Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Obstruct Vesicular/Plasmalemmal Recycling in Astrocytes Down-Stream of Calcium Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transfection
2.3. Imaging
2.4. Ca2+ Dynamics
2.5. Glutamate Release
2.6. Plasma Membrane Recycling
2.7. Statistics
3. Results
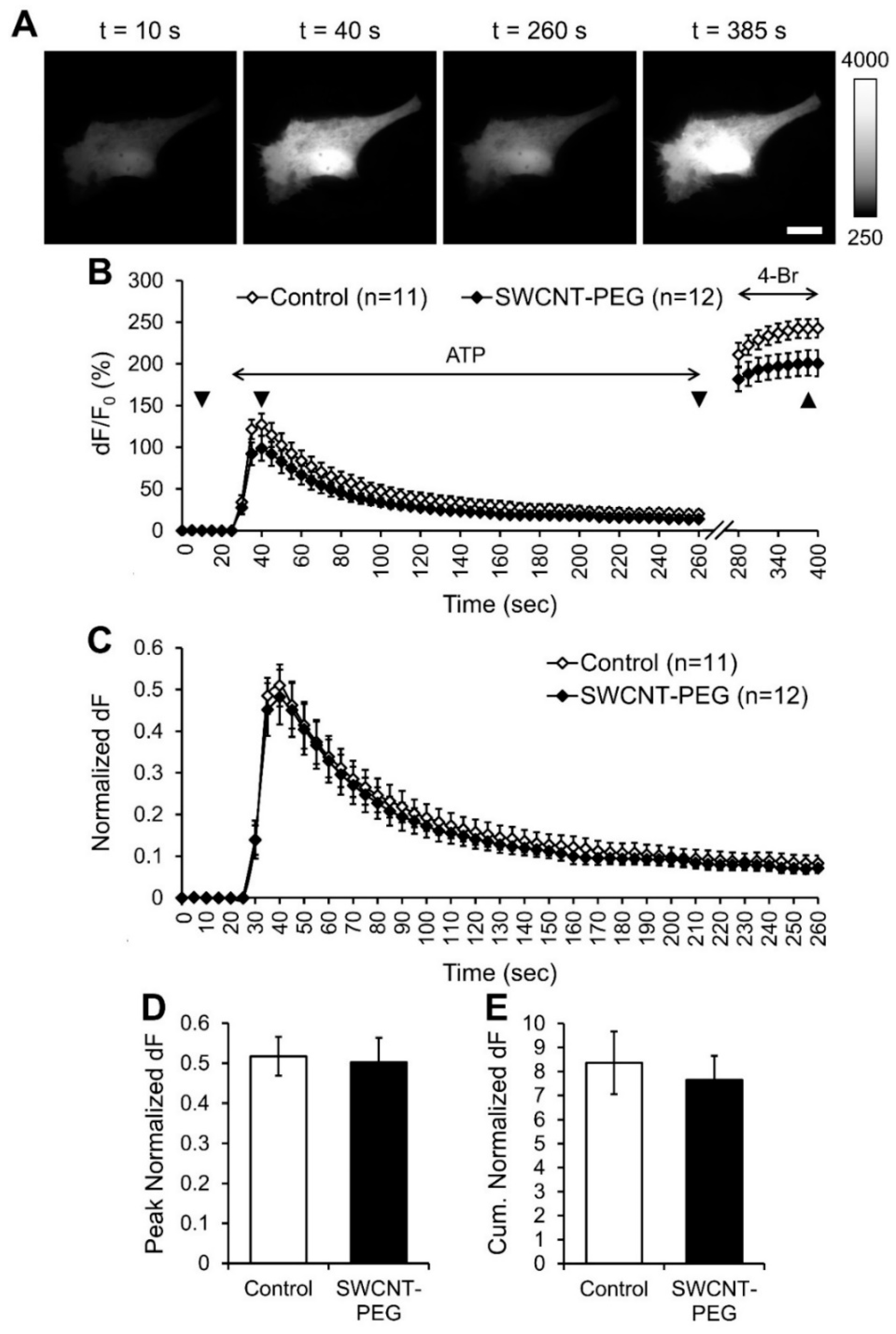
3.1. SWCNT-PEG does not Affect ATP-evoked Ca2+ Dynamics in Astrocytes
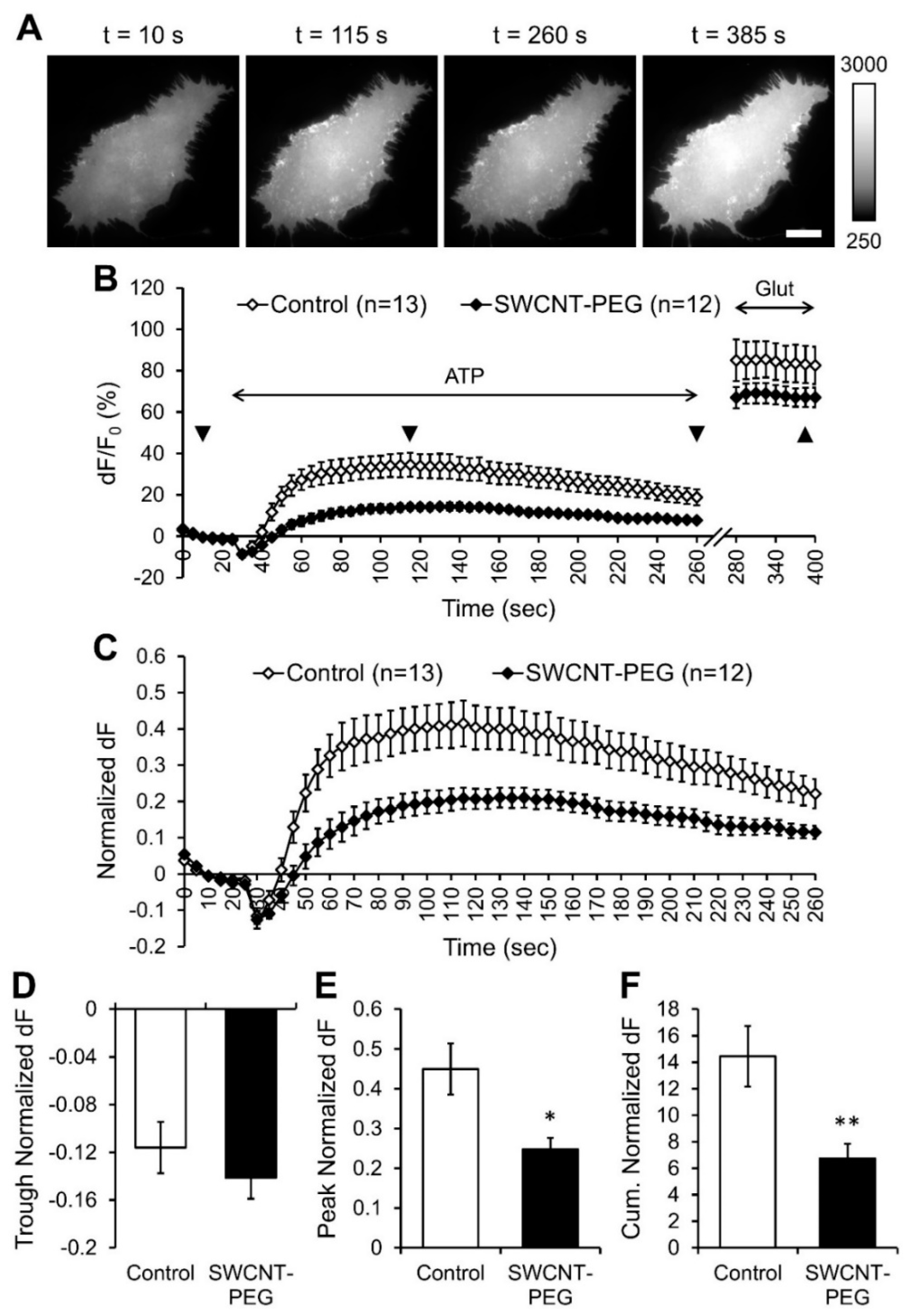
3.2. SWCNT-PEG Hampers ATP-evoked Glutamate Release from Astrocytes
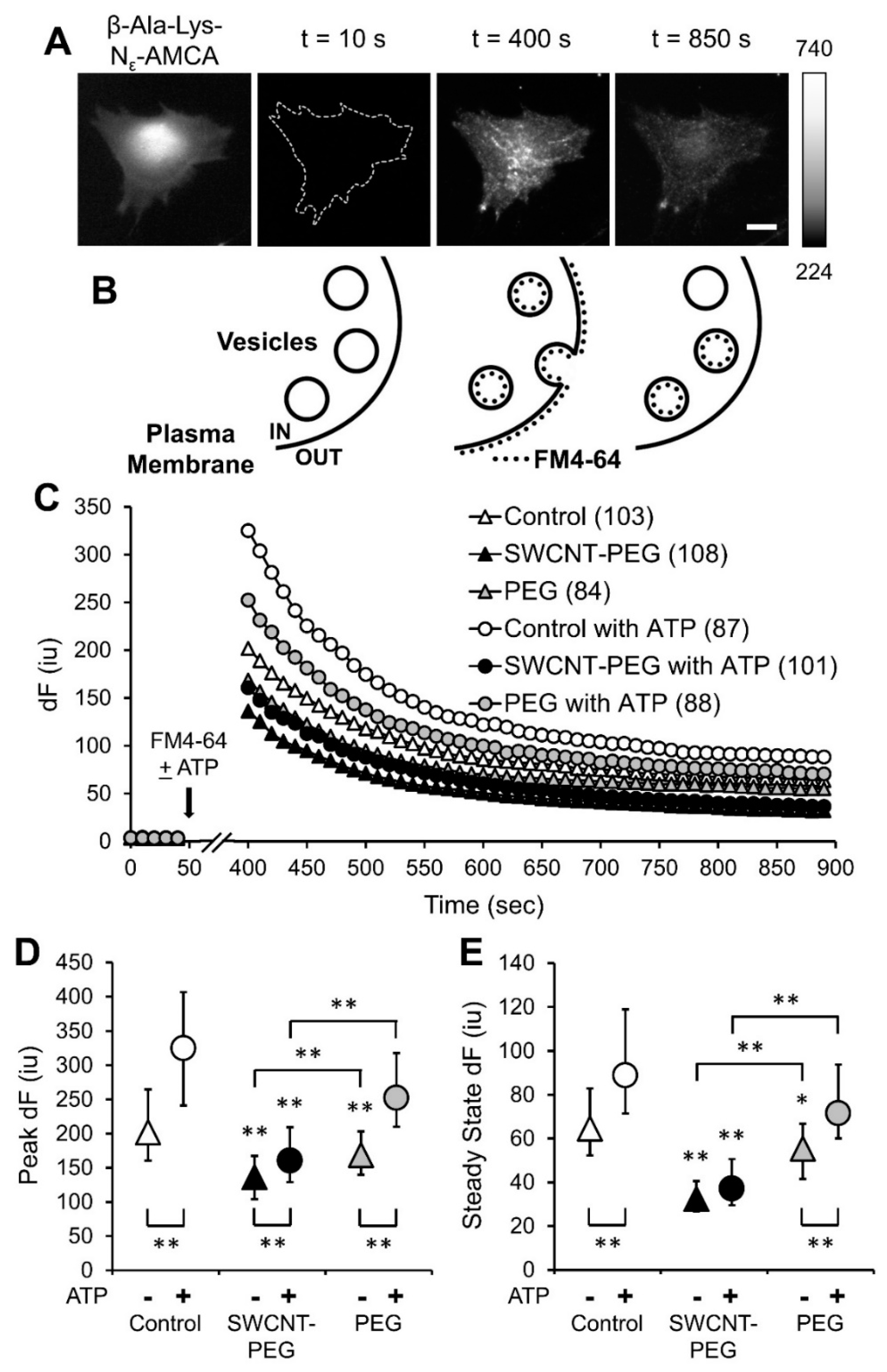
3.3. SWCNT-PEG Inhibits Constitutive and ATP-stimulated Membrane Recycling in Astrocytes
3.4. SWCNT-PEG Obstructs ATP-evoked Exocytosis in Astrocytes
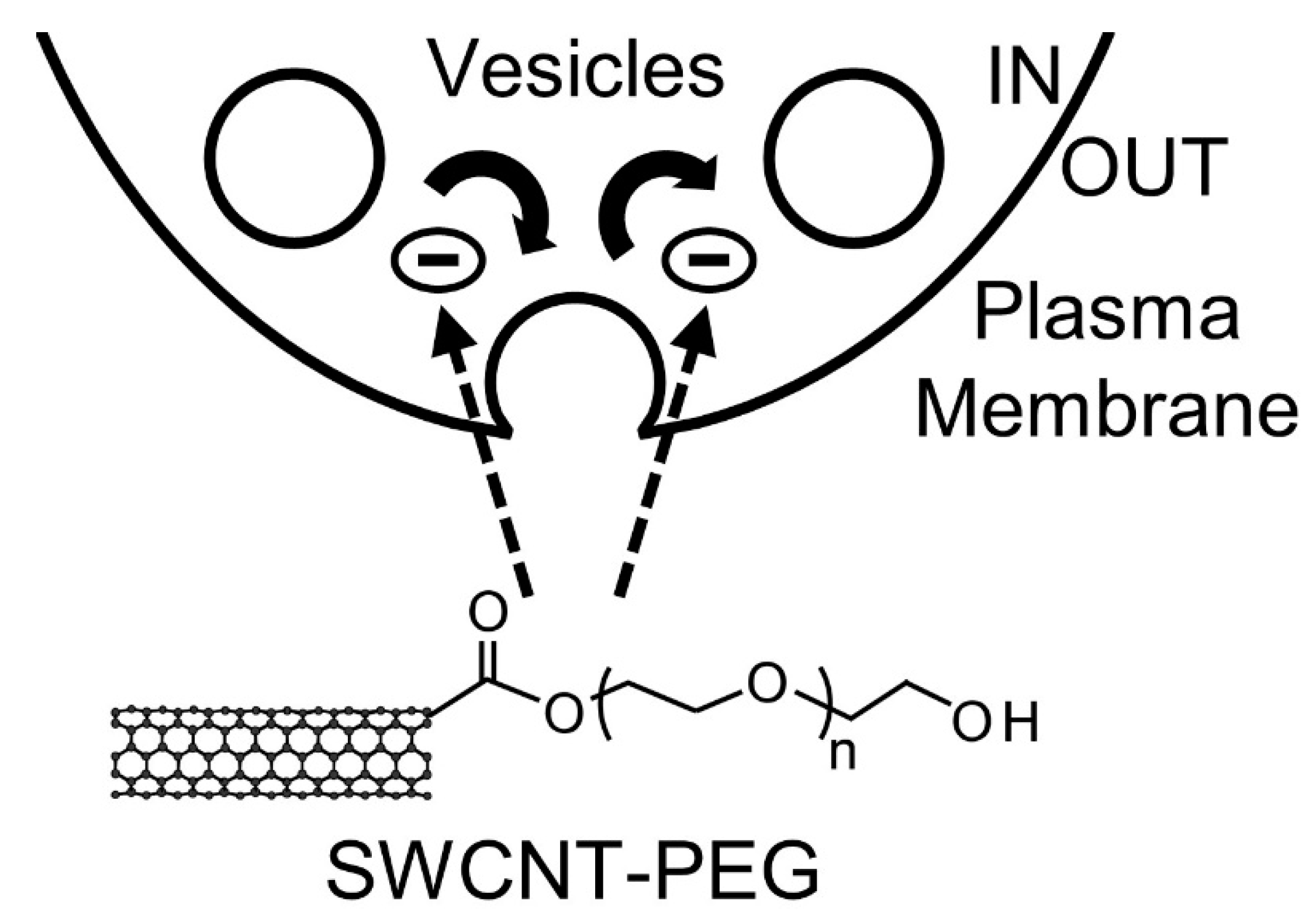
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Haddon, R.C.; Parpura, V. Biofunctionalization of Carbon Nanotubes. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Malarkey, E.B.; Parpura, V. Applications of carbon nanotubes in neurobiology. Neurodegener. Dis. 2007, 4, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Verkhratsky, A.; Parpura, V. Probing astroglia with carbon nanotubes: Modulation of form and function. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Hu, H.; Malarkey, E.B.; Zhao, B.; Montana, V.; Haddon, R.C.; Parpura, V. Chemically Functionalized Water Soluble Single-Walled Carbon Nanotubes Modulate Neurite Outgrowth. J. Nanosci. Nanotechnol. 2005, 5, 1707–1712. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano. Lett. 2012, 12. [Google Scholar] [CrossRef]
- Malarkey, E.B.; Reyes, R.C.; Zhao, B.; Haddon, R.C.; Parpura, V. Water soluble single-walled carbon nanotubes inhibit stimulated endocytosis in neurons. Nano. Lett. 2008, 8, 3538–3542. [Google Scholar] [CrossRef][Green Version]
- Roman, J.A.; Niedzielko, T.L.; Haddon, R.C.; Parpura, V.; Floyd, C.L. Single-Walled Carbon Nanotubes Chemically Functionalized with Polyethylene Glycol Promote Tissue Repair in a Rat Model of Spinal Cord Injury. J. Neurotrauma. 2011, 28, 2349–2362. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Bekyarova, E.; Brenner, M.; Haddon, R.C.; Parpura, V. Changes in the morphology and proliferation of astrocytes induced by two modalities of chemically functionalized single-walled carbon nanotubes are differentially mediated by glial fibrillary acidic protein. Nano. Lett. 2014, 14, 3720–3727. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized single-walled carbon nanotubes enhance the glutamate uptake characteristics of mouse cortical astrocytes. Amino Acids 2015, 47. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Malarkey, E.B.; Verderio, C.; Matteoli, M.; Parpura, V. Vesicular transmitter release from astrocytes. Glia 2006, 54, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.F.; Azmi, H.; Takano, T.; Xu, Q.; Peng, W.; Lin, J.; Oberheim, N.; Lou, N.; Wang, X.; Zielke, H.R.; et al. An astrocytic basis of epilepsy. Nat. Med. 2005, 11, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Xu, J.; Xu, Q.; Nedergaard, M.; Kang, J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2005, 94, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Fellin, T.; Zhu, Y.; Lee, S.Y.; Auberson, Y.P.; Meaney, D.F.; Coulter, D.A.; Carmignoto, G.; Haydon, P.G. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci 2007, 27, 10674–10684. [Google Scholar] [CrossRef]
- Lee, W.; Reyes, R.C.; Gottipati, M.K.; Lewis, K.; Lesort, M.; Parpura, V.; Gray, M. Enhanced Ca2+-dependent glutamate release from astrocytes of the BACHD Huntington’s disease mouse model. Neurobiol. Dis. 2013, 58, 192–199. [Google Scholar] [CrossRef]
- Parpura, V.; Grubisic, V.; Verkhratsky, A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta 2011, 1813, 984–991. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodriguez, J.J.; Parpura, V. Calcium signalling in astroglia. Mol. Cell Endocrinol. 2012, 353, 45–56. [Google Scholar] [CrossRef]
- Malarkey, E.B.; Ni, Y.; Parpura, V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 2008, 56, 821–835. [Google Scholar] [CrossRef]
- Parpura, V.; Zorec, R. Gliotransmission: Exocytotic release from astrocytes. Brain Res. Rev. 2010, 63, 83–92. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.P.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Orkand, R.K.; Kettenmann, H. Glial calcium: Homeostasis and signaling function. Physiol. Rev. 1998, 78, 99–141. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Ni, Y.; Sunjara, V.; Hua, X.; Parpura, V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 2004, 24, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Malarkey, E.B.; Parpura, V. Temporal characteristics of vesicular fusion in astrocytes: Examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J. Physiol. 2011, 589, 4271–4300. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Samuelson, J.J.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano. Lett. 2013, 13, 4387–4392. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderon, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolo, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef]
- Buscemi, L.; Ginet, V.; Lopatar, J.; Montana, V.; Pucci, L.; Spagnuolo, P.; Zehnder, T.; Grubisic, V.; Truttman, A.; Sala, C.; et al. Homer1 Scaffold Proteins Govern Ca2+ Dynamics in Normal and Reactive Astrocytes. Cereb. Cortex 2017, 27, 2365–2384. [Google Scholar] [CrossRef]
- Marvin, J.S.; Borghuis, B.G.; Tian, L.; Cichon, J.; Harnett, M.T.; Akerboom, J.; Gordus, A.; Renninger, S.L.; Chen, T.W.; Bargmann, C.I.; et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 2013, 10, 162–170. [Google Scholar] [CrossRef]
- Lee, W.; Parpura, V. Spatio-temporal characteristics of metabotropic glutamate receptor 5 traffic at or near the plasma membrane in astrocytes. Glia 2016, 64, 1050–1065. [Google Scholar] [CrossRef]
- Benediktsson, A.M.; Schachtele, S.J.; Green, S.H.; Dailey, M.E. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J. Neurosci. Methods 2005, 141, 41–53. [Google Scholar] [CrossRef]
- Parpura, V.; Haydon, P.G. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Grubisic, V.; Gottipati, M.K.; Stout, R.F., Jr.; Grammer, J.R.; Parpura, V. Heterogeneity of myotubes generated by the MyoD and E12 basic helix-loop-helix transcription factors in otherwise non-differentiation growth conditions. Biomaterials 2014, 35, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.C.; Perry, G.; Lesort, M.; Parpura, V. Immunophilin deficiency augments Ca2+-dependent glutamate release from mouse cortical astrocytes. Cell Calcium 2011, 49, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Parpura, V. Dual regulation of Ca2+-dependent glutamate release from astrocytes: Vesicular glutamate transporters and cytosolic glutamate levels. Glia 2009, 57, 1296–1305. [Google Scholar] [CrossRef]
- West, A.P.; Willison, K.R. Brefeldin A and mannose 6-phosphate regulation of acrosomic related vesicular trafficking. Eur. J. Cell Biol. 1996, 70, 315–321. [Google Scholar]
- Gaffield, M.A.; Tabares, L.; Betz, W.J. Preferred sites of exocytosis and endocytosis colocalize during high- but not lower-frequency stimulation in mouse motor nerve terminals. J. Neurosci. 2009, 29, 15308–15316. [Google Scholar] [CrossRef]
- Dieck, S.T.; Heuer, H.; Ehrchen, J.; Otto, C.; Bauer, K. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala-Lys-Nepsilon-AMCA in astrocytes. Glia 1999, 25, 10–20. [Google Scholar] [CrossRef]
- Betz, W.J.; Bewick, G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 1992, 255, 200–203. [Google Scholar] [CrossRef]
- Neher, E. Introduction: Regulated exocytosis. Cell Calcium 2012, 52, 196–198. [Google Scholar] [CrossRef][Green Version]
- Zorec, R.; Verkhratsky, A.; Rodriguez, J.J.; Parpura, V. Astrocytic vesicles and gliotransmitters: Slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience 2016, 323, 67–75. [Google Scholar] [CrossRef]
- Park, K.H.; Chhowalla, M.; Iqbal, Z.; Sesti, F. Single-walled carbon nanotubes are a new class of ion channel blockers. J. Biol. Chem. 2003, 278, 50212–50216. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jorgacevski, J.; Kreft, M.; Grubisic, V.; Stout, R.F., Jr.; Potokar, M.; Parpura, V.; Zorec, R. Single-vesicle architecture of synaptobrevin2 in astrocytes. Nat. Commun. 2014, 5, 3780. [Google Scholar] [CrossRef] [PubMed]
- Gucek, A.; Jorgacevski, J.; Gorska, U.; Rituper, B.; Kreft, M.; Zorec, R. Local electrostatic interactions determine the diameter of fusion pores. Channels (Austin) 2015, 9, 96–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gucek, A.; Jorgacevski, J.; Singh, P.; Geisler, C.; Lisjak, M.; Vardjan, N.; Kreft, M.; Egner, A.; Zorec, R. Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state. Cell Mol. Life Sci. 2016, 73, 3719–3731. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Kam, N.W.; Liu, Z.; Dai, H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef]
- Krajcik, R.; Jung, A.; Hirsch, A.; Neuhuber, W.; Zolk, O. Functionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genes. Biochem. Biophys. Res. Commun. 2008, 369, 595–602. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. 2004, 16–17. [Google Scholar] [CrossRef]
- Stenovec, M.; Solmajer, T.; Perdih, A.; Vardjan, N.; Kreft, M.; Zorec, R. Distinct labelling of fusion events in rat lactotrophs by FM 1-43 and FM 4-64 is associated with conformational differences. Acta Physiol. 2007, 191, 35–42. [Google Scholar] [CrossRef]
- Robinson, M.B. Acute regulation of sodium-dependent glutamate transporters: A focus on constitutive and regulated trafficking. Handb. Exp. Pharm. 2006, 251–275. [Google Scholar]
- Gonzalez, M.I.; Robinson, M.B. Protein kinase C-dependent remodeling of glutamate transporter function. Mol. Interv. 2004, 4, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cavender, C.E.; Gottipati, M.K.; Parpura, V. Trafficking of excitatory amino acid transporter 2-laden vesicles in cultured astrocytes: A comparison between approximate and exact determination of trajectory angles. Amino Acids 2015, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, K.M.; Sultan, P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology 1995, 34, 1387–1395. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Gronborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brugger, B.; Ringler, P.; et al. Molecular anatomy of a trafficking organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Liu, W.; Mohideen, U.; Parpura, V. Single molecule measurements of mechanical interactions within ternary SNARE complexes and dynamics of their disassembly: SNAP25 vs. SNAP23. J. Physiol. 2009, 587, 1943–1960. [Google Scholar] [CrossRef] [PubMed]
- Couchoud, M.; Der, C.; Girodet, S.; Vernoud, V.; Prudent, M.; Leborgne-Castel, N. Drought stress stimulates endocytosis and modifies membrane lipid order of rhizodermal cells of Medicago truncatula in a genotype-dependent manner. BMC Plant. Biol 2019, 19, 221. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S. Physiological and Proteomic Responses of Contrasting Alfalfa (Medicago sativa L.) Varieties to PEG-Induced Osmotic Stress. Front. Plant. Sci. 2018, 9, 242. [Google Scholar] [CrossRef]
- Wang, Y.F.; Parpura, V. Astroglial Modulation of Hydromineral Balance and Cerebral Edema. Front. Mol. Neurosci. 2018, 11, 204. [Google Scholar] [CrossRef]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottipati, M.K.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Obstruct Vesicular/Plasmalemmal Recycling in Astrocytes Down-Stream of Calcium Ions. Cells 2020, 9, 1597. https://doi.org/10.3390/cells9071597
Gottipati MK, Bekyarova E, Haddon RC, Parpura V. Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Obstruct Vesicular/Plasmalemmal Recycling in Astrocytes Down-Stream of Calcium Ions. Cells. 2020; 9(7):1597. https://doi.org/10.3390/cells9071597
Chicago/Turabian StyleGottipati, Manoj K., Elena Bekyarova, Robert C. Haddon, and Vladimir Parpura. 2020. "Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Obstruct Vesicular/Plasmalemmal Recycling in Astrocytes Down-Stream of Calcium Ions" Cells 9, no. 7: 1597. https://doi.org/10.3390/cells9071597
APA StyleGottipati, M. K., Bekyarova, E., Haddon, R. C., & Parpura, V. (2020). Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Obstruct Vesicular/Plasmalemmal Recycling in Astrocytes Down-Stream of Calcium Ions. Cells, 9(7), 1597. https://doi.org/10.3390/cells9071597






