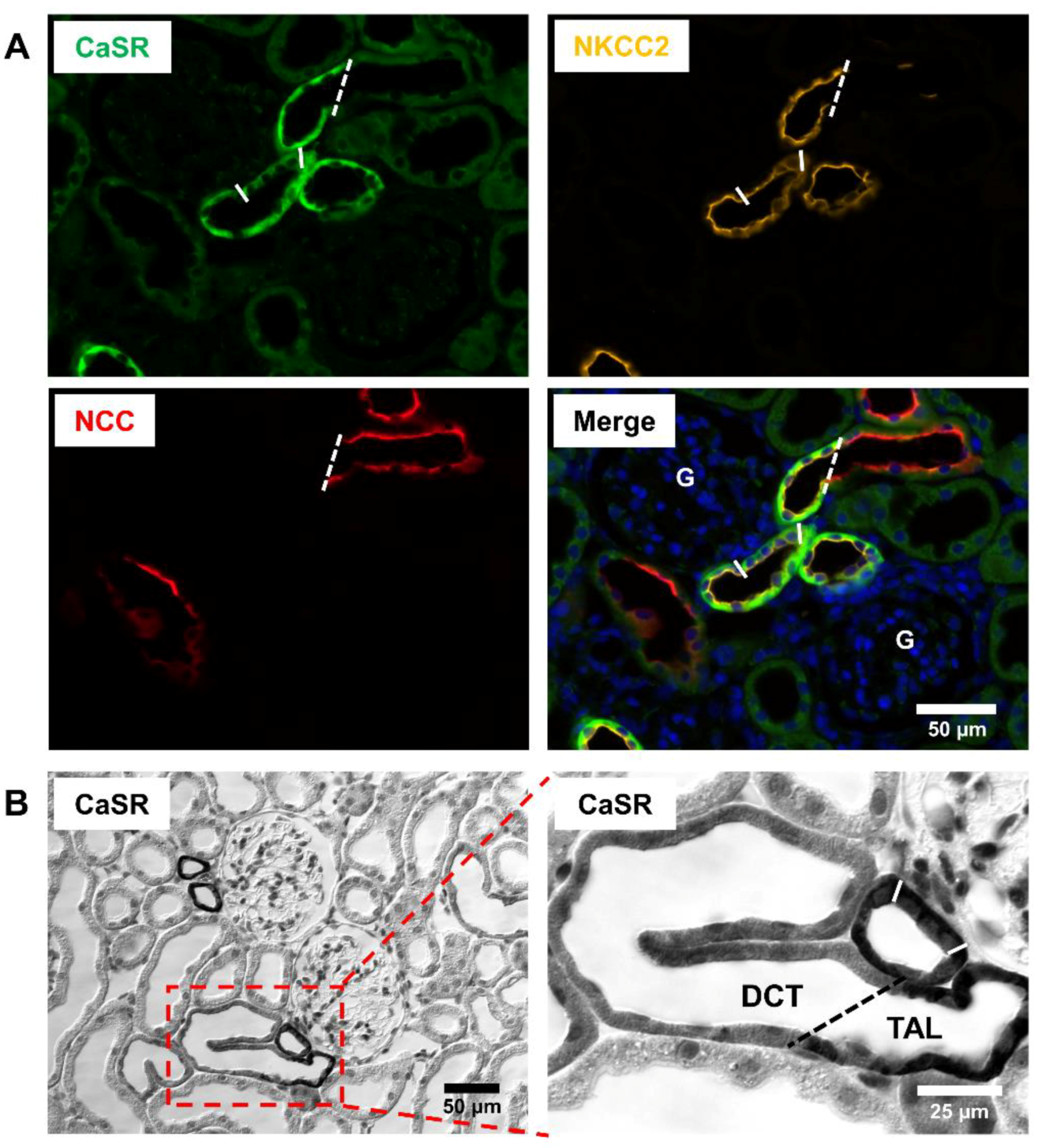
2. Renal Distribution of CaSR
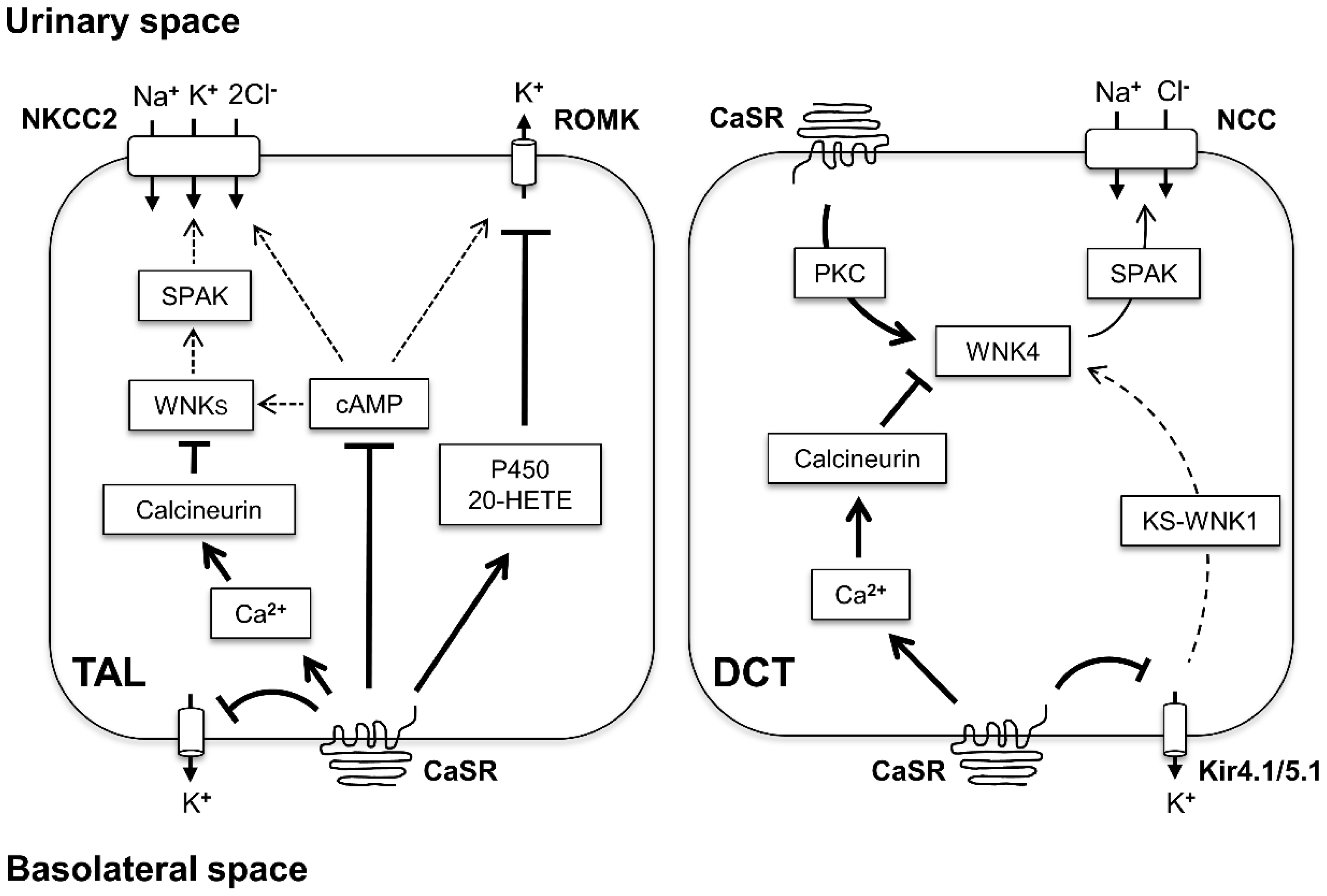
3. CaSR Function in TAL
3.1. CaSR Inhibits the Transcellular NaCl Reabsorption
3.2. CaSR Inhibits the Paracellular Ca2+ and Mg2+ Reabsorption
4. CaSR Function in JGA
5. CaSR Function in DCT
6. CaSR Function in CNT and CD
7. Translational Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lee, K.; Brown, D.; Ureña, P.; Ardaillou, N.; Ardaillou, R.; Deeds, J.; Segre, G.V. Localization of parathyroid hormone/parathyroid hormone-related peptide receptor mRNA in kidney. Am. J. Physiol. 1996, 270, F186–F191. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Lee, W.S.; Lee, K.; Segre, G.V.; Brown, E.M.; Hebert, S.C. Localization of the extracellular Ca(2+)-sensing receptor and PTH/PTHrP receptor in rat kidney. Am. J. Physiol. 1996, 271, F951–F956. [Google Scholar] [CrossRef]
- Melamed, M.L.; Thadhani, R.I. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Sexton, P.M.; Adam, W.R.; Moseley, J.M.; Martin, T.J.; Mendelsohn, F.A.O. Localization and characterization of renal calcitonin receptors by in vitro autoradiography. Kidney Int. 1987, 32, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Graca, J.A.Z.; Schepelmann, M.; Brennan, S.C.; Reens, J.; Chang, W.; Yan, P.; Toka, H.; Riccardi, D.; Price, S.A. Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am. J. Physiol. Ren. Physiol. 2016, 310, F518–F533. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Brown, E.M. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Ren. Physiol. 2010, 298, F485–F499. [Google Scholar] [CrossRef]
- Riccardi, D.; Valenti, G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016, 12, 414–425. [Google Scholar] [CrossRef]
- Wu, L.-G.; Hamid, E.; Shin, W.; Chiang, H.-C. Exocytosis and endocytosis: Modes, functions, and coupling mechanisms. Annu. Rev. Physiol. 2014, 76, 301–331. [Google Scholar] [CrossRef]
- Topala, C.N.; Schoeber, J.P.H.; Searchfield, L.E.; Riccardi, D.; Hoenderop, J.G.J.; Bindels, R.J.M. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 2009, 45, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Desai, P.; Borovac, J.; Lau, A.; Pan, W.; Alexander, R.T. Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am. J. Physiol. Ren. Physiol. 2013, 304, F761–F769. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hou, J. Claudin-14 Underlies Ca++-Sensing Receptor-Mediated Ca++ Metabolism via NFAT-microRNA-Based Mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Carmosino, M.; Gerbino, A.; Hendy, G.N.; Torretta, S.; Rizzo, F.; Debellis, L.; Procino, G.; Svelto, M. NKCC2 activity is inhibited by the Bartter’s syndrome type 5 gain-of-function CaR-A843E mutant in renal cells. Biol. Cell 2015, 107, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Bazúa-Valenti, S.; Rojas-Vega, L.; Castañeda-Bueno, M.; Barrera-Chimal, J.; Bautista, R.; Cervantes-Pérez, L.G.; Vázquez, N.; Plata, C.; Murillo-de-Ozores, A.R.; González-Mariscal, L.; et al. The calcium-sensing receptor increases activity of the renal NCC through the WNK4-SPAK pathway. J. Am. Soc. Nephrol. 2018, 29, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Smajilovic, S.; Tfelt-Hansen, J. Novel role of the calcium-sensing receptor in blood pressure modulation. Hypertension 2008, 52, 994–1000. [Google Scholar] [CrossRef]
- Kusano, E.; Murayama, N.; Werness, J.L.; Christensen, S.; Homma, S.; Yusufi, A.N.; Dousa, T.P. Effects of calcium on the vasopressin-sensitive cAMP metabolism in medullary tubules. Am. J. Physiol. Ren. Physiol. 1985, 249, F956–F966. [Google Scholar] [CrossRef]
- Reilly, R.F.; Ellison, D.H. Mammalian distal tubule: Physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 2000, 80, 277–313. [Google Scholar] [CrossRef]
- Madsen, K.M.; Tisher, C.C. Structural-functional relationships along the distal nephron. Am. J. Physiol. 1986, 250, F1–F15. [Google Scholar] [CrossRef]
- Chen, L.; Clark, J.Z.; Nelson, J.W.; Kaissling, B.; Ellison, D.H.; Knepper, M.A. Renal-Tubule epithelial cell nomenclature for single-cell RNA-sequencing studies. J. Am. Soc. Nephrol. 2019. [Google Scholar] [CrossRef]
- Gamba, G.; Friedman, P.A. Thick ascending limb: The Na+:K+:2Cl− co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflüg. Arch. Eur. J. Physiol. 2009, 458, 61–76. [Google Scholar] [CrossRef]
- Mount, D.B. Thick ascending limb of the loop of henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1974–1986. [Google Scholar] [CrossRef]
- Mutig, K.; Borowski, T.; Boldt, C.; Borschewski, A.; Paliege, A.; Popova, E.; Bader, M.; Bachmann, S. Demonstration of the functional impact of vasopressin signaling in the thick ascending limb by a targeted transgenic rat approach. Am. J. Physiol. Ren. Physiol. 2016, 311, F411–F423. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G. Regulation of the renal Na+-Cl− cotransporter by phosphorylation and ubiquitylation. Am. J. Physiol. Ren. Physiol. 2012, 303, F1573–F1583. [Google Scholar] [CrossRef] [PubMed]
- Terker, A.S.; Zhang, C.; McCormick, J.A.; Lazelle, R.A.; Zhang, C.; Meermeier, N.P.; Siler, D.A.; Park, H.J.; Fu, Y.; Cohen, D.M.; et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015, 21, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Frid, M.; Castañeda-Bueno, M.; Gamba, G. Regulation of the renal NaCl cotransporter by the WNK/SPAK pathway: Lessons learned from genetically altered animals. Am. J. Physiol. Ren. Physiol. 2019, 316, F146–F158. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.; Soundararajan, R.; Trimpert, C.; Kashlan, O.B.; Deen, P.M.T.; Kohan, D.E. Collecting duct principal cell transport processes and their regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 135–146. [Google Scholar] [CrossRef]
- Simon, D.B.; Karet, F.E.; Hamdan, J.M.; DiPietro, A.; Sanjad, S.A.; Lifton, R.P. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Genet. 1996, 13, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.B.; Nelson-Williams, C.; Bia, M.J.; Ellison, D.; Karet, F.E.; Molina, A.M.; Vaara, I.; Iwata, F.; Cushner, H.M.; Koolen, M.; et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. 1996, 12, 24–30. [Google Scholar] [CrossRef]
- Watanabe, S.; Fukumoto, S.; Chang, H.; Takeuchi, Y.; Hasegawa, Y.; Okazaki, R.; Chikatsu, N.; Fujita, T. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet Lond. Engl. 2002, 360, 692–694. [Google Scholar] [CrossRef]
- Fujita, T.; Ando, K.; Sato, Y.; Yamashita, K.; Nomura, M.; Fukui, T. Independent roles of prostaglandins and the renin-angiotensin system in abnormal vascular reactivity in Bartter’s syndrome. Am. J. Med. 1982, 73, 71–76. [Google Scholar] [CrossRef]
- Toka, H.R.; Al-Romaih, K.; Koshy, J.M.; DiBartolo, S.; Kos, C.H.; Quinn, S.J.; Curhan, G.C.; Mount, D.B.; Brown, E.M.; Pollak, M.R. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J. Am. Soc. Nephrol. 2012, 23, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Trepiccione, F.; Zacchia, M.; Capasso, G. The role of the kidney in salt-sensitive hypertension. Clin. Exp. Nephrol. 2012, 16, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Majid, D.S.A.; Prieto, M.C.; Navar, L.G. Salt-sensitive hypertension: Perspectives on intrarenal mechanisms. Curr. Hypertens. Rev. 2015, 11, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hadchouel, J.; Ellison, D.H.; Gamba, G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 Kinases. Annu. Rev. Physiol. 2016, 78, 367–389. [Google Scholar] [CrossRef]
- Wilson, F.H.; Disse-Nicodème, S.; Choate, K.A.; Ishikawa, K.; Nelson-Williams, C.; Desitter, I.; Gunel, M.; Milford, D.V.; Lipkin, G.W.; Achard, J.M.; et al. Human hypertension caused by mutations in WNK kinases. Science 2001, 293, 1107–1112. [Google Scholar] [CrossRef]
- Shibata, S.; Zhang, J.; Puthumana, J.; Stone, K.L.; Lifton, R.P. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc. Natl. Acad. Sci. USA 2013, 110, 7838–7843. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Shimosawa, T.; Ogura, S.; Wang, H.; Uetake, Y.; Kawakami-Mori, F.; Marumo, T.; Yatomi, Y.; Geller, D.S.; Tanaka, H.; et al. Epigenetic modulation of the renal β-adrenergic–WNK4 pathway in salt-sensitive hypertension. Nat. Med. 2011, 17, 573–580. [Google Scholar] [CrossRef] [PubMed]
- San-Cristobal, P.; Pacheco-Alvarez, D.; Richardson, C.; Ring, A.M.; Vazquez, N.; Rafiqi, F.H.; Chari, D.; Kahle, K.T.; Leng, Q.; Bobadilla, N.A.; et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 4384–4389. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Walsh, S.B.; McCormick, J.A.; Fürstenberg, A.; Yang, C.-L.; Roeschel, T.; Paliege, A.; Howie, A.J.; Conley, J.; Bachmann, S.; et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat. Med. 2011, 17, 1304–1309. [Google Scholar] [CrossRef]
- Borschewski, A.; Himmerkus, N.; Boldt, C.; Blankenstein, K.I.; McCormick, J.A.; Lazelle, R.; Willnow, T.E.; Jankowski, V.; Plain, A.; Bleich, M.; et al. Calcineurin and sorting-related receptor with a-type repeats interact to regulate the renal Na+-K+-2Cl− Cotransporter. J. Am. Soc. Nephrol. 2016, 27, 107–119. [Google Scholar] [CrossRef]
- Blankenstein, K.I.; Borschewski, A.; Labes, R.; Paliege, A.; Boldt, C.; McCormick, J.A.; Ellison, D.H.; Bader, M.; Bachmann, S.; Mutig, K. Calcineurin inhibitor cyclosporine a activates renal Na-(K)-Cl Cotransporters via Local and Systemic Mechanisms. Am. J. Physiol. Ren. Physiol. 2016, 312, F489–F501. [Google Scholar] [CrossRef]
- Ishizawa, K.; Wang, Q.; Li, J.; Yamazaki, O.; Tamura, Y.; Fujigaki, Y.; Uchida, S.; Lifton, R.P.; Shibata, S. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc. Natl. Acad. Sci. USA 2019, 116, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Hatton, D.C.; McCarron, D.A. Dietary calcium and blood pressure in experimental models of hypertension. A review. Hypertension 1994, 23, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sano, H.; Furuta, Y.; Yamanishi, J.; Omatsu, T.; Ito, Y.; Fukuzaki, H. Calcium supplementation in salt-dependent hypertension. Contrib. Nephrol. 1991, 90, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sano, H.; Furuta, Y.; Fukuzaki, H. Effect of oral calcium on blood pressure response in salt-loaded borderline hypertensive patients. Hypertens. Dallas Tex 1979 1989, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H.; Wagner, U.L.; Fineberg, N.S. The blood pressure effects of calcium supplementation in humans of known sodium responsiveness. Am. J. Hypertens. 1993, 6, 799–805. [Google Scholar] [CrossRef]
- Riccardi, D.; Park, J.; Lee, W.S.; Gamba, G.; Brown, E.M.; Hebert, S.C. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 131–135. [Google Scholar] [CrossRef]
- Yang, T.; Hassan, S.; Huang, Y.G.; Smart, A.M.; Briggs, J.P.; Schnermann, J.B. Expression of PTHrP, PTH/PTHrP receptor, and Ca(2+)-sensing receptor mRNAs along the rat nephron. Am. J. Physiol. 1997, 272, F751–F758. [Google Scholar] [CrossRef]
- Riccardi, D.; Hall, A.E.; Chattopadhyay, N.; Xu, J.Z.; Brown, E.M.; Hebert, S.C. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. 1998, 274, F611–F622. [Google Scholar] [CrossRef]
- Saritas, T.; Borschewski, A.; McCormick, J.A.; Paliege, A.; Dathe, C.; Uchida, S.; Terker, A.; Himmerkus, N.; Bleich, M.; Demaretz, S.; et al. SPAK differentially mediates vasopressin effects on sodium cotransporters. J. Am. Soc. Nephrol. 2013, 24, 407–418. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Function and regulation of claudins in the thick ascending limb of Henle. Pflug. Arch. 2009, 458, 77–88. [Google Scholar] [CrossRef]
- Ferreri, N.R.; Hao, S.; Pedraza, P.L.; Escalante, B.; Vio, C.P. Eicosanoids and tumor necrosis factor-alpha in the kidney. Prostaglandins Other Lipid Mediat. 2012, 98, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Lu, M.; Hebert, S.C. Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am. J. Physiol. Cell Physiol. 1996, 271, C103–C111. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, M.; Balazy, M.; Hebert, S.C. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am. J. Physiol. Ren. Physiol. 1997, 273, F421–F429. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, C.; Li, W.; Wang, L.; Luan, H.; Wang, W.-H.; Gu, R. Stimulation of Ca2+-sensing receptor inhibits the basolateral 50-pS K channels in the thick ascending limb of rat kidney. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-K.; Huang, C.; Ding, Y.; Qi, X.; Huang, C.-L.; Miller, R.T. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J. Biol. Chem. 2011, 286, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sindic, A.; Hill, C.E.; Hujer, K.M.; Chan, K.W.; Sassen, M.; Wu, Z.; Kurachi, Y.; Nielsen, S.; Romero, M.F.; et al. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am. J. Physiol. Ren. Physiol. 2007, 292, F1073–F1081. [Google Scholar] [CrossRef]
- Su, X.-T.; Wang, W.-H. The expression, regulation, and function of Kir4.1 (Kcnj10) in the mammalian kidney. Am. J. Physiol. Ren. Physiol. 2016, 311, F12–F15. [Google Scholar] [CrossRef]
- Abdullah, H.I.; Pedraza, P.L.; McGiff, J.C.; Ferreri, N.R. Calcium-sensing receptor signaling pathways in medullary thick ascending limb cells mediate COX-2-derived PGE2 production: Functional significance. Am. J. Physiol. Ren. Physiol. 2008, 295, F1082–F1089. [Google Scholar] [CrossRef]
- Reinalter, S.C.; Jeck, N.; Brochhausen, C.; Watzer, B.; Nüsing, R.M.; Seyberth, H.W.; Kömhoff, M. Role of cyclooxygenase-2 in hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int. 2002, 62, 253–260. [Google Scholar] [CrossRef][Green Version]
- Kammerl, M.C.; Nüsing, R.M.; Richthammer, W.; Krämer, B.K.; Kurtz, A. Inhibition of COX-2 counteracts the effects of diuretics in rats. Kidney Int. 2001, 60, 1684–1691. [Google Scholar] [CrossRef]
- Lazelle, R.A.; McCully, B.H.; Terker, A.S.; Himmerkus, N.; Blankenstein, K.I.; Mutig, K.; Bleich, M.; Bachmann, S.; Yang, C.-L.; Ellison, D.H. Renal deletion of 12 kDa FK506-binding protein attenuates tacrolimus-induced hypertension. J. Am. Soc. Nephrol. 2016, 27, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Terker, A.S.; Zhang, C.; Erspamer, K.J.; Gamba, G.; Yang, C.-L.; Ellison, D.H. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- de Ferreira, M.C.J.; Héliès-Toussaint, C.; Imbert-Teboul, M.; Bailly, C.; Verbavatz, J.-M.; Bellanger, A.-C.; Chabardès, D. Co-expression of a Ca2+ -inhibitable adenylyl cyclase and of a Ca2+ -sensing receptor in the cortical thick ascending limb cell of the rat kidney: Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J. Biol. Chem. 1998, 273, 15192–15202. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, R.; Braucht, D.W.W.; Rinschen, M.M.; Chou, C.-L.; Hoffert, J.D.; Pisitkun, T.; Knepper, M.A. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc. Natl. Acad. Sci. USA 2010, 107, 15653–15658. [Google Scholar] [CrossRef]
- Gerbino, A.; Ruder, W.C.; Curci, S.; Pozzan, T.; Zaccolo, M.; Hofer, A.M. Termination of cAMP signals by Ca2+ and Gαi via extracellular Ca2+ sensors. J. Cell Biol. 2005, 171, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Na, T.; Wu, G.; Peng, J.-B. Disease-causing mutations in the acidic motif of WNK4 impair the sensitivity of WNK4 kinase to calcium ions. Biochem. Biophys. Res. Commun. 2012, 419, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, M.; Mori, T.; Isobe, K.; Sohara, E.; Susa, K.; Araki, Y.; Chiga, M.; Kikuchi, E.; Nomura, N.; Mori, Y.; et al. Impaired KLHL3-Mediated Ubiquitination of WNK4 Causes Human Hypertension. Cell Rep. 2013, 3, 858–868. [Google Scholar] [CrossRef]
- Louis-Dit-Picard, H.; Barc, J.; Trujillano, D.; Miserey-Lenkei, S.; Bouatia-Naji, N.; Pylypenko, O.; Beaurain, G.; Bonnefond, A.; Sand, O.; Simian, C.; et al. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat. Genet. 2012, 44, 456–460, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Na, T.; Wu, G.; Zhang, W.; Dong, W.-J.; Peng, J.-B. Disease-causing R1185C mutation of WNK4 disrupts a regulatory mechanism involving calmodulin binding and SGK1 phosphorylation sites. Am. J. Physiol. Ren. Physiol. 2013, 304, F8–F18. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway: Claudin-14 function and regulation. EMBO J. 2012, 31, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; MacGregor, G.G.; Wilson, F.H.; Van Hoek, A.N.; Brown, D.; Ardito, T.; Kashgarian, M.; Giebisch, G.; Hebert, S.C.; Boulpaep, E.L.; et al. Paracellular Cl- permeability is regulated by WNK4 kinase: Insight into normal physiology and hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 14877–14882. [Google Scholar] [CrossRef]
- Ohta, A.; Yang, S.-S.; Rai, T.; Chiga, M.; Sasaki, S.; Uchida, S. Overexpression of human WNK1 increases paracellular chloride permeability and phosphorylation of claudin-4 in MDCKII cells. Biochem. Biophys. Res. Commun. 2006, 349, 804–808. [Google Scholar] [CrossRef]
- Yamauchi, K.; Rai, T.; Kobayashi, K.; Sohara, E.; Suzuki, T.; Itoh, T.; Suda, S.; Hayama, A.; Sasaki, S.; Uchida, S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc. Natl. Acad. Sci. USA 2004, 101, 4690–4694. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hou, J. Claudins in barrier and transport function-the kidney. Pflug. Arch. 2017, 469, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.; Machura, K.; Schneider, A.; Hugo, C.; Todorov, V.T.; Kurtz, A. COX-2-derived PGE2 triggers hyperplastic renin expression and hyperreninemia in aldosterone synthase-deficient mice. Pflug. Arch. 2018, 470, 1127–1137. [Google Scholar] [CrossRef]
- Vargas-Poussou, R. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a bartter-like syndrome. J. Am. Soc. Nephrol. 2002, 13, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Atchison, D.K.; Ortiz-Capisano, M.C.; Beierwaltes, W.H. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1020–R1026. [Google Scholar] [CrossRef][Green Version]
- Maillard, M.P.; Tedjani, A.; Perregaux, C.; Burnier, M. Calcium-sensing receptors modulate renin release in vivo and in vitro in the rat. J. Hypertens. 2009, 27, 1980–1987. [Google Scholar] [CrossRef]
- Kurtz, A.; Della Bruna, R.; Kühn, K. Cyclosporine A enhances renin secretion and production in isolated juxtaglomerular cells. Kidney Int. 1988, 33, 947–953. [Google Scholar] [CrossRef]
- Madsen, K.; Friis, U.G.; Gooch, J.L.; Hansen, P.B.; Holmgaard, L.; Skøtt, O.; Jensen, B.L. Inhibition of calcineurin phosphatase promotes exocytosis of renin from juxtaglomerular cells. Kidney Int. 2010, 77, 110–117. [Google Scholar] [CrossRef]
- McCormick, J.A.; Ellison, D.H. Distal convoluted tubule. Compr. Physiol. 2015, 5, 45–98. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Bostanjoglo, M.; Schmitt, R.; Ellison, D.H. Sodium transport-related proteins in the mammalian distal nephron-distribution, ontogeny and functional aspects. Anat. Embryol. 1999, 200, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.C.; Wynne, B.M.; Yu, L.; Tomilin, V.; Yue, Q.; Zhou, Y.; Al-Khalili, O.; Mallick, R.; Cai, H.; Alli, A.A.; et al. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem. J. 2016, 473, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Wynne, B.M.; Mistry, A.C.; Al-Khalili, O.; Mallick, R.; Theilig, F.; Eaton, D.C.; Hoover, R.S. Aldosterone modulates the association between NCC and ENaC. Sci. Rep. 2017, 7, 4149. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Hoenderop, J.G.J.; Bindels, R.J.M. Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J. Physiol. 2011, 589, 1535–1542. [Google Scholar] [CrossRef]
- Loffing, J.; Loffing-Cueni, D.; Valderrabano, V.; Kläusli, L.; Hebert, S.C.; Rossier, B.C.; Hoenderop, J.G.J.; Bindels, R.J.M.; Kaissling, B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am. J. Physiol. Ren. Physiol. 2001, 281, F1021–F1027. [Google Scholar] [CrossRef]
- Bostanjoglo, M.; Reeves, W.B.; Reilly, R.F.; Velázquez, H.; Robertson, N.; Litwack, G.; Morsing, P.; Dørup, J.; Bachmann, S.; Ellison, D.H.; et al. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J. Am. Soc. Nephrol. 1998, 9, 1347–1358. [Google Scholar]
- Hoover, R.S.; Tomilin, V.; Hanson, L.; Pochynyuk, O.; Ko, B. PTH modulation of NCC activity regulates TRPV5 Ca2+ reabsorption. Am. J. Physiol. Ren. Physiol. 2016, 310, F144–F151. [Google Scholar] [CrossRef]
- Reilly, R.F.; Huang, C.-L. The mechanism of hypocalciuria with NaCl cotransporter inhibition. Nat. Rev. Nephrol. 2011, 7, 669–674. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Vallon, V.; van der Kemp, A.W.C.M.; Loffing, J.; Hoenderop, J.G.J.; Bindels, R.J.M. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J. Clin. Invest. 2005, 115, 1651–1658. [Google Scholar] [CrossRef]
- Castañeda-Bueno, M.; Arroyo, J.P.; Zhang, J.; Puthumana, J.; Yarborough, O.; Shibata, S.; Rojas-Vega, L.; Gamba, G.; Rinehart, J.; Lifton, R.P. Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc. Natl. Acad. Sci. USA 2017, 114, E879–E886. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Uchida, K.; Ohashi, T.; Nitta, K.; Ohta, A.; Chiga, M.; Sasaki, S.; Uchida, S. Immunolocalization of WNK4 in mouse kidney. Histochem. Cell Biol. 2011, 136, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Terker, A.S.; Castañeda-Bueno, M.; Ferdaus, M.Z.; Cornelius, R.J.; Erspamer, K.J.; Su, X.-T.; Miller, L.N.; McCormick, J.A.; Wang, W.-H.; Gamba, G.; et al. With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am. J. Physiol. Ren. Physiol. 2018, 315, F781–F790. [Google Scholar] [CrossRef] [PubMed]
- Murillo-de-Ozores, A.R.; Rodríguez-Gama, A.; Bazúa-Valenti, S.; Leyva-Ríos, K.; Vázquez, N.; Pacheco-Álvarez, D.; De La Rosa-Velázquez, I.A.; Wengi, A.; Stone, K.L.; Zhang, J.; et al. C-terminally truncated, kidney-specific variants of the WNK4 kinase lack several sites that regulate its activity. J. Biol. Chem. 2018, 293, 12209–12221. [Google Scholar] [CrossRef]
- Boyd-Shiwarski, C.R.; Shiwarski, D.J.; Roy, A.; Namboodiri, H.N.; Nkashama, L.J.; Xie, J.; McClain, K.L.; Marciszyn, A.; Kleyman, T.R.; Tan, R.J.; et al. Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent. Mol. Biol. Cell 2018, 29, 499–509. [Google Scholar] [CrossRef]
- Thomson, M.N.; Cuevas, C.A.; Bewarder, T.M.; Dittmayer, C.; Miller, L.N.; Si, J.; Cornelius, R.J.; Su, X.-T.; Yang, C.-L.; McCormick, J.A.; et al. WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am. J. Physiol. Ren. Physiol. 2019. [Google Scholar] [CrossRef]
- Thomson, M.N.; Schneider, W.; Mutig, K.; Ellison, D.H.; Kettritz, R.; Bachmann, S. Patients with hypokalemia develop WNK bodies in the distal convoluted tubule of the kidney. Am. J. Physiol. Ren. Physiol. 2019, 316, F292–F300. [Google Scholar] [CrossRef]
- Subramanya, A.R.; Yang, C.-L.; Zhu, X.; Ellison, D.H. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am. J. Physiol. Ren. Physiol. 2006, 290, F619–F624. [Google Scholar] [CrossRef]
- Argaiz, E.R.; Chavez-Canales, M.; Ostrosky-Frid, M.; Rodríguez-Gama, A.; Vázquez, N.; Gonzalez-Rodriguez, X.; Garcia-Valdes, J.; Hadchouel, J.; Ellison, D.; Gamba, G. Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC. Am. J. Physiol. Ren. Physiol. 2018, 315, F734–F745. [Google Scholar] [CrossRef]
- Cuevas, C.A.; Su, X.-T.; Wang, M.-X.; Terker, A.S.; Lin, D.-H.; McCormick, J.A.; Yang, C.-L.; Ellison, D.H.; Wang, W.-H. Potassium Sensing by Renal Distal Tubules Requires Kir4.1. J. Am. Soc. Nephrol. 2017, 28, 1814–1825. [Google Scholar] [CrossRef]
- Wang, M.-X.; Cuevas, C.A.; Su, X.-T.; Wu, P.; Gao, Z.-X.; Lin, D.-H.; McCormick, J.A.; Yang, C.-L.; Wang, W.-H.; Ellison, D.H. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018, 93, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ferdaus, M.Z.; Gratreak, B.D.K.; Miller, L.; Si, J.; McCormick, J.A.; Yang, C.; Ellison, D.H.; Terker, A.S. WNK4 limits distal calcium losses following acute furosemide treatment. Physiol. Rep. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cong, P.; Williams, S.R.; Zhang, W.; Na, T.; Ma, H.-P.; Peng, J.-B. WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem. Biophys. Res. Commun. 2008, 375, 225–229. [Google Scholar] [CrossRef]
- Cha, S.-K.; Huang, C.-L. WNK4 kinase stimulates caveola-mediated endocytosis of TRPV5 amplifying the dynamic range of regulation of the channel by Protein Kinase C. J. Biol. Chem. 2010, 285, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ferguson, W.B.; Peng, J.-B. WNK4 enhances TRPV5-mediated calcium transport: Potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am. J. Physiol. Ren. Physiol. 2007, 292, F545–F554. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Na, T.; Zhang, W.; Wu, G.; Liu, C.; Peng, J.-B. Concerted actions of NHERF2 and WNK4 in regulating TRPV5. Biochem. Biophys. Res. Commun. 2011, 404, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.F.; Wu, X.-R.; Huang, C.-L. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int. 2013, 84, 130–137. [Google Scholar] [CrossRef]
- Breton, S.; Lisanti, M.P.; Tyszkowski, R.; McLaughlin, M.; Brown, D. Basolateral distribution of caveolin-1 in the kidney: Absence from H+ -ATPase-coated endocytic vesicles in intercalated cells. J. Histochem. Cytochem. 1998, 46, 205–214. [Google Scholar] [CrossRef]
- Willière, Y.; Borschewski, A.; Patzak, A.; Nikitina, T.; Dittmayer, C.; Daigeler, A.L.; Schuelke, M.; Bachmann, S.; Mutig, K. Caveolin 1 promotes renal water and salt reabsorption. Sci. Rep. 2018, 8, 545. [Google Scholar] [CrossRef]
- Păunescu, T.G.; Lu, H.A.J.; Russo, L.M.; Pastor-Soler, N.M.; McKee, M.; McLaughlin, M.M.; Bartlett, B.E.; Breton, S.; Brown, D. Vasopressin induces apical expression of caveolin in rat kidney collecting duct principal cells. Am. J. Physiol. Ren. Physiol. 2013, 305, F1783–F1795. [Google Scholar] [CrossRef]
- Roy, A.; Al-bataineh, M.M.; Pastor-Soler, N.M. Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Renkema, K.Y.; Velic, A.; Dijkman, H.B.; Verkaart, S.; van der Kemp, A.W.; Nowik, M.; Timmermans, K.; Doucet, A.; Wagner, C.A.; Bindels, R.J.; et al. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J. Am. Soc. Nephrol. 2009, 20, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Himmerkus, N.; Leipziger, J.; Bleich, M.; Koshimizu, T.-A.; Fähling, M.; Smorodchenko, A.; Shpak, J.; Knappe, C.; Isermann, J.; et al. Vasopressin increases urinary acidification via v1a receptors in collecting duct intercalated cells. J. Am. Soc. Nephrol. 2019, 30, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Liu, Z.; Huang, C.-L. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc. Natl. Acad. Sci. USA 2006, 103, 1615–1620. [Google Scholar] [CrossRef]
- Hadchouel, J.; Soukaseum, C.; Büsst, C.; Zhou, X.; Baudrie, V.; Zürrer, T.; Cambillau, M.; Elghozi, J.-L.; Lifton, R.P.; Loffing, J.; et al. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc. Natl. Acad. Sci. USA 2010, 107, 18109–18114. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, G.; Macrina, L.; Magni, G.; Arcidiacono, T. Calcium-sensing receptor: Evidence and hypothesis for its role in nephrolithiasis. Urolithiasis 2019, 47, 23–33. [Google Scholar] [CrossRef]
- Pollak, M.R.; Brown, E.M.; Chou, Y.-H.W.; Hebert, S.C.; Marx, S.J.; Stelnmann, B.; Levi, T.; Seidman, C.E.; Seidman, J.G. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 1993, 75, 1297–1303. [Google Scholar] [CrossRef]
- Pollak, M.R.; Chou, Y.H.; Marx, S.J.; Steinmann, B.; Cole, D.E.; Brandi, M.L.; Papapoulos, S.E.; Menko, F.H.; Hendy, G.N.; Brown, E.M. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J. Clin. Invest. 1994, 93, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Conner, D.A.; Pollak, M.R.; Ladd, D.J.; Kifor, O.; Warren, H.B.; Brown, E.M.; Seidman, J.G.; Seidman, C.E. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat. Genet. 1995, 11, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Hebert, S.C. Therapeutic use of calcimimetics. Annu. Rev. Med. 2006, 57, 349–364. [Google Scholar] [CrossRef]
- Torres, P.A.U.; De Broe, M. Calcium-sensing receptor, calcimimetics, and cardiovascular calcifications in chronic kidney disease. Kidney Int. 2012, 82, 19–25. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostroverkhova, D.S.; Hu, J.; Tarasov, V.V.; Melnikova, T.I.; Porozov, Y.B.; Mutig, K. Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney. Cells 2020, 9, 1644. https://doi.org/10.3390/cells9071644
Ostroverkhova DS, Hu J, Tarasov VV, Melnikova TI, Porozov YB, Mutig K. Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney. Cells. 2020; 9(7):1644. https://doi.org/10.3390/cells9071644
Chicago/Turabian StyleOstroverkhova, Daria S., Junda Hu, Vadim V. Tarasov, Tatiana I. Melnikova, Yuri B. Porozov, and Kerim Mutig. 2020. "Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney" Cells 9, no. 7: 1644. https://doi.org/10.3390/cells9071644
APA StyleOstroverkhova, D. S., Hu, J., Tarasov, V. V., Melnikova, T. I., Porozov, Y. B., & Mutig, K. (2020). Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney. Cells, 9(7), 1644. https://doi.org/10.3390/cells9071644




