Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
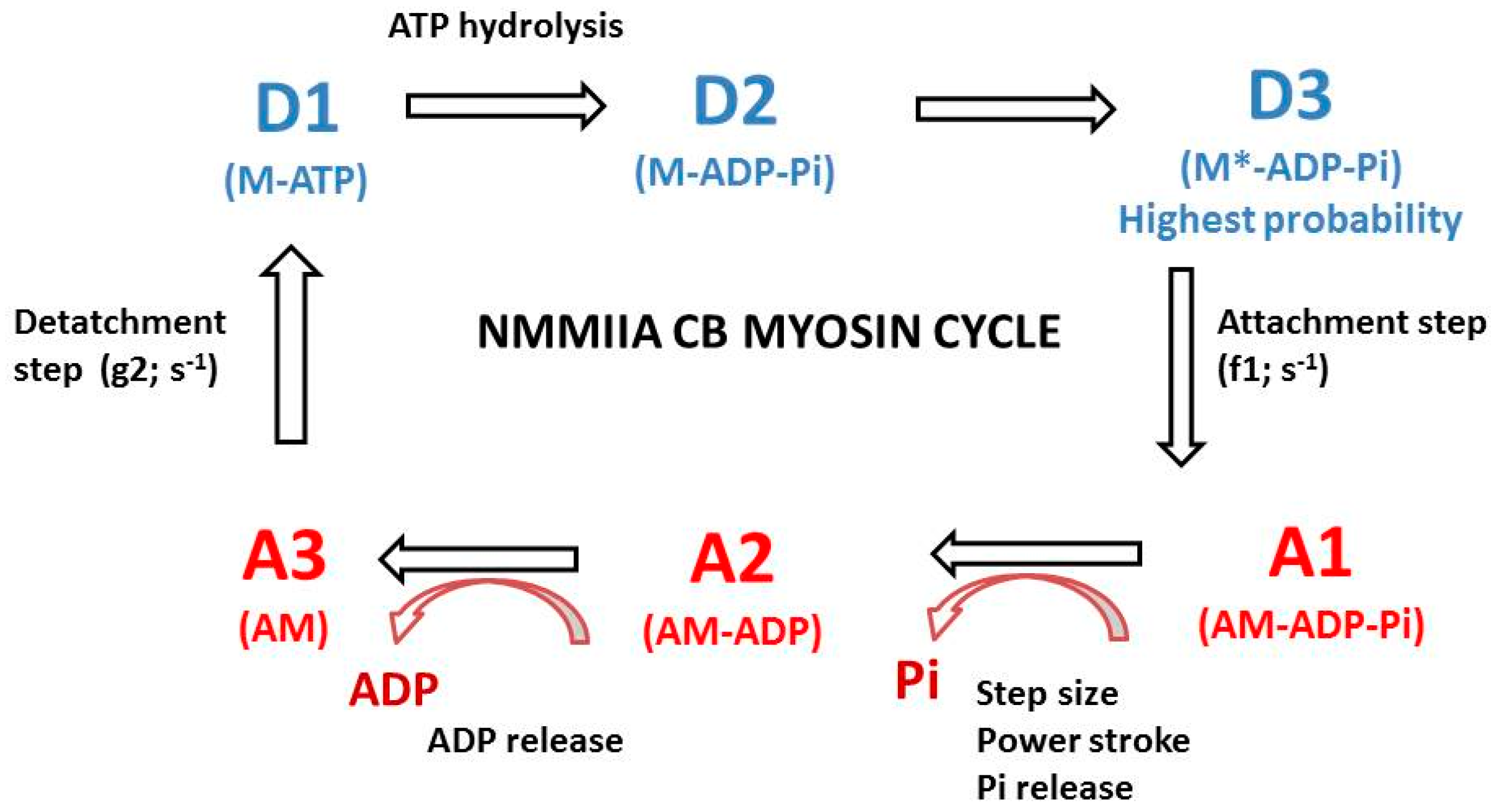
2.2. The A. Huxley Formalism
2.3. Computation of CB Probabilities of the 6 States of the NMMIIA-CB Cycle
2.4. Statistical Mechanics
2.5. Statistical Analysis
3. Results
3.1. Near-Equilibrium Collagen Scaffolds Operated in a Linear Stationary Regime
3.2. Mechanical Parameters of COL-MSC and COL-MSC-RGD Scaffolds
3.3. Probabilities of the Six Conformational Steps of the NMMIIA CB Cycle
3.4. Molecular NMMIIA-CB Parameters of COL-MSC and COL-MSC-RGD Scaffolds
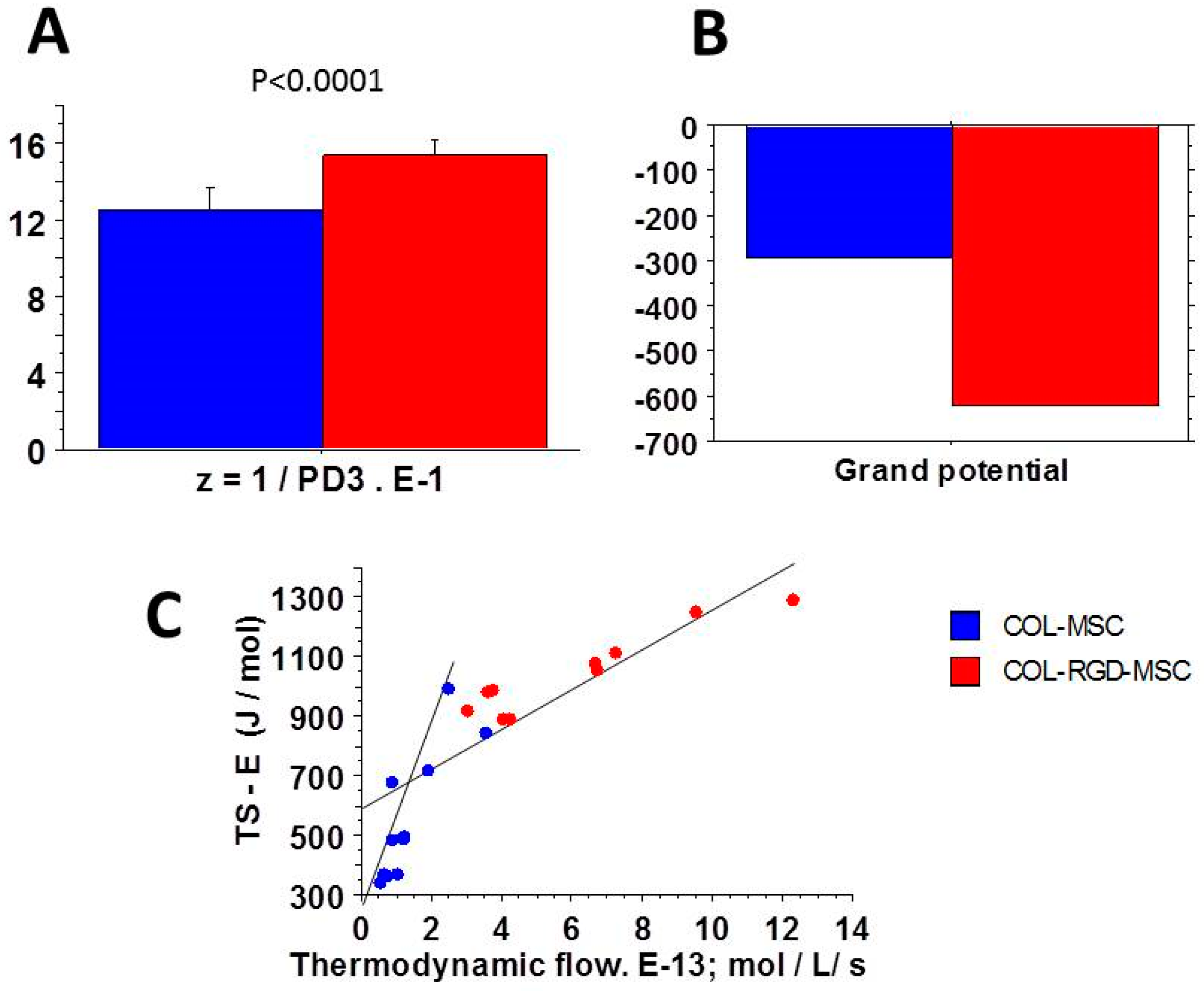
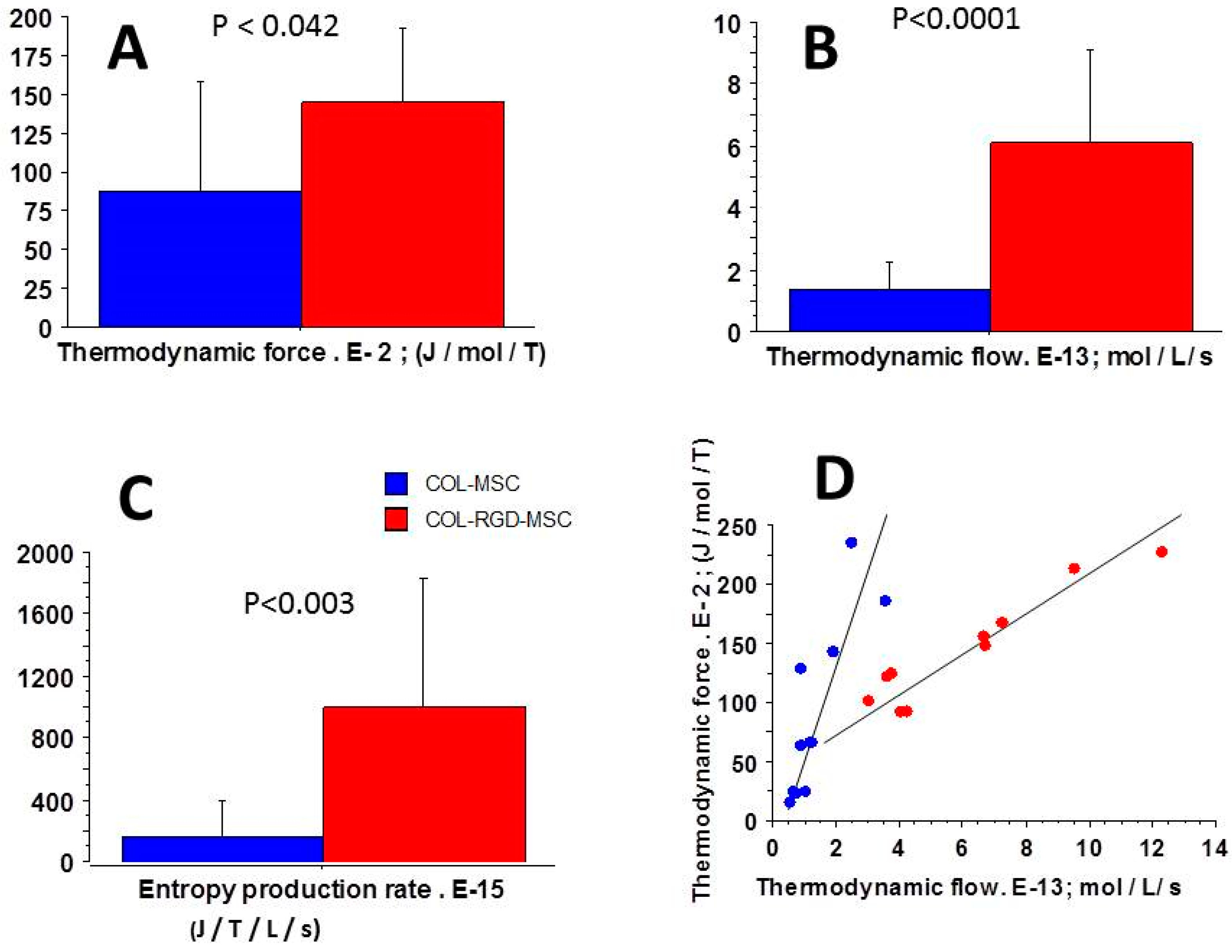
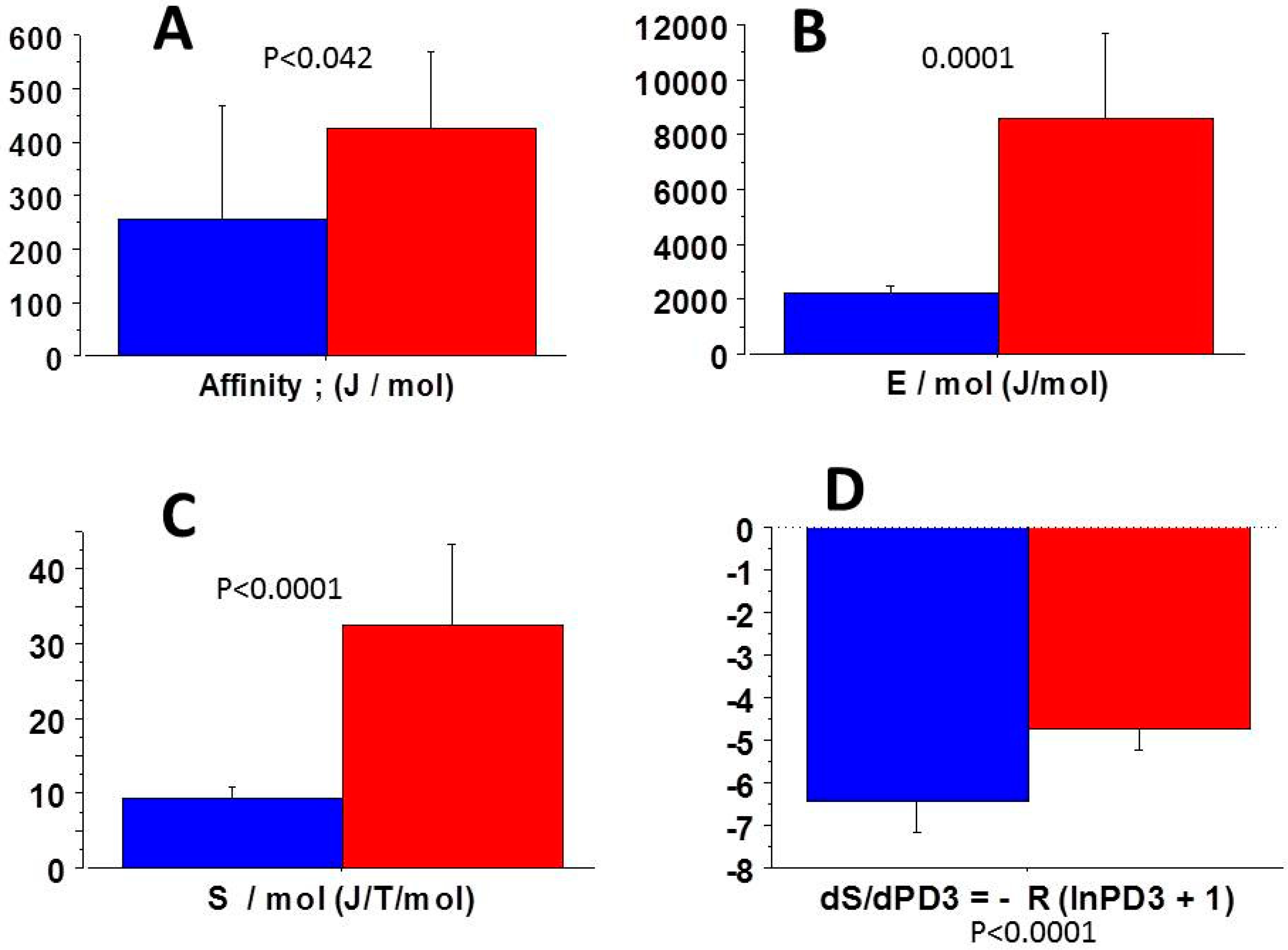
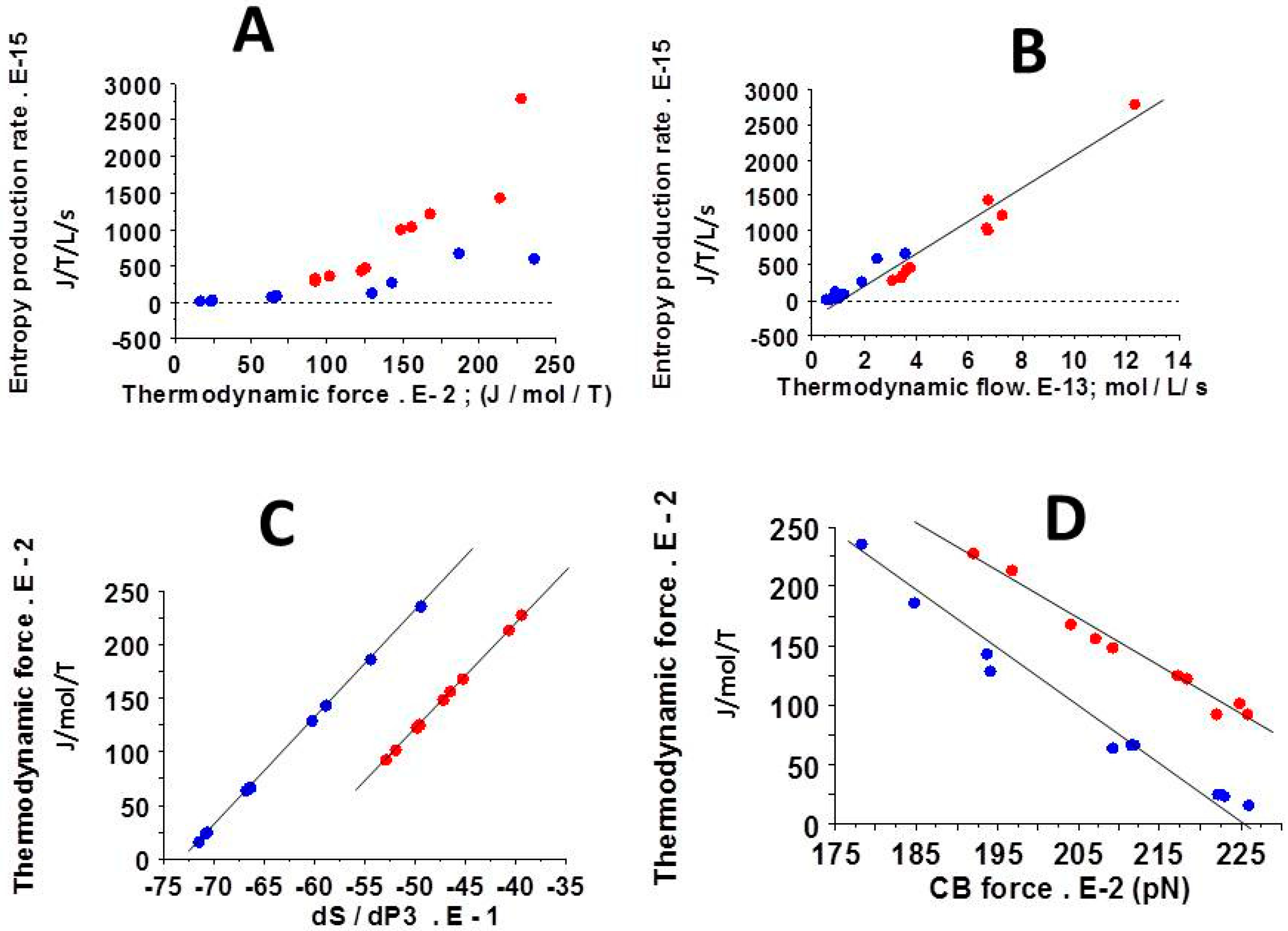
3.5. Relationships Between Thermodynamic Quantities
4. Discussion
4.1. Applying Statistical Mechanics (SM) to Collagen Scaffolds
4.2. Near-Equilibrium Thermodynamics
4.3. The Linear Stationary Regime
4.4. Entropy Production Rate
4.5. Probabilities of the Steps of the NMMIIA CB Cycle
4.6. Thermodynamic Specificity of the Non-Muscle Myosin NMMIIA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef]
- Gabbiani, G.; Hirschel, B.J.; Ryan, G.B.; Statkov, P.R.; Majno, G. Granulation tissue as a contractile organ. A study of structure and function. J. Exp. Med. 1972, 135, 719–734. [Google Scholar] [CrossRef]
- Feller, A.C.; Schneider, H.; Schmidt, D.; Parwaresch, M.R. Myofibroblast as a major cellular constituent of villous stroma in human placenta. Placenta 1985, 6, 405–415. [Google Scholar] [CrossRef]
- Krantz, E.K.; Parker, J.C. Contractile properties of the smooth muscle in the human placenta. Clin. Obs. Gynecol. 1963, 6, 26–38. [Google Scholar] [CrossRef]
- Farley, A.E.; Graham, C.H.; Smith, G.N. Contractile properties of human placental anchoring villi. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R680–R685. [Google Scholar] [CrossRef]
- Lecarpentier, E.; Claes, V.; Timbely, O.; Hebert, J.L.; Arsalane, A.; Moumen, A.; Guerin, C.; Guizard, M.; Michel, F.; Lecarpentier, Y. Role of both actin-myosin cross bridges and NO-cGMP pathway modulators in the contraction and relaxation of human placental stem villi. Placenta 2013, 34, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Schussler, O.; Claes, V.; Vallée, A. The Myofibroblast: TGFβ-1, A Conductor which Plays a Key Role in Fibrosis by Regulating the Balance between PPARγ and the Canonical WNT Pathway. Nucl. Recept. Res. 2017, 4. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Schussler, O.; Sakic, A.; Rincon-Garriz, J.M.; Soulie, P.; Bochaton-Piallat, M.L.; Kindler, V. Human Bone Marrow Contains Mesenchymal Stromal Stem Cells That Differentiate In Vitro into Contractile Myofibroblasts Controlling T Lymphocyte Proliferation. Stem Cells Int. 2018, 2018, 6134787. [Google Scholar] [CrossRef]
- Conti, M.A.; Adelstein, R.S. Nonmuscle myosin II moves in new directions. J. Cell Sci. 2008, 121, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Sakurai, K.; Shinomiya, T.; Fujitani, N.; Key, K.; Ohashi, M. Biochemical and immunohistochemical characterization of the isoforms of myosin and actin in human placenta. Placenta 2011, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Wang, F.; Hu, A.; Zhang, Y.; Sellers, J.R. Functional divergence of human cytoplasmic myosin II: Kinetic characterization of the non-muscle IIA isoform. J. Biol. Chem. 2003, 278, 38132–38140. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Claes, V.; Lecarpentier, E.; Guerin, C.; Hebert, J.L.; Arsalane, A.; Moumen, A.; Krokidis, X.; Michel, F.; Timbely, O. Ultraslow myosin molecular motors of placental contractile stem villi in humans. PLoS ONE 2014, 9, e108814. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.W. Physical Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 1990; pp. 1–1031. [Google Scholar]
- Lecarpentier, Y.; Claes, V.; Krokidis, X.; A Vallée, A. Comparative Statistical Mechanics of Muscle and Non-Muscle Contractile Systems: Stationary States of Near-Equilibrium Systems in A Linear Regime. Entropy J. 2017, 19, 558. [Google Scholar] [CrossRef]
- Huxley, A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Lymn, R.W.; Taylor, E.W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 1971, 10, 4617–4624. [Google Scholar] [CrossRef]
- Cooke, R. Actomyosin interaction in striated muscle. Physiol. Rev. 1997, 77, 671–697. [Google Scholar] [CrossRef]
- Levine, I.N. Physical Chemistry, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2003; pp. 1–986. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics from Heat Engines to Dissipative Structures; Wiley & Sons: New York, NY, USA, 1999; pp. 1–486. [Google Scholar]
- Onsager, L. Reciprocal relations in irreversible processes II. Phys. Rev. 1931, 38, 405–426. [Google Scholar] [CrossRef]
- Demirel, Y.; Sandler, S.I. Thermodynamics and bioenergetics. Biophys. Chem. 2002, 97, 87–111. [Google Scholar] [CrossRef]
- Stucki, J.W. The thermodynamic-buffer enzymes. Eur. J. Biochem. 1980, 109, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Devin, A.; Espie, P.; Guerin, B.; Fontaine, E.; Piquet, M.A.; Nogueira, V.; Leverve, X. Flux-force relationships in intact cells: A helpful tool for understanding the mechanism of oxidative phosphorylation alterations? Biochim. Biophys. Acta 1998, 1365, 117–124. [Google Scholar] [CrossRef]
- Dewey, T.G.; Delle Donne, M. Non-equilibrium thermodynamics of molecular evolution. J. Theor. Biol. 1998, 193, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Schussler, O.; Coirault, C.; Louis-Tisserand, M.; Al-Chare, W.; Oliviero, P.; Menard, C.; Michelot, R.; Bochet, P.; Salomon, D.R.; Chachques, J.C.; et al. Use of arginine-glycine-aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 240–249. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Kindler, V.; Bochaton-Piallat, M.L.; Sakic, A.; Claes, V.; Hebert, J.L.; Vallee, A.; Schussler, O. Tripeptide Arg-Gly-Asp (RGD) modifies the molecular mechanical properties of the non-muscle myosin IIA in human bone marrow-derived myofibroblasts seeded in a collagen scaffold. PLoS ONE 2019, 14, e0222683. [Google Scholar] [CrossRef]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Biol. Sci. 1938, 126, 136–195. [Google Scholar]
- Veech, R.L.; Lawson, J.W.; Cornell, N.W.; Krebs, H.A. Cytosolic phosphorylation potential. J. Biol. Chem. 1979, 254, 6538–6547. [Google Scholar]
- Lecarpentier, Y.; Blanc, F.X.; Quillard, J.; Hebert, J.L.; Krokidis, X.; Coirault, C. Statistical mechanics of myosin molecular motors in skeletal muscles. J. Theor. Biol. 2005, 235, 381–392. [Google Scholar] [CrossRef]
- Leibler, S.; Huse, D.A. Porters versus rowers: A unified stochastic model of motor proteins. J. Cell Biol. 1993, 121, 1357–1368. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Chemla, D.; Pourny, J.C.; Blanc, F.X.; Coirault, C. Myosin cross bridges in skeletal muscles: “rower” molecular motors. J. Appl. Physiol. 2001, 91, 2479–2486. [Google Scholar] [CrossRef]
- Woledge, R.C.; Curtin, A.N.; Homsher, E. Energetic Aspects of Muscle Contraction; Academic Press: London, UK, 1985; Volume 41, pp. 1–357. [Google Scholar]
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes; John Wiley & Sons, Inc.: New York, NY, USA, 1967. [Google Scholar]
- De Donder, T. L’ Affinité; Gauthiers-Villars: Paris, France, 1927. [Google Scholar]
- Marshall, W.E.; Omachi, A. Measured and calculated NAD+-NADH ratios in human erythrocytes. Biochim. Biophys. Acta 1974, 354, 1–10. [Google Scholar] [CrossRef]
- Masuda, T.; Dobson, G.P.; Veech, R.L. The Gibbs-Donnan near-equilibrium system of heart. J. Biol. Chem. 1990, 265, 20321–20334. [Google Scholar] [PubMed]
- Lecarpentier, Y.; Claes, V.; Hebert, J.L.; Krokidis, X.; Blanc, F.X.; Michel, F.; Timbely, O. Statistical Mechanics of the Human Placenta: A Stationary State of a Near-Equilibrium System in a Linear Regime. PLoS ONE 2015, 10, e0142471. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Krokidis, X.; Martin, P.; Pineau, T.; Hebert, J.L.; Quillard, J.; Cortes-Morichetti, M.; Coirault, C. Increased entropy production in diaphragm muscle of PPAR alpha knockout mice. J. Theor. Biol. 2008, 250, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Stucki, J.W. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur. J. Biochem. 1980, 109, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Rayment, I.; Holden, H.M.; Whittaker, M.; Yohn, C.B.; Lorenz, M.; Holmes, K.C.; Milligan, R.A. Structure of the actin-myosin complex and its implications for muscle contraction. Science 1993, 261, 58–65. [Google Scholar] [CrossRef]
- Rayment, I.; Rypniewski, W.R.; Schmidt-Base, K.; Smith, R.; Tomchick, D.R.; Benning, M.M.; Winkelmann, D.A.; Wesenberg, G.; Holden, H.M. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science 1993, 261, 50–58. [Google Scholar] [CrossRef]
- Lauzon, A.M.; Tyska, M.J.; Rovner, A.S.; Freyzon, Y.; Warshaw, D.M.; Trybus, K.M. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J. Muscle Res. Cell Motil. 1998, 19, 825–837. [Google Scholar] [CrossRef]
- Winegrad, S. How actin-myosin interactions differ with different isoforms of myosin. Circ. Res. 1998, 82, 1109–1110. [Google Scholar] [CrossRef]
- Trybus, K.M. Role of myosin light chains. J. Muscle Res. Cell Motil. 1994, 15, 587–594. [Google Scholar] [CrossRef]
- Spudich, J.A.; Finer, J.; Simmons, B.; Ruppel, K.; Patterson, B.; Uyeda, T. Myosin structure and function. Cold Spring Harb. Symp. Quant. Biol. 1995, 60, 783–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michael, M.; Parsons, M. New perspectives on integrin-dependent adhesions. Curr. Opin. Cell Biol. 2020, 63, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, M.; Ivaska, J. Tensins: Bridging AMP-Activated Protein Kinase with Integrin Activation. Trends Cell Biol. 2017, 27, 703–711. [Google Scholar] [CrossRef] [PubMed]
| COL-MSC n = 14 | COL-MSC-RGD n = 10 | p | |
|---|---|---|---|
| Tension (TT) (mN/mm2) | 0.266 ± 0.103 | 0.642 ± 0.212 | 0.0001 |
| max SL (L/Lo) | 0.012 ± 0.008 | 0.020 ± 0.014 | 0.020 |
| Vmax (Lo/s) | 0.002 ± 0.001 | 0.004 ± 0.001 | 0.006 |
| Eff. max (%) | 38 ± 3 | 38 ± 3 | 0.857; NS |
| CB force (po) (pN) | 2.1 ± 0.2 | 2.1 ± 0.1 | 0.865; NS |
| CB mole/L (E-11) | 4.23 ± 1.55 | 10.27 ± 30 | 0.0001 |
| CB number/L (E13) | 2.55 ± 0.90 | 6.18 ± 1.99 | 0.0001 |
| COL-MSC n = 14 | COL-MSC-RGD n = 10 | p | |
|---|---|---|---|
| PD1 | 0.008 ± 0.006 | 0.009 ± 0.005 | 0.618; NS |
| PD2 | 0.082 ± 0.056 | 0.094 ± 0.053 | 0.618; NS |
| PD3 | 0.825 ± 0.068 | 0.813 ± 0.062 | 0.658; NS |
| PA1 | 0.037 ± 0.011 | 0.036 ± 0.010 | 0.865; NS |
| PA2 | 0.041 ± 0.003 | 0.042 ± 0.003 | 0.865; NS |
| PA3. E-11 | 1.794 ± 0.147 | 1.766 ± 0.134 | 0.653; NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecarpentier, Y.; Kindler, V.; Krokidis, X.; Bochaton-Piallat, M.-L.; Claes, V.; Hébert, J.-L.; Vallée, A.; Schussler, O. Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD). Cells 2020, 9, 1510. https://doi.org/10.3390/cells9061510
Lecarpentier Y, Kindler V, Krokidis X, Bochaton-Piallat M-L, Claes V, Hébert J-L, Vallée A, Schussler O. Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD). Cells. 2020; 9(6):1510. https://doi.org/10.3390/cells9061510
Chicago/Turabian StyleLecarpentier, Yves, Vincent Kindler, Xénophon Krokidis, Marie-Luce Bochaton-Piallat, Victor Claes, Jean-Louis Hébert, Alexandre Vallée, and Olivier Schussler. 2020. "Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD)" Cells 9, no. 6: 1510. https://doi.org/10.3390/cells9061510
APA StyleLecarpentier, Y., Kindler, V., Krokidis, X., Bochaton-Piallat, M.-L., Claes, V., Hébert, J.-L., Vallée, A., & Schussler, O. (2020). Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD). Cells, 9(6), 1510. https://doi.org/10.3390/cells9061510





