Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis
Abstract
1. Introduction
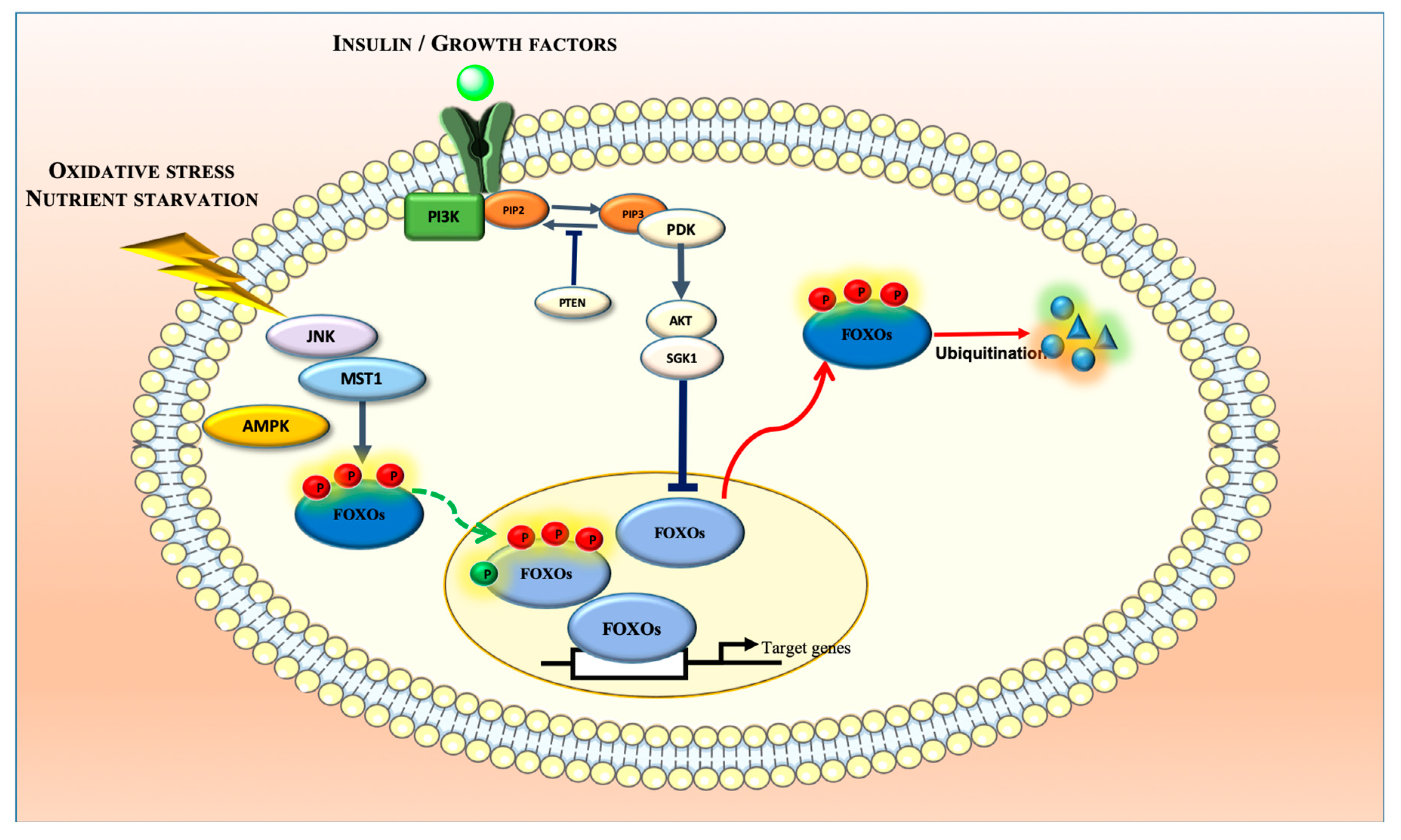
2. Biochemistry and Regulatory Mechanisms of FOXOs
3. Roles of FOXOs in Cancer Regulation
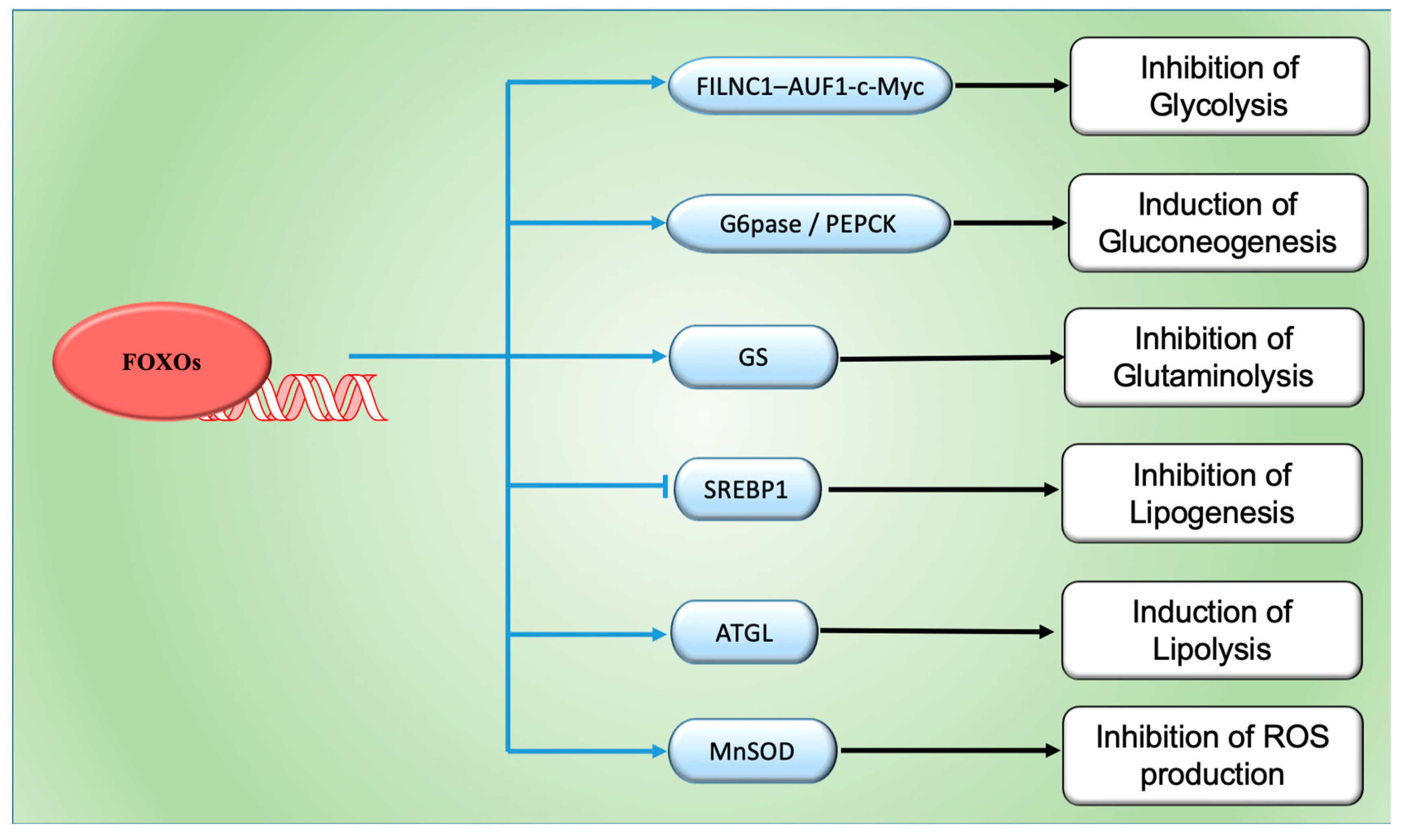
4. FOXOs and Cancer Metabolism
5. FOXOs and Angiogenesis
5.1. Role of Angiogenesis in Cancer
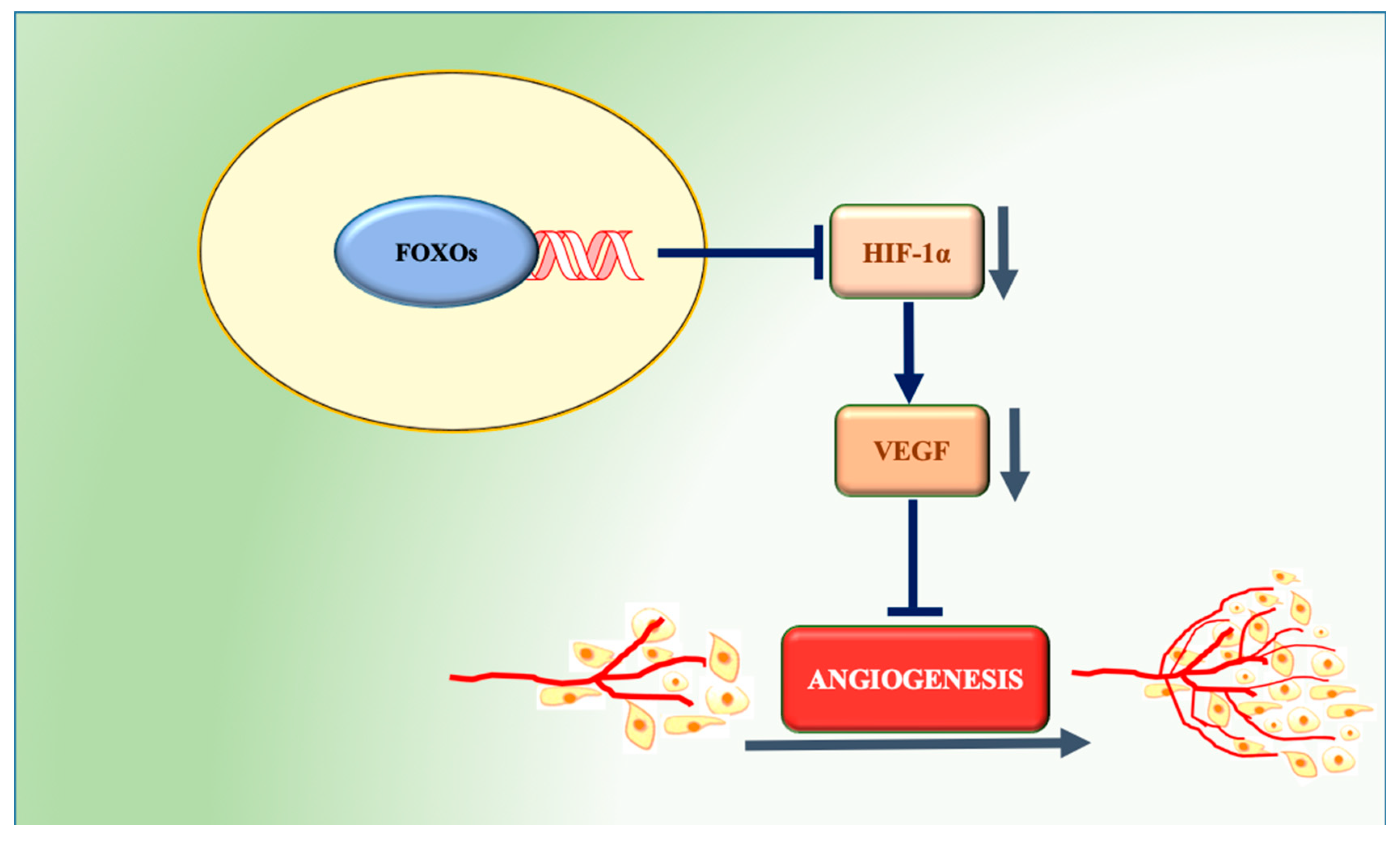
5.2. Role of FOXOs in Angiogenesis
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| AMPK | AMP-activated protein kinase |
| AKT | Protein Kinase B |
| ATGL | Adipose triacylglycerol lipase |
| Bim | Bcl-2-like protein 11 |
| CK1 | Casein kinase 1 alpha 1 |
| CML | Chronic myeloid leukemia |
| CPT1A | Carnitine palmitoyltransferase 1A |
| CREB | cAMP response element-binding protein |
| DYRK1A | Dual-specificity tyrosine phosphorylation regulated kinase 1A |
| EC | Endothelial cells |
| ECM | Extracellular matrix |
| EGF | Endothelial growth factor |
| EMT | Epithelial-Mesenchymal Transitions |
| ERK | Extracellular signal-regulated kinase |
| FAO | Fatty acid β-oxidation |
| FILCN1 | FOXO induced long non-coding RNA |
| FOXO | Forkhead box transcription factors O |
| FGF | Fibroblast growth factor |
| GADD45 | Growth Arrest and DNA Damage-inducible 45 |
| GEMM | Genetically engineered mouse model |
| GLUT | Glucose transporter |
| GLS | Glutaminase |
| GS | Glutamine synthetase |
| G6Pase | Glucose-6-phosphatase |
| HIF-1α | Hypoxia inducible factor-1α |
| IL-8 | Interleukin-8 |
| IGF-1 | Insulin-like growth factor 1 |
| IKK | I kappa B kinase |
| JNK | c-Jun N-terminal kinase |
| LIC | Leukemia initiating cell |
| MDR1 | Multi drug response pump 1 |
| MMP | Matrix metalloproteinase |
| MnSOD | Manganese superoxide dismutase |
| MST1 | Mammalian sterile 20-like kinase |
| PCK1 | Phosphoenolpyruvate carboxykinase 1 |
| PDGF | Platelet derived growth factor |
| PDK1 | 3-phosphoinositide-dependent kinase-1 |
| PEPCK | Phosphoenolpyruvate carboxykinase 2 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol (4,5)-bisphosphate |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PUMA | p53 upregulated modulator of apoptosis |
| ROS | Reactive oxygen species |
| RTK | Receptor tyrosine kinase |
| SGK | Serum- and glucocorticoid-inducing kinase |
| SREBP1 | Sterol regulatory element-binding transcription factor 1 |
| TCA | Tricarboxylic acid |
| TNF-α | Tumor necrosis factor α |
| VDUP1 | Vitamin D3 up- regulated protein-1 |
| VEGF | Vascular endothelial growth factor |
| VCAM-1 | Vascular cell adhesion molecule |
References
- Weigel, D.; Jackle, H. The Fork Head Domain: A Novel DNA Binding Motif of Eukaryotic Transcription Factors? Cell 1990, 63, 455–456. [Google Scholar] [CrossRef]
- Kaestner, K.H.; Knochel, W.; Martinez, D.E. Unified Nomenclature for the Winged Helix/Forkhead Transcription Factors. Genes Dev. 2000, 14, 142–146. [Google Scholar] [PubMed]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C.; et al. FoxOs Are Critical Mediators of Hematopoietic Stem Cell Resistance to Physiologic Oxidative Stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, S.M.; Hess Michelini, R.; Doedens, A.L.; Goldrath, A.W.; Stone, E.L. FOXO Transcription Factors Throughout T Cell Biology. Nat. Rev. Immunol. 2012, 12, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Kim, H.-N.; Ambrogini, E.; Han, L.; Iyer, S.; Serra Ucer, S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO Proteins Restrain Osteoclastogenesis and Bone Resorption by Attenuating H2O2 Accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R.; Brunet, A. The FoxO Code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef]
- Furuyama, T.; Yamashita, H.; Kitayama, K.; Higami, Y.; Shimokawa, I.; Mori, N. Effects of Aging and Caloric Restriction on the Gene Expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the Rat Skeletal Muscles. Microsc. Res. Tech. 2002, 59, 331–334. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M.T. FOXOs: Signalling Integrators for Homeostasis Maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. FOXO Transcription Factors at the Interface between Longevity and Tumor Suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, B.; Liu, D.; Paik, J.-H. FoxO Family Members in Cancer. Cancer Biol. Ther. 2011, 12, 253–259. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of Cancer: Next Generation. Cells 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Woods, Y.L.; Prescott, A.R.; Peggie, M.; Unterman, T.G.; Williams, M.R.; Cohen, P. Two Novel Phosphorylation Sites on FKHR That Are Critical for Its Nuclear Exclusion. EMBO J. 2002, 21, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Jacobs, F.M.J.; Van der Heide, L.P.; Wijchers, P.J.E.C.; Burbach, J.P.H.; Hoekman, M.F.M.; Smidt, M.P. FoxO6, a Novel Member of the FoxO Class of Transcription Factors with Distinct Shuttling Dynamics. J. Biol. Chem. 2003, 278, 35959–35967. [Google Scholar] [CrossRef]
- Brunet, A.; Kanai, F.; Stehn, J.; Xu, J.; Sarbassova, D.; Frangioni, J.V.; Dalal, S.N.; DeCaprio, J.A.; Greenberg, M.E.; Yaffe, M.B. 14-3-3 Transits to the Nucleus and Participates in Dynamic Nucleocytoplasmic Transport. J. Cell Biol. 2002, 156, 817–828. [Google Scholar] [CrossRef]
- Huang, H.; Regan, K.M.; Wang, F.; Smith, D.I.; van Deursen, J.M.; Tindall, D.J. Skp2 Inhibits FOXO1 in Tumor Suppression Through Ubiquitin-Mediated Degradation. Proc. Natl. Acad. Sci. USA 2005, 102, 1649–1654. [Google Scholar] [CrossRef]
- Van der Heide, L.P.; Hoekman, M.F.M.; Smidt, M.P. The Ins and Outs of FoxO Shuttling: Mechanisms of FoxO Translocation and Transcriptional Regulation. Biochem. J. 2004, 380, 297–309. [Google Scholar] [CrossRef]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-Evaluating the Role of FOXOs in Cancer. Semin. Cancer Biol. 2018, 50, 90–100. [Google Scholar] [CrossRef]
- Essers, M.A.G.; Weijzen, S.; de Vries-Smits, A.M.M.; Saarloos, I.; de Ruiter, N.D.; Bos, J.L.; Burgering, B.M.T. FOXO Transcription Factor Activation by Oxidative Stress Mediated by the Small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and Acetylation Modifications of FOXO3a: Independently or Synergistically? Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Sunayama, J.; Tsuruta, F.; Masuyama, N.; Gotoh, Y. JNK Antagonizes Akt-Mediated Survival Signals by Phosphorylating 14-3-3. J. Cell Biol. 2005, 170, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Yuan, Z.; Boag, P.R.; Yang, Y.; Villen, J.; Becker, E.B.E.; DiBacco, S.; de la Iglesia, N.; Gygi, S.; Blackwell, T.K.; et al. A Conserved MST-FOXO Signaling Pathway Mediates Oxidative-Stress Responses and Extends Life Span. Cell 2006, 125, 987–1001. [Google Scholar] [CrossRef]
- Greer, E.L.; Oskoui, P.R.; Banko, M.R.; Maniar, J.M.; Gygi, M.P.; Gygi, S.P.; Brunet, A. The Energy Sensor AMP-Activated Protein Kinase Directly Regulates the Mammalian FOXO3 Transcription Factor. J. Biol. Chem. 2007, 282, 30107–30119. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sanchez-Ramos, C.; Prieto-Arroyo, I.; Urbanek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Fukuoka, M.; Daitoku, H.; Hatta, M.; Matsuzaki, H.; Umemura, S.; Fukamizu, A. Negative Regulation of Forkhead Transcription Factor AFX (Foxo4) by CBP-Induced Acetylation. Int. J. Mol. Med. 2003, 12, 503–508. [Google Scholar] [CrossRef]
- Daitoku, H.; Hatta, M.; Matsuzaki, H.; Aratani, S.; Ohshima, T.; Miyagishi, M.; Nakajima, T.; Fukamizu, A. Silent Information Regulator 2 Potentiates Foxo1-Mediated Transcription through Its Deacetylase Activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10042–10047. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Aoyama, H.; Yoshimoshi, K.; Fukamizu, A. Acetylation of Foxo1 Alters Its DNA-Binding Ability and Sensitivity to Phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 11278–11283. [Google Scholar] [CrossRef]
- van der Horst, A.; de Vries-Smits, A.M.M.; Brenkman, A.B.; van Triest, M.H.; van den Broek, N.; Colland, F.; Maurice, M.M.; Burgering, B.M.T. FOXO4 Transcriptional Activity Is Regulated by Monoubiquitination and USP7/HAUSP. Nat. Cell Biol. 2006, 8, 1064–1073. [Google Scholar] [CrossRef]
- Yamagata, K.; Daitoku, H.; Takahashi, Y.; Namiki, K.; Hisatake, K.; Kako, K.; Mukai, H.; Kasuya, Y.; Fukamizu, A. Arginine Methylation of FOXO Transcription Factors Inhibits Their Phosphorylation by Akt. Mol. Cell 2008, 32, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.; Zilberfarb, V.; Gangneux, N.; Christeff, N.; Issad, T. O-Glycosylation of FoxO1 Increases Its Transcriptional Activity towards the Glucose 6-Phosphatase Gene. FEBS Lett. 2008, 582, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, R.; Hong, H.; Yang, Z.; Sun, D.; Sun, S.; Guo, X.; Ye, J.; Li, Z.; Liu, P. The Poly(ADP-Ribosyl)Ation of FoxO3 Mediated by PARP1 Participates in Isoproterenol-Induced Cardiac Hypertrophy. Biochim. Biophys. Acta 2016, 1863, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Dansen, T.B.; Burgering, B.M.T. Unravelling the Tumor-Suppressive Functions of FOXO Proteins. Trends Cell Biol. 2008, 18, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- de Keizer, P.L.J.; Packer, L.M.; Szypowska, A.A.; Riedl-Polderman, P.E.; van den Broek, N.J.F.; de Bruin, A.; Dansen, T.B.; Marais, R.; Brenkman, A.B.; Burgering, B.M.T. Activation of Forkhead Box O Transcription Factors by Oncogenic BRAF Promotes P21cip1-Dependent Senescence. Cancer Res. 2010, 70, 8526–8536. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and Regulation of Apoptosis. BBA Mol. Cell Res. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Modur, V.; Nagarajan, R.; Evers, B.M.; Milbrandt, J. FOXO Proteins Regulate Tumor Necrosis Factor-Related Apoptosis Inducing Ligand Expression. J. Biol. Chem. 2002, 277, 47928–47937. [Google Scholar] [CrossRef]
- Zhang, B.; Tomita, Y.; Ch’ng, E.; Qiu, Y.; He, J.; Jin, Y.-F.; Tomoeda, M.; Hamada, K.-I.; Ueda, T.; Aozasa, K. Prognostic Significance of Phosphorylated FOXO1 Expression in Soft Tissue Sarcoma. Ann. Surg. Oncol. 2009, 16, 1925–1937. [Google Scholar] [CrossRef]
- Cheong, J.-W.; Eom, J.I.; Maeng, H.-Y.; Lee, S.T.; Hahn, J.S.; Ko, Y.W.; Min, Y.H. Constitutive Phosphorylation of FKHR Transcription Factor as a Prognostic Variable in Acute Myeloid Leukemia. Leuk. Res. 2003, 27, 1159–1162. [Google Scholar] [CrossRef]
- Wu, Y.; Elshimali, Y.; Sarkissyan, M.; Mohamed, H.; Clayton, S.; Vadgama, J.V. Expression of FOXO1 Is Associated with GATA3 and Annexin-1 and Predicts Disease-Free Survival in Breast Cancer. Am. J. Cancer Res. 2012, 2, 104–115. [Google Scholar] [PubMed]
- Santo, E.E.; Stroeken, P.; Sluis, P.V.; Koster, J.; Versteeg, R.; Westerhout, E.M. FOXO3a Is a Major Target of Inactivation by PI3K/AKT Signaling in Aggressive Neuroblastoma. Cancer Res. 2013, 73, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Habashy, H.O.; Rakha, E.A.; Aleskandarany, M.; Ahmed, M.A.; Green, A.R.; Ellis, I.O.; Powe, D.G. FOXO3a Nuclear Localisation Is Associated with Good Prognosis in Luminal-Like Breast Cancer. Breast Cancer Res. Treat 2011, 129, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.D.; Bruce, A.; Sreekumar, R.; Curtis, N.; Cheung, T.; Reading, I.; Primrose, J.N.; Ottensmeier, C.; Packham, G.K.; Thomas, G.; et al. FOXO3 Expression During Colorectal Cancerprogression: Biomarker Potential Reflects a Tumour Suppressor Role. Br. J. Cancer 2013, 109, 387–394. [Google Scholar] [CrossRef]
- Paik, J.-H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs Are Lineage-Restricted Redundant Tumor Suppressors and Critical Regulators of Endothelial Cell Homeostasis. Cell 2008, 182, 309–323. [Google Scholar]
- Dong, T.; Zhang, Y.; Chen, Y.; Liu, P.; An, T.; Zhang, J.; Yang, H.; Zhu, W.; Yang, X. FOXO1 Inhibits the Invasion and Metastasis of Hepatocellular Carcinoma by Reversing ZEB2-Induced Epithelial-Mesenchymal Transition. Oncotarget 2017, 8, 1703–1713. [Google Scholar] [CrossRef]
- Ni, D.; Ma, X.; Li, H.Z.; Gao, Y.; Li, X.T.; Zhang, Y.; Ai, Q.; Zhang, P.; Song, E.L.; Huang, Q.B.; et al. Downregulation of FOXO3a Promotes Tumor Metastasis and Is Associated with Metastasis-Free Survival of Patients with Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2014, 20, 1779–1790. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chauhan, A.S.; Zhuang, L.; Gan, B. FoxO Transcription Factors in Cancer Metabolism. Semin. Cancer Biol. 2018, 50, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Procaccia, S.; Ordan, M.; Cohen, I.; Bendetz-Nezer, S.; Seger, R. Direct Binding of MEK1 and MEK2 to AKT Induces Foxo1 Phosphorylation, Cellular Migration and Metastasis. Sci. Rep. 2017, 7, 43078. [Google Scholar] [CrossRef]
- Su, B.; Gao, L.; Baranowski, C.; Gillard, B.; Wang, J.; Ransom, R.; Ko, H.-K.; Gelman, I.H. A Genome-Wide RNAi Screen Identifies FOXO4 as a Metastasis-Suppressor Through Counteracting PI3K/AKT Signal Pathway in Prostate Cancer. PLoS ONE 2014, 9, e101411. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.K.; Lee, H.E.; Cho, S.J.; Cho, Y.J.; Lee, B.L.; Lee, H.S.; Nam, S.Y.; Lee, J.-S.; Kim, W.H. Constitutive Phosphorylation of the FOXO1A Transcription Factor as a Prognostic Variable in Gastric Cancer. Mod. Pathol. 2007, 20, 835–842. [Google Scholar] [CrossRef]
- Trinh, D.L.; Scott, D.W.; Morin, R.D.; Mendez-Lago, M.; An, J.; Jones, S.J.M.; Mungall, A.J.; Zhao, Y.; Schein, J.; Steidl, C.; et al. Analysis of FOXO1 Mutations in Diffuse Large B-Cell Lymphoma. Blood 2013, 121, 3666–3674. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, C.M.; Chillón, M.C.; García-Sanz, R.; Pérez, C.; Caballero, M.D.; Ramos, F.; de Coca, A.G.; Alonso, J.M.; Giraldo, P.; Bernal, T.; et al. High FOXO3a Expression Is Associated with a Poorer Prognosis in AML with Normal Cytogenetics. Leuk. Res. 2009, 33, 1706–1709. [Google Scholar] [CrossRef]
- Kumazoe, M.; Takai, M.; Bae, J.; Hiroi, S.; Huang, Y.; Takamatsu, K.; Won, Y.; Yamashita, M.; Hidaka, S.; Yamashita, S.; et al. FOXO3 Is Essential for CD44 Expression in Pancreatic Cancer Cells. Oncogene 2017, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Ren, L.; Wu, D.; Yang, X.; Zhou, Z.; Nie, Q.; Jiang, G.; Xue, S.; Weng, W.; Qiu, Y.; et al. Overexpression of FoxO3a Is Associated with Glioblastoma Progression and Predicts Poor Patient Prognosis. Int. J. Cancer 2017, 140, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Tenbaum, S.P.; Ordonez-Moran, P.; Puig, I.; Chicote, I.; Arques, O.; Landolfi, S.; Fernandez, Y.; Herance, J.R.; Gispert, J.D.; Mendizabal, L.; et al. Beta-Catenin Confers Resistance to PI3K and AKT Inhibitors and Subverts FOXO3a to Promote Metastasis in Colon Cancer. Nat. Med. 2012, 18, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gomes, A.R.; Monteiro, L.J.; Wong, S.Y.; Wu, L.H.; Ng, T.-T.; Karadedou, C.T.; Millour, J.; Ip, Y.-C.; Cheung, Y.N. Constitutively Nuclear FOXO3a Localization Predicts Poor Survival and Promotes Akt Phosphorylation in Breast Cancer. PLoS ONE 2010, 5, e12293. [Google Scholar] [CrossRef]
- Storz, P.; Doppler, H.; Copland, J.A.; Simpson, K.J.; Toker, A. FOXO3a Promotes Tumor Cell Invasion through the Induction of Matrix Metalloproteinases. Mol. Cell. Biol. 2009, 29, 4906–4917. [Google Scholar] [CrossRef]
- Feng, X.; Wu, Z.; Wu, Y.; Hankey, W.; Prior, T.W.; Li, L.; Ganju, R.K.; Shen, R.; Zou, X. Cdc25A Regulates Matrix Metalloprotease 1 through Foxo1 and Mediates Metastasis of Breast Cancer Cells. Mol. Cell. Biol. 2011, 31, 3457–3471. [Google Scholar] [CrossRef]
- Sunters, A.; Madureira, P.A.; Pomeranz, K.M.; Aubert, M.; Brosens, J.J.; Cook, S.J.; Burgering, B.M.T.; Coombes, R.C.; Lam, E.W.-F. Paclitaxel-Induced Nuclear Translocation of FOXO3a in Breast Cancer Cells Is Mediated by C-Jun NH2-Terminal Kinase and Akt. Cancer Res. 2006, 66, 212–220. [Google Scholar] [CrossRef]
- Essafi, A.; Fernandez de Mattos, S.; Hassen, Y.A.M.; Soeiro, I.; Mufti, G.J.; Thomas, N.S.B.; Medema, R.H.; Lam, E.W.-F. Direct Transcriptional Regulation of Bim by FoxO3a Mediates STI571-Induced Apoptosis in Bcr-Abl-Expressing Cells. Oncogene 2005, 24, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ganapathy, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol Induces Growth Arrest and Apoptosis through Activation of FOXO Transcription Factors in Prostate Cancer Cells. PLoS ONE 2010, 5, e15288. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Srivastava, R.K.; Shankar, S. Inhibition of PI3K/AKT and MAPK/ERK Pathways Causes Activation of FOXO Transcription Factor, Leading to Cell Cycle Arrest and Apoptosis in Pancreatic Cancer. J. Mol. Signal 2010, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; DU, D.; Fu, S.; Chen, Y.; Yu, F.; Gao, P. Involvement of Post-Transcriptional Regulation of FOXO1 by HuR in 5-FU-Induced Apoptosis in Breast Cancer Cells. Oncol. Lett. 2013, 6, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-Y.; Cho, K.-B.; Choi, H.-S.; Han, H.-K.; Kang, K.-W. Role of FoxO1 Activation in MDR1 Expression in Adriamycin-Resistant Breast Cancer Cells. Carcinogenesis 2008, 29, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.C.-Y.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W.-F. Doxorubicin Activates FOXO3a to Induce the Expression of Multidrug Resistance Gene ABCB1 (MDR1) in K562 Leukemic Cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef]
- Goto, T.; Takano, M.; Hirata, J.; Tsuda, H. The Involvement of FOXO1 in Cytotoxic Stress and Drug-Resistance Induced by Paclitaxel in Ovarian Cancers. Br. J. Cancer 2008, 98, 1068–1075. [Google Scholar] [CrossRef]
- Naka, K.; Hoshii, T.; Muraguchi, T.; Tadokoro, Y.; Ooshio, T.; Kondo, Y.; Nakao, S.; Motoyama, N.; Hirao, A. TGF-B–FOXO Signalling Maintains Leukaemia- Initiating Cells in Chronic Myeloid Leukaemia. Nature 2010, 463, 676–680. [Google Scholar] [CrossRef]
- Sykes, S.M.; Lane, S.W.; Bullinger, L.; Kalaitzidis, D.; Yusuf, R.; Saez, B.; Ferraro, F.; Mercier, F.; Singh, H.; Brumme, K.M.; et al. AKT/FOXO Signaling Enforces Reversible Differentiation Blockade in Myeloid Leukemias. Cell 2011, 146, 697–708. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s Contributions to Current Concepts of Cancer Metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Ancey, P.-B.; Contat, C.; Meylan, E. Glucose Transporters in Cancer—From Tumor Cells to the Tumor Microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Gordan, J.D.; Thompson, C.B.; Simon, M.C. HIF and C-Myc: Sibling Rivals for Control of Cancer Cell Metabolism and Proliferation. Cancer Cell 2007, 12, 108–113. [Google Scholar] [CrossRef]
- Peck, B.; Ferber, E.C.; Schulze, A. Antagonism between FOXO and MYC Regulates Cellular Powerhouse. Front. Oncol. 2013, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. The Interplay between MYC and HIF in the Warburg Effect. Ernst Scher. Found. Symp. Proc. 2007, 35–53. [Google Scholar]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef]
- Delpuech, O.; Griffiths, B.; East, P.; Essafi, A.; Lam, E.W.-F.; Burgering, B.; Downward, J.; Schulze, A. Induction of Mxi1-SR Alpha by FOXO3a Contributes to Repression of Myc-Dependent Gene Expression. Mol. Cell. Biol. 2007, 27, 4917–4930. [Google Scholar] [CrossRef]
- Gan, B.; Lim, C.; Chu, G.; Hua, S.; Ding, Z.; Collins, M.; Hu, J.; Jiang, S.; Fletcher-Sananikone, E.; Zhuang, L.; et al. FoxOs Enforce a Progression Checkpoint to Constrain mTORC1-Activated Renal Tumorigenesis. Cancer Cell 2010, 18, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Marquardt, J.; Bras, A.; Medema, R.H.; Eilers, M. Myc-Induced Proliferation and Transformation Require Akt-Mediated Phosphorylation of FoxO Proteins. EMBO J. 2004, 23, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- Kress, T.R.; Cannell, I.G.; Brenkman, A.B.; Samans, B.; Gaestel, M.; Roepman, P.; Burgering, B.M.; Bushell, M.; Rosenwald, A.; Eilers, M. The MK5/PRAK Kinase and Myc Form a Negative Feedback Loop That Is Disrupted During Colorectal Tumorigenesis. Mol. Cell 2011, 41, 445–457. [Google Scholar] [CrossRef]
- Ferber, E.C.; Peck, B.; Delpuech, O.; Bell, G.P.; East, P.; Schulze, A. FOXO3a Regulates Reactive Oxygen Metabolism by Inhibiting Mitochondrial Gene Expression. Cell Death Differ. 2012, 19, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-D.; Han, L.; Lee, H.; Zhuang, L.; Zhang, Y.; Baddour, J.; Nagrath, D.; Wood, C.G.; Gu, J.; Wu, X.; et al. Energy Stress-Induced lncRNA FILNC1 Represses C-Myc-Mediated Energy Metabolism and Inhibits Renal Tumor Development. Nat. Commun. 2017, 8, 783. [Google Scholar] [CrossRef]
- Masui, K.; Tanaka, K.; Akhavan, D.; Babic, I.; Gini, B.; Matsutani, T.; Iwanami, A.; Liu, F.; Villa, G.R.; Gu, Y.; et al. mTOR Complex 2 Controls Glycolytic Metabolism in Glioblastoma Through FoxO Acetylation and Upregulation of C-Myc. Cell Metab. 2013, 18, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Amente, S.; Zhang, J.; Lavadera, M.L.; Lania, L.; Avvedimento, E.V.; Majello, B. Myc and PI3K/AKT Signaling Cooperatively Repress FOXO3a-Dependent PUMA and GADD45a Gene Expression. Nucleic Acids Res. 2011, 39, 9498–9507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tu, B.; Wang, H.; Cao, Z.; Tang, M.; Zhang, C.; Gu, B.; Li, Z.; Wang, L.; Yang, Y.; et al. Tumor Suppressor P53 Cooperates with SIRT6 to Regulate Gluconeogenesis by Promoting FoxO1 Nuclear Exclusion. Proc. Natl. Acad. Sci. USA 2014, 111, 10684–10689. [Google Scholar] [CrossRef]
- Khan, M.W.; Chakrabarti, P. Gluconeogenesis Combats Cancer: Opening New Doors in Cancer Biology. Cell Death Dis. 2015, 6, e1872. [Google Scholar] [CrossRef]
- Stein, W.H.; Moore, S. The Free Amino Acids of Human Blood Plasma. J. Biol. Chem. 1954, 211, 915–926. [Google Scholar]
- Biolo, G.; Fleming, R.Y.; Maggi, S.P.; Wolfe, R.R. Transmembrane Transport and Intracellular Kinetics of Amino Acids in Human Skeletal Muscle. Am. J. Physiol. 1995, 268, E75–E84. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive Carboxylation Supports Growth in Tumour Cells with Defective Mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino Acid Transporters ASCT2 and LAT1 in Cancer: Partners in Crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shi, X.; Meng, G.; Chen, J.; Yan, C.; Jiang, Y.; Wei, J.; Ding, Y. Kidney-Type Glutaminase (GLS1) Is a Biomarker for Pathologic Diagnosis and Prognosis of Hepatocellular Carcinoma. Oncotarget 2015, 6, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Mou, J.; Shao, B.; Wei, Y.; Liang, H.; Takano, N.; Semenza, G.L.; Xie, G. Glutaminase 1 Expression in Colorectal Cancer Cells Is Induced by Hypoxia and Required for Tumor Growth, Invasion, and Metastatic Colonization. Cell Death Dis. 2019, 10, 40. [Google Scholar] [CrossRef]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc Regulates a Transcriptional Program That Stimulates Mitochondrial Glutaminolysis and Leads to Glutamine Addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and Cancer: Cell Biology, Physiology, and Clinical Opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Issaq, S.H.; Mendoza, A.; Fox, S.D.; Helman, L.J. Glutamine Synthetase Is Necessary for Sarcoma Adaptation to Glutamine Deprivation and Tumor Growth. Oncogenesis 2019, 8, 20. [Google Scholar] [CrossRef]
- Long, J.; Lang, Z.-W.; Wang, H.-G.; Wang, T.-L.; Wang, B.-E.; Liu, S.-Q. Glutamine Synthetase as an Early Marker for Hepatocellular Carcinoma Based on Proteomic Analysis of Resected Small Hepatocellular Carcinomas. Hepatobiliary Pancreat Dis. Int. 2010, 9, 296–305. [Google Scholar]
- Rosati, A.; Poliani, P.L.; Todeschini, A.; Cominelli, M.; Medicina, D.; Cenzato, M.; Simoncini, E.L.; Magrini, S.M.; Buglione, M.; Grisanti, S.; et al. Glutamine Synthetase Expression as a Valuable Marker of Epilepsy and Longer Survival in Newly Diagnosed Glioblastoma Multiforme. Neuro Oncol. 2013, 15, 618–625. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Coffer, P.J. Glutamine Metabolism Links Growth Factor Signaling to the Regulation of Autophagy. Cell Death Differ. 2012, 8, 1862–1864. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent Insights into the Function of Autophagy in Cancer. Genes Dev. 2016, 30, 1913–1930. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in Malignant Transformation and Cancer Progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Eisenberg-Lerner, A.; Kimchi, A. The Paradox of Autophagy and Its Implication in Cancer Etiology and Therapy. Apoptosis 2009, 14, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liao, W.; Liu, X.; Zhang, H.; Wang, S.; Wang, D.; Feng, J.; Yu, L.; Zhu, W.-G. Cytosolic FoxO1 Is Essential for the Induction of Autophagy and Tumour Suppressor Activity. Nat. Cell Biol. 2010, 12, 665–675. [Google Scholar] [CrossRef]
- Fitzwalter, B.E.; Thorburn, A. Autophagy Inhibition Improves Anti-Cancer Drugs via FOXO3a Activation. Oncotarget 2018, 9, 25384–25385. [Google Scholar] [CrossRef] [PubMed]
- Fitzwalter, B.E.; Towers, C.G.; Sullivan, K.D.; Andrysik, Z.; Hoh, M.; Ludwig, M.; O’Prey, J.; Ryan, K.M.; Espinosa, J.M.; Morgan, M.J.; et al. Autophagy Inhibition Mediates Apoptosis Sensitization in Cancer Therapy by Relieving FOXO3a Turnover. Dev. Cell 2018, 44, 555–565.e3. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid Metabolism in Cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Lipid Metabolism in Cancer Cells under Metabolic Stress. Br. J. Cancer 2019, 120, 1090–1098. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, W.; O-Sullivan, I.; Williams, J.B.; Dong, Q.; Park, E.A.; Raghow, R.; Unterman, T.G.; Elam, M.B. FoxO1 Inhibits Sterol Regulatory Element-Binding Protein-1c (SREBP-1c) Gene Expression via Transcription Factors Sp1 and SREBP-1c. J. Biol. Chem. 2012, 287, 20132–20143. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Kandror, K.V. FoxO1 Controls Insulin-Dependent Adipose Triglyceride Lipase (ATGL) Expression and Lipolysis in Adipocytes. J. Biol. Chem. 2009, 284, 13296–13300. [Google Scholar] [CrossRef]
- Shao, H.; Mohamed, E.M.; Xu, G.G.; Waters, M.; Jing, K.; Ma, Y.; Zhang, Y.; Spiegel, S.; Idowu, M.O.; Fang, X. Carnitine Palmitoyltransferase 1A Functions to Repress FoxO Transcription Factors to Allow Cell Cycle Progression in Ovarian Cancer. Oncotarget 2016, 7, 3832–3846. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in Cancer: Initiators, Amplifiers or an Achilles’ Heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Storz, P. Reactive Oxygen Species in Tumor Progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective Killing of Oncogenically Transformed Cells Through a ROS-Mediated Mechanism by Beta-Phenylethyl Isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-Dependent Antitumorigenic Effect of Antioxidants in Vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Putker, M.; Vos, H.R.; van Dorenmalen, K.; de Ruiter, H.; Duran, A.G.; Snel, B.; Burgering, B.M.T.; Vermeulen, M.; Dansen, T.B. Evolutionary Acquisition of Cysteines Determines FOXO Paralog-Specific Redox Signaling. Antioxid. Redox Signal 2015, 22, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Putker, M.; Madl, T.; Vos, H.R.; de Ruiter, H.; Visscher, M.; van den Berg, M.C.W.; Kaplan, M.; Korswagen, H.C.; Boelens, R.; Vermeulen, M.; et al. Redox-Dependent Control of FOXO/DAF-16 by Transportin-1. Mol. Cell 2013, 49, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hartmann, D.; Braren, R.; Gupta, A.; Wang, B.; Wang, Y.; Mogler, C.; Cheng, Z.; Wirth, T.; Friess, H.; et al. Oncogenic Akt-FOXO3 Loop Favors Tumor-Promoting Modes and Enhances Oxidative Damage-Associated Hepatocellular Carcinogenesis. BMC Cancer 2019, 19, 887. [Google Scholar] [CrossRef]
- Breier, G. Angiogenesis in Embryonic Development--a Review. Placenta 2000, 21 (Suppl. A), S11–S15. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in Wound Repair: Angiogenic Growth Factors and the Extracellular Matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and Wound Repair: When Enough Is Enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Eichhorn, M.E.; Kleespies, A.; Angele, M.K.; Jauch, K.-W.; Bruns, C.J. Angiogenesis in Cancer: Molecular Mechanisms, Clinical Impact. Langenbecks Arch. Surg. 2007, 392, 371–379. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Lu, W.; Chen, M.; Ye, W.; Zhang, D. The Tumor Vessel Targeting Strategy: A Double-Edged Sword in Tumor Metastasis. Cells 2019, 8, 1602. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Pavlakovic, H.; Havers, W.; Schweigerer, L. Multiple Angiogenesis Stimulators in a Single Malignancy: Implications for Anti-Angiogenic Tumour Therapy. Angiogenesis 2001, 4, 259–262. [Google Scholar] [CrossRef]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular Endothelial Growth Factor (VEGF) and Its Receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Mezquita, P.; Parghi, S.S.; Brandvold, K.A.; Ruddell, A. Myc Regulates VEGF Production in B Cells by Stimulating Initiation of VEGF mRNA Translation. Oncogene 2005, 24, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Mizejewski, G.J. Role of Integrins in Cancer: Survey of Expression Patterns. Proc. Soc. Exp. Biol. Med. 1999, 222, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular Endothelial Growth Factor-C-Mediated Lymphangiogenesis Promotes Tumour Metastasis. EMBO J. 2001, 20, 672–682. [Google Scholar] [CrossRef]
- Rafii, S.; Skobe, M. Splitting Vessels: Keeping Lymph Apart from Blood. Nat. Med. 2003, 9, 166–168. [Google Scholar] [CrossRef]
- Hosaka, T.; Biggs, W.H.; Tieu, D.; Boyer, A.D.; Varki, N.M.; Cavenee, W.K.; Arden, K.C. Disruption of Forkhead Transcription Factor (FOXO) Family Members in Mice Reveals Their Functional Diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 2975–2980. [Google Scholar] [CrossRef]
- Furuyama, T.; Kitayama, K.; Shimoda, Y.; Ogawa, M.; Sone, K.; Yoshida-Araki, K.; Hisatsune, H.; Nishikawa, S.-I.; Nakayama, K.; Nakayama, K.; et al. Abnormal Angiogenesis in Foxo1 (Fkhr)-Deficient Mice. J. Biol. Chem. 2004, 279, 34741–34749. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.R.; Guo, S.; Minami, T.; Spokes, K.C.; Ueki, K.; Skurk, C.; Walsh, K.; Aird, W.C. Vascular Endothelial Growth Factor Activates PI3K/Akt/Forkhead Signaling in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.R.; Shih, S.-C.; Otu, H.H.; Spokes, K.C.; Okada, Y.; Curiel, D.T.; Minami, T.; Aird, W.C. A Novel Class of Vascular Endothelial Growth Factor-Responsive Genes That Require Forkhead Activity for Expression. J. Biol. Chem. 2006, 281, 35544–35553. [Google Scholar] [CrossRef]
- Skurk, C.; Maatz, H.; Kim, H.-S.; Yang, J.; Abid, M.R.; Aird, W.C.; Walsh, K. The Akt-Regulated Forkhead Transcription Factor FOXO3a Controls Endothelial Cell Viability Through Modulation of the Caspase-8 Inhibitor FLIP. J. Biol. Chem. 2004, 279, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Fisslthaler, B.; Busse, R.; Fleming, I. 11,12-Epoxyeicosatrienoic Acid-Induced Inhibition of FOXO Factors Promotes Endothelial Proliferation by Down-Regulating p27Kip1. J. Biol. Chem. 2003, 278, 29619–29625. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Knau, A.; Fichtlscherer, S.; Walter, D.H.; Bruhl, T.; Potente, M.; Hofmann, W.K.; de Vos, S.; Zeiher, A.M.; Dimmeler, S. FOXO-Dependent Expression of the Proapoptotic Protein Bim: Pivotal Role for Apoptosis Signaling in Endothelial Progenitor Cells. FASEB J. 2005, 19, 974–976. [Google Scholar] [CrossRef]
- Potente, M.; Urbich, C.; Sasaki, K.-I.; Hofmann, W.K.; Heeschen, C.; Aicher, A.; Kollipara, R.; DePinho, R.A.; Zeiher, A.M.; Dimmeler, S. Involvement of Foxo Transcription Factors in Angiogenesis and Postnatal Neovascularization. J. Clin. Investig. 2005, 115, 2382–2392. [Google Scholar] [CrossRef]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 Couples Metabolic Activity and Growth State in the Vascular Endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, J.; Ko, Y.S.; Chang, M.S.; Park, J.-W.; Lee, H.E.; Kim, M.A.; Kim, J.H.; Kim, W.H.; Lee, B.L. Constitutive Phosphorylation of the FOXO1 Transcription Factor in Gastric Cancer Cells Correlates with Microvessel Area and the Expressions of Angiogenesis-Related Molecules. BMC Cancer 2011, 11, 264. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ko, Y.S.; Park, J.; Choi, Y.; Park, J.-W.; Kim, Y.; Pyo, J.-S.; Yoo, Y.B.; Lee, J.-S.; Lee, B.L. Forkhead Transcription Factor FOXO1 Inhibits Angiogenesis in Gastric Cancer in Relation to SIRT1. Cancer Res. Treat 2016, 48, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.-L.; Lasky, L.A. The Forkhead Transcription Factor FOXO4 Induces the Down-Regulation of Hypoxia-Inducible Factor 1 Alpha by a Von Hippel-Lindau Protein-Independent Mechanism. J. Biol. Chem. 2003, 278, 30125–30135. [Google Scholar] [CrossRef] [PubMed]
- Karadedou, C.T.; Gomes, A.R.; Chen, J.; Petkovic, M.; Ho, K.K.; Zwolinska, A.K.; Feltes, A.; Wong, S.Y.; Chan, K.Y.K.; Cheung, Y.-N.; et al. FOXO3a Represses VEGF Expression Through FOXM1-Dependent and -Independent Mechanisms in Breast Cancer. Oncogene 2012, 31, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.; Zhan, S.; Chen, L.; Wang, Y.; Zhang, Y.; Du, J.; Wu, Y.; Gu, L. Arsenic Trioxide Suppressed Migration and Angiogenesis by Targeting FOXO3a in Gastric Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3739. [Google Scholar] [CrossRef]
- Hagenbuchner, J.; Rupp, M.; Salvador, C.; Meister, B.; Kiechl-Kohlendorfer, U.; Muller, T.; Geiger, K.; Sergi, C.; Obexer, P.; Ausserlechner, M.J. Nuclear FOXO3 Predicts Adverse Clinical Outcome and Promotes Tumor Angiogenesis in Neuroblastoma. Oncotarget 2016, 7, 77591–77606. [Google Scholar] [CrossRef]
- Li, J.; Wang, E.; Rinaldo, F.; Datta, K. Upregulation of VEGF-C by Androgen Depletion: The Involvement of IGF-IR-FOXO Pathway. Oncogene 2005, 24, 5510–5520. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor Microvasculature and Microenvironment: Targets for Anti-Angiogenesis and Normalization. Microvasc. Res. 2007, 74, 72–84. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; Silva, M.; Xingan, X.; Huang, Y.; Zheng, W. Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis. Cells 2020, 9, 1586. https://doi.org/10.3390/cells9071586
Farhan M, Silva M, Xingan X, Huang Y, Zheng W. Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis. Cells. 2020; 9(7):1586. https://doi.org/10.3390/cells9071586
Chicago/Turabian StyleFarhan, Mohd, Marta Silva, Xing Xingan, Yu Huang, and Wenhua Zheng. 2020. "Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis" Cells 9, no. 7: 1586. https://doi.org/10.3390/cells9071586
APA StyleFarhan, M., Silva, M., Xingan, X., Huang, Y., & Zheng, W. (2020). Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis. Cells, 9(7), 1586. https://doi.org/10.3390/cells9071586





