Overlap of Peak Growth Activity and Peak IGF-1 to IGFBP Ratio: Delayed Increase of IGFBPs Versus IGF-1 in Serum as a Mechanism to Speed up and down Postnatal Weight Gain in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Husbandry, and Study Design
2.2. Longitudinal Analysis of IGFs and IGFBPs in Mouse Serum
2.3. Statistical Analyses
3. Results
3.1. Longitudinal Growth in Non-Inbred Mouse Models
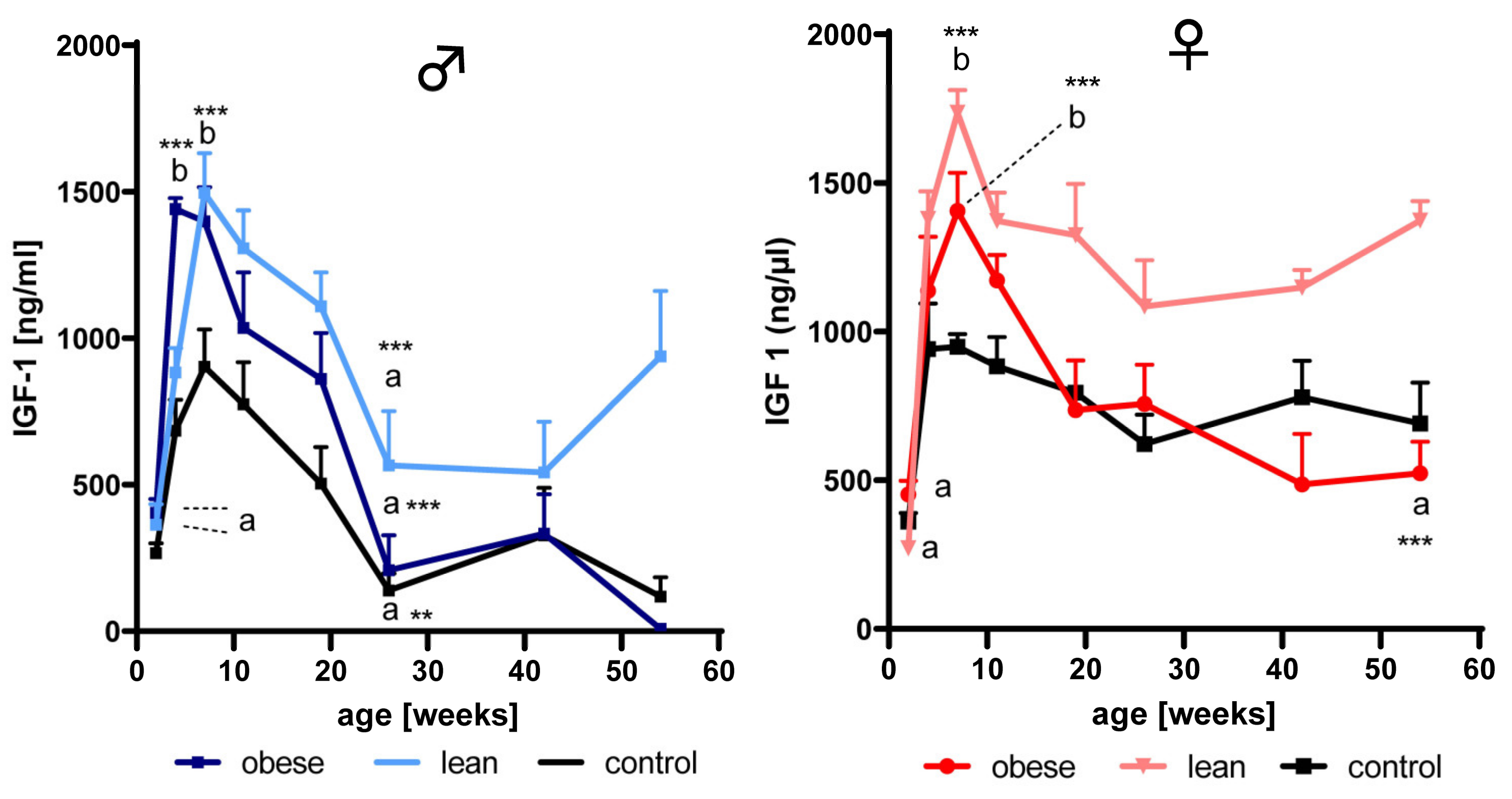
3.2. Effects of Age and Growth Selection on the Concentrations of IGF-1
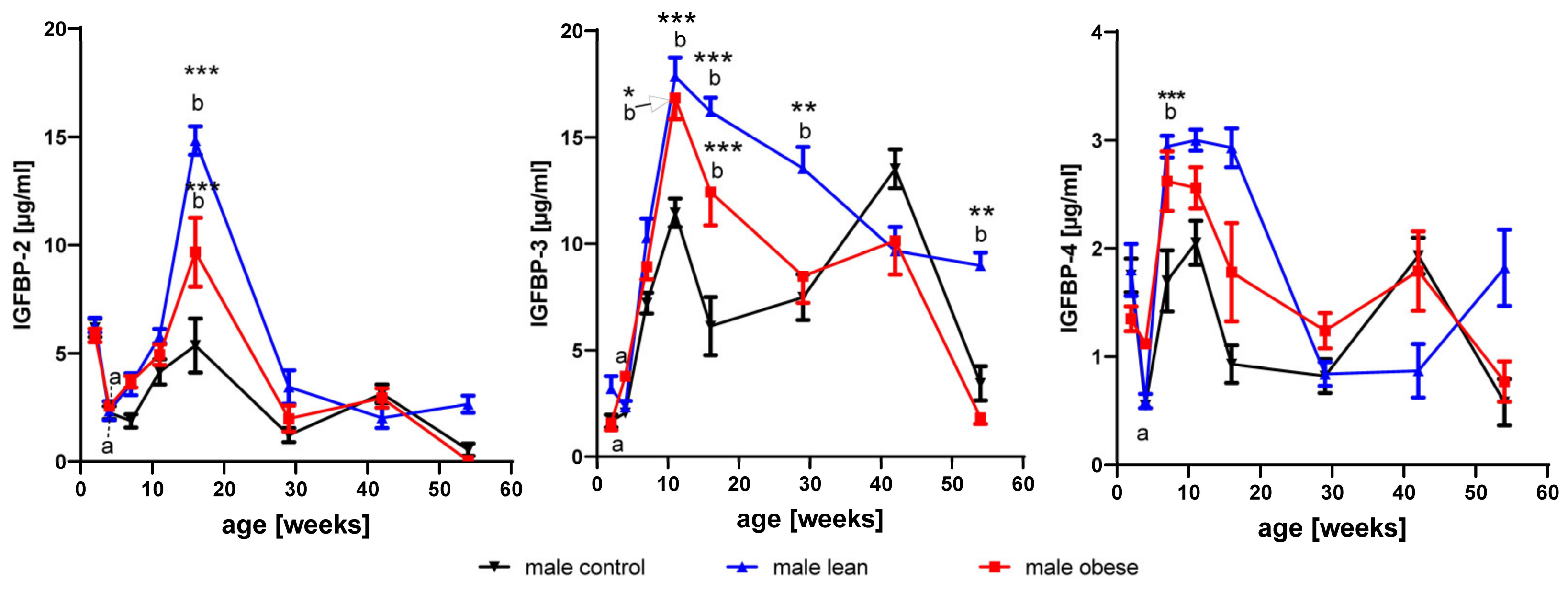
3.3. Effects of Age and Gender on Levels of IGFBP2- to 4
3.3.1. IGFBP-2
3.3.2. IGFBP-3
3.3.3. IGFBP-4
3.4. Effects of Growth Selection on the Concentrations of IGFBP-2 to -4
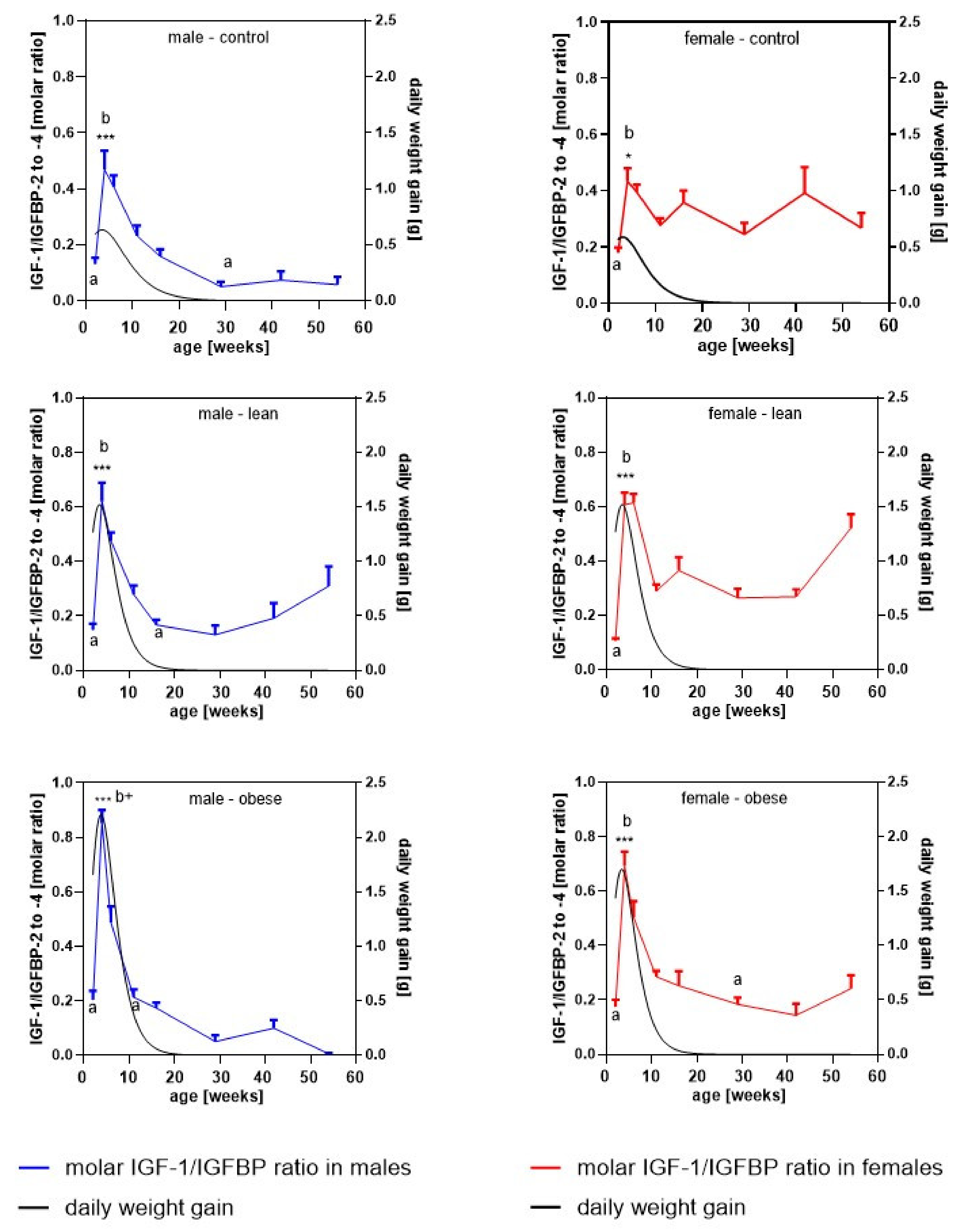
3.5. Longitudinal Molar Ratio of IGF-1 and IGFBP Concentrations in Serum
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodale, H.D. A Study of the Inheritance of Body Weight in the Albino Mouse by Selection. J. Hered. 1938, 29, 101–112. [Google Scholar] [CrossRef]
- Wilson, S.P.; Goodale, H.D.; Kyle, W.H.; Godfrey, E.F. Long term selection for bodyweight in mice. J. Hered. 1971, 62, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Renne, U.; Langhammer, M.; Brenmoehl, J.; Walz, C.; Zeissler, A.; Tuchscherer, A.; Piechotta, M.; Wiesner, R.J.; Bielohuby, M.; Hoeflich, A. Lifelong Obesity in a Polygenic Mouse Model Prevents Age- and Diet-Induced Glucose Intolerance– Obesity Is No Road to Late-Onset Diabetes in Mice. PLoS ONE 2013, 8, e79788. [Google Scholar] [CrossRef] [PubMed]
- Marouli, E.; The EPIC-InterAct Consortium; Graff, M.; Medina-Gomez, C.; Lo, K.S.; Wood, A.R.; Kjaer, T.R.; Fine, R.; Lu, Y.; Schurmann, C. Rare and low-frequency coding variants alter human adult height. Nature 2017, 542, 186–190. [Google Scholar] [CrossRef]
- Brockmann, A.G.; Haley, C.S.; Renne, U.; Knott, A.S.; Schwerin, M. Quantitative trait loci affecting body weight and fatness from a mouse line selected for extreme high growth. Genetics 1998, 150, 369–381. [Google Scholar]
- Bunger, L.; Laidlaw, A.; Bulfield, G.; Eisen, E.J.; Medrano, J.F.; Bradford, G.E.; Pirchner, F.; Renne, U.; Schlote, W.; Hill, W.G. Inbred lines of mice derived from long-term growth selected lines: Unique resources for mapping growth genes. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2001, 12, 678–686. [Google Scholar] [CrossRef]
- Bunger, L.; Renne, U.; Dietl, G.; Kuhla, S. Long-term selection for protein amount over 70 generations in mice. Genet. Res. 1998, 72, 93–109. [Google Scholar] [CrossRef]
- Sawitzky, M.; Zeissler, A.; Langhammer, M.; Bielohuby, M.; Stock, P.; Hammon, H.M.; Görs, S.; Metges, C.C.; Stoehr, B.J.M.; Bidlingmaier, M.; et al. Phenotype Selection Reveals Coevolution of Muscle Glycogen and Protein and PTEN as a Gate Keeper for the Accretion of Muscle Mass in Adult Female Mice. PLoS ONE 2012, 7, e39711. [Google Scholar] [CrossRef]
- Schüler, L. Der Mäuseauszuchtstamm Fzt:DU und seine Anwendung als Modell in der Tierzuchtforschung. Arch. Tierz. 1985, 28, 357–363. [Google Scholar]
- Dietl, G.; Langhammer, M.; Renne, U. Model simulations for genetic random drift in the outbred strain Fzt: DU. Arch. Tierz. 2004, 47, 595–604. [Google Scholar] [CrossRef]
- Hoeflich, A.; Bunger, L.; Nedbal, S.; Renne, U.; Elmlinger, M.W.; Blum, W.F.; Bruley, C.; Kolb, H.J.; Wolf, E. Growth selection in mice reveals conserved and redundant expression patterns of the insulin-like growth factor system. Gen. Comp. Endocrinol. 2004, 136, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Ahmed, I.; Liu, L.; Liu, Y.; Xu, Z.; Duan, X.; Li, Q.; Dou, T.; Gu, D.; Rong, H.; et al. Selection for growth rate and body size have altered the expression profiles of somatotropic axis genes in chickens. PLoS ONE 2018, 13, e0195378. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.T.; Freriksen, J.; Van Bijnen, A.; Gerritsen, C.; Bosch, T.V.D.; Harders, F.; Verburg, F.; Visscher, A.; De Greef, K. Selection for growth rate or against back fat thickness in pigs is associated with changes in growth hormone axis plasma protein concentration and mRNA level. Domest. Anim. Endocrinol. 2001, 20, 165–184. [Google Scholar] [CrossRef]
- Anh, N.T.L.; Kunhareang, S.; Duangjinda, M. Association of Chicken Growth Hormones and Insulin-like Growth Factor Gene Polymorphisms with Growth Performance and Carcass Traits in Thai Broilers. Asian Australas. J. Anim. Sci. 2015, 28, 1686–1695. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Saleh, A.A.; Nafeaa, A.A.; El-Komy, S.M.; Afifi, M. Polymorphisms of the IGF1 gene and their association with growth traits, serum concentration and expression rate of IGF1 and IGF1R in buffalo. J. Zhejiang Univ. Sci. B 2017, 18, 1064–1074. [Google Scholar] [CrossRef]
- Bidlingmaier, M.; Friedrich, N.; Emeny, R.T.; Spranger, J.; Wolthers, O.D.; Roswall, J.; Körner, A.; Obermayer-Pietsch, B.; Hübener, C.; Dahlgren, J.; et al. Reference Intervals for Insulin-like Growth Factor-1 (IGF-I) From Birth to Senescence: Results From a Multicenter Study Using a New Automated Chemiluminescence IGF-I Immunoassay Conforming to Recent International Recommendations. J. Clin. Endocrinol. Metab. 2014, 99, 1712–1721. [Google Scholar] [CrossRef]
- Friedrich, N.; Wolthers, O.D.; Arafat, A.; Emeny, R.T.; Spranger, J.; Roswall, J.; Kratzsch, J.; Grabe, H.J.; Hübener, C.; Pfeiffer, A.F.; et al. Age- and Sex-Specific Reference Intervals Across Life Span for Insulin-Like Growth Factor Binding Protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 Ratio Measured by New Automated Chemiluminescence Assays. J. Clin. Endocrinol. Metab. 2014, 99, 1675–1686. [Google Scholar] [CrossRef]
- Hossenlopp, P.; Segovia, B.; Lassarre, C.; Roghani, M.; Bredon, M.; Binoux, M. Evidence of Enzymatic Degradation of Insulin-Like Growth Factor-Binding Proteins in the 150K Complex during Pregnancy. J. Clin. Endocrinol. Metab. 1990, 71, 797–805. [Google Scholar] [CrossRef]
- Hoeflich, A.; David, R.; Hjortebjerg, R. Current IGFBP-Related Biomarker Research in Cardiovascular Disease—We Need More Structural and Functional Information in Clinical Studies. Front. Endocrinol. 2018, 9, 388. [Google Scholar] [CrossRef]
- Lawrence, J.B.; Oxvig, C.; Overgaard, M.T.; Sottrup-Jensen, L.; Gleich, G.J.; Hays, L.G.; Yates, J.R.; Conover, C.A. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-a. Proc. Natl. Acad. Sci. USA 1999, 96, 3149–3153. [Google Scholar] [CrossRef]
- Botkjaer, J.A.; Noer, P.R.; Oxvig, C.; Yding Andersen, C. A common variant of the pregnancy-associated plasma protein-a (pappa) gene encodes a protein with reduced proteolytic activity towards igf-binding proteins. Sci. Rep. 2019, 9, 13231. [Google Scholar] [CrossRef]
- Conover, C.A.; Oxvig, C. 40 YEARS OF IGF1: PAPP-A and cancer. J. Mol. Endocrinol. 2018, 61, T1–T10. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Khoury, J.C.; Khoury, P.R.; Kalra, B.; Kumar, A.; Sluss, P.; Oxvig, C.; Hwa, V.; Dauber, A. Anthropometric and biochemical correlates of PAPP-A2, free IGF-I, and IGFBP-3 in childhood. Eur. J. Endocrinol. 2020, 182, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Saenger, S.; Goeldner, C.; Frey, J.; Ozmen, L.; Ostrowitzki, S.; Spooren, W.; Ballard, T.; Prinssen, E.; Borroni, E.; Metzger, F. PEGylation enhances the therapeutic potential for insulin-like growth factor I in central nervous system disorders. Growth Horm. IGF Res. 2011, 21, 292–303. [Google Scholar] [CrossRef]
- Wirthgen, E.; Höflich, C.; Spitschak, M.; Helmer, C.; Brand, B.; Langbein, J.; Metzger, F.; Hoeflich, A. Quantitative Western ligand blotting reveals common patterns and differential features of IGFBP-fingerprints in domestic ruminant breeds and species. Growth Horm. IGF Res. 2016, 26, 42–49. [Google Scholar] [CrossRef]
- Efstratiadis, A. Genetics of mouse growth. Int. J. Dev. Biol. 1998, 42, 955–976. [Google Scholar]
- Pintar, J.E.; Cerro, J.A.; Wood, T.L. Genetic Approaches to the Function of Insulin-Like Growth Factor-Binding Proteins during Rodent Development. Horm. Res. 1996, 45, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Leroith, D.; Roberts, J.C.T. Insulin-like Growth Factors. Ann. N. Y. Acad. Sci. 1993, 692, 1–9. [Google Scholar] [CrossRef]
- Timtchenko, D.; Kratzsch, J.; Sauerwein, H.; Wegner, J.; Souffrant, W.; Schwerin, M.; Brockmann, G. Fat storage capacity in growth-selected and control mouse lines is associated with line-specific gene expression and plasma hormone levels. Int. J. Obes. 1999, 23, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of Growth Hormone and Insulin-like Growth Factor 1 in Mouse Postnatal Growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Moerth, C.; Schneider, M.R.; Renner-Mueller, I.; Blutke, A.; Elmlinger, M.W.; Erben, R.G.; Camacho-Hübner, C.; Hoeflich, A.; Wolf, E. Postnatally Elevated Levels of Insulin-Like Growth Factor (IGF)-II Fail to Rescue the Dwarfism of IGF-I-Deficient Mice except Kidney Weight. Endocrinology 2007, 148, 441–451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogol, A.D.; A Clark, P.; Roemmich, J.N. Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am. J. Clin. Nutr. 2000, 72, S521–S528. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Fetus into Man: Physical Growth from Conception to Maturity; Harward University Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Bereket, A.; Turan, S.; Omar, A.; Berber, M.; Ozen, A.; Akbenlioglu, C.; Haklar, G. Serum IGF-I and IGFBP-3 Levels of Turkish Children during Childhood and Adolescence: Establishment of Reference Ranges with Emphasis on Puberty. Horm. Res. Paediatr. 2006, 65, 96–105. [Google Scholar] [CrossRef]
- Smith, W.J.; Nam, T.J.; Underwood, L.E.; Busby, W.H.; Celnicker, A.; Clemmons, D.R. Use of insulin-like growth factor-binding protein-2 (igfbp-2), igfbp-3, and igf-i for assessing growth hormone status in short children. J. Clin. Endocrinol. Metab. 1993, 77, 1294–1299. [Google Scholar]
- Mattsson, A.; Svensson, D.; Schuett, B.; Osterziel, K.J.; Ranke, M.B. Multidimensional reference regions for IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum of healthy adults. Growth Horm. IGF Res. 2008, 18, 506–516. [Google Scholar] [CrossRef]
- Beld, A.W.V.D.; Carlson, O.D.; Doyle, M.E.; Rizopoulos, D.; Ferrucci, L.; Van Der Lely, A.J.; Egan, J.M. IGFBP-2 and aging: A 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur. J. Endocrinol. 2019, 180, 109–116. [Google Scholar] [CrossRef]
- Van Doorn, J.; Cornelissen, A.J.F.H.; Van Buul-Offers, S.C. Plasma levels of insulin-like growth factor binding protein-4 (IGFBP-4) under normal and pathological conditions. Clin. Endocrinol. 2001, 54, 655–664. [Google Scholar] [CrossRef]
- Christians, J.K.; Hoeflich, A.; Keightley, P.D. PAPPA2, an Enzyme That Cleaves an Insulin-Like Growth-Factor-Binding Protein, Is a Candidate Gene for a Quantitative Trait Locus Affecting Body Size in Mice. Genetics 2006, 173, 1547–1553. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walz, M.; Chau, L.; Walz, C.; Sawitzky, M.; Ohde, D.; Brenmoehl, J.; Tuchscherer, A.; Langhammer, M.; Metzger, F.; Höflich, C.; et al. Overlap of Peak Growth Activity and Peak IGF-1 to IGFBP Ratio: Delayed Increase of IGFBPs Versus IGF-1 in Serum as a Mechanism to Speed up and down Postnatal Weight Gain in Mice. Cells 2020, 9, 1516. https://doi.org/10.3390/cells9061516
Walz M, Chau L, Walz C, Sawitzky M, Ohde D, Brenmoehl J, Tuchscherer A, Langhammer M, Metzger F, Höflich C, et al. Overlap of Peak Growth Activity and Peak IGF-1 to IGFBP Ratio: Delayed Increase of IGFBPs Versus IGF-1 in Serum as a Mechanism to Speed up and down Postnatal Weight Gain in Mice. Cells. 2020; 9(6):1516. https://doi.org/10.3390/cells9061516
Chicago/Turabian StyleWalz, Michael, Luong Chau, Christina Walz, Mandy Sawitzky, Daniela Ohde, Julia Brenmoehl, Armin Tuchscherer, Martina Langhammer, Friedrich Metzger, Christine Höflich, and et al. 2020. "Overlap of Peak Growth Activity and Peak IGF-1 to IGFBP Ratio: Delayed Increase of IGFBPs Versus IGF-1 in Serum as a Mechanism to Speed up and down Postnatal Weight Gain in Mice" Cells 9, no. 6: 1516. https://doi.org/10.3390/cells9061516
APA StyleWalz, M., Chau, L., Walz, C., Sawitzky, M., Ohde, D., Brenmoehl, J., Tuchscherer, A., Langhammer, M., Metzger, F., Höflich, C., & Hoeflich, A. (2020). Overlap of Peak Growth Activity and Peak IGF-1 to IGFBP Ratio: Delayed Increase of IGFBPs Versus IGF-1 in Serum as a Mechanism to Speed up and down Postnatal Weight Gain in Mice. Cells, 9(6), 1516. https://doi.org/10.3390/cells9061516





