Reaction Cycles of Halogen Species in the Immune Defense: Implications for Human Health and Diseases and the Pathology and Treatment of COVID-19
Abstract
1. Introduction
2. Theoretical Studies
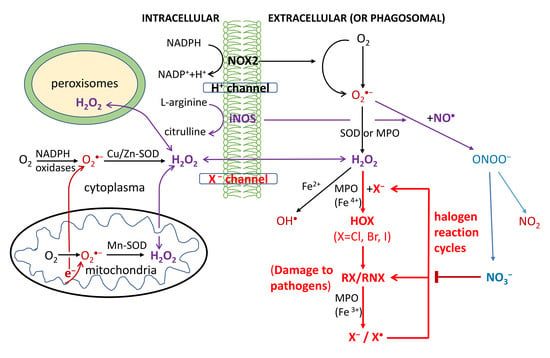
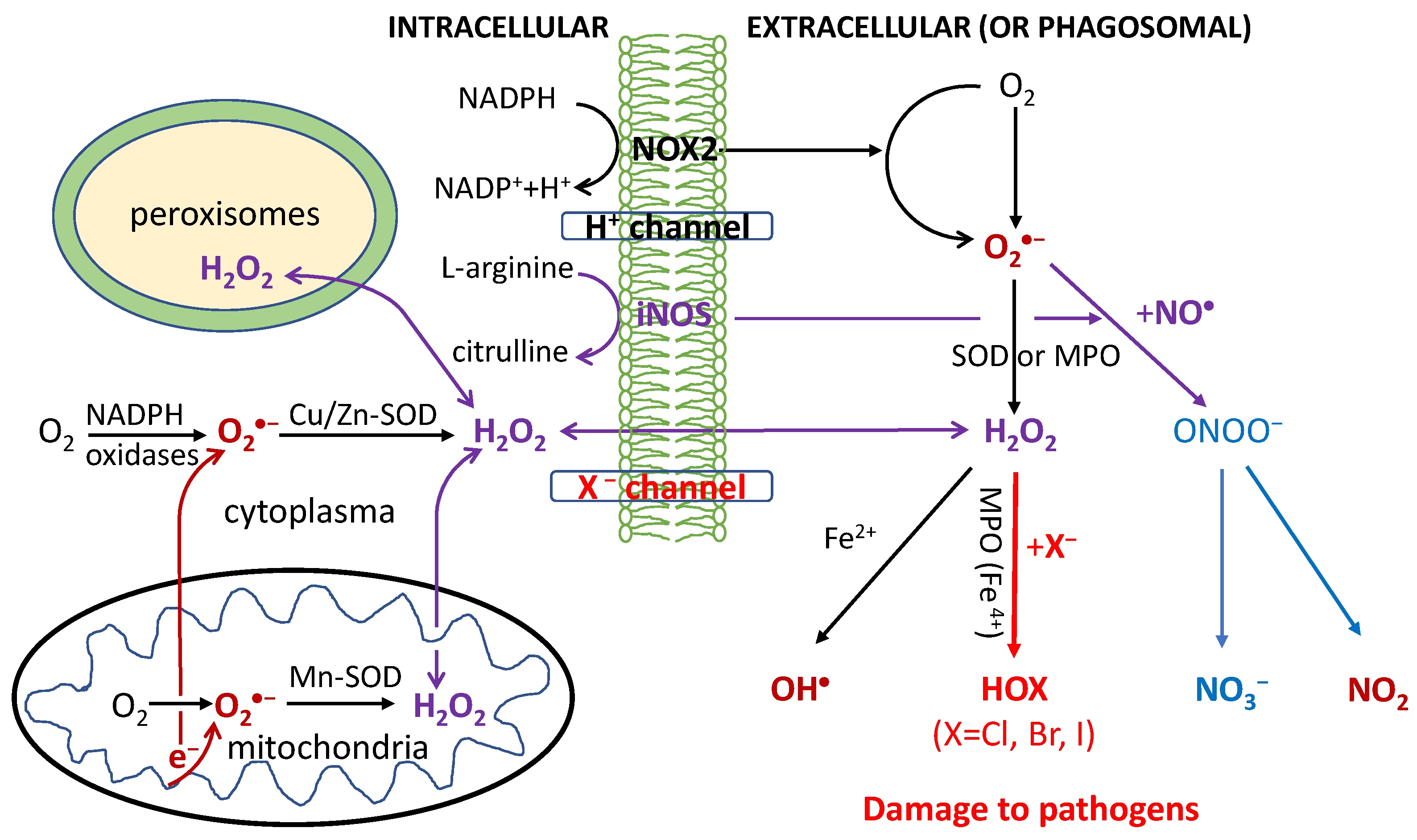
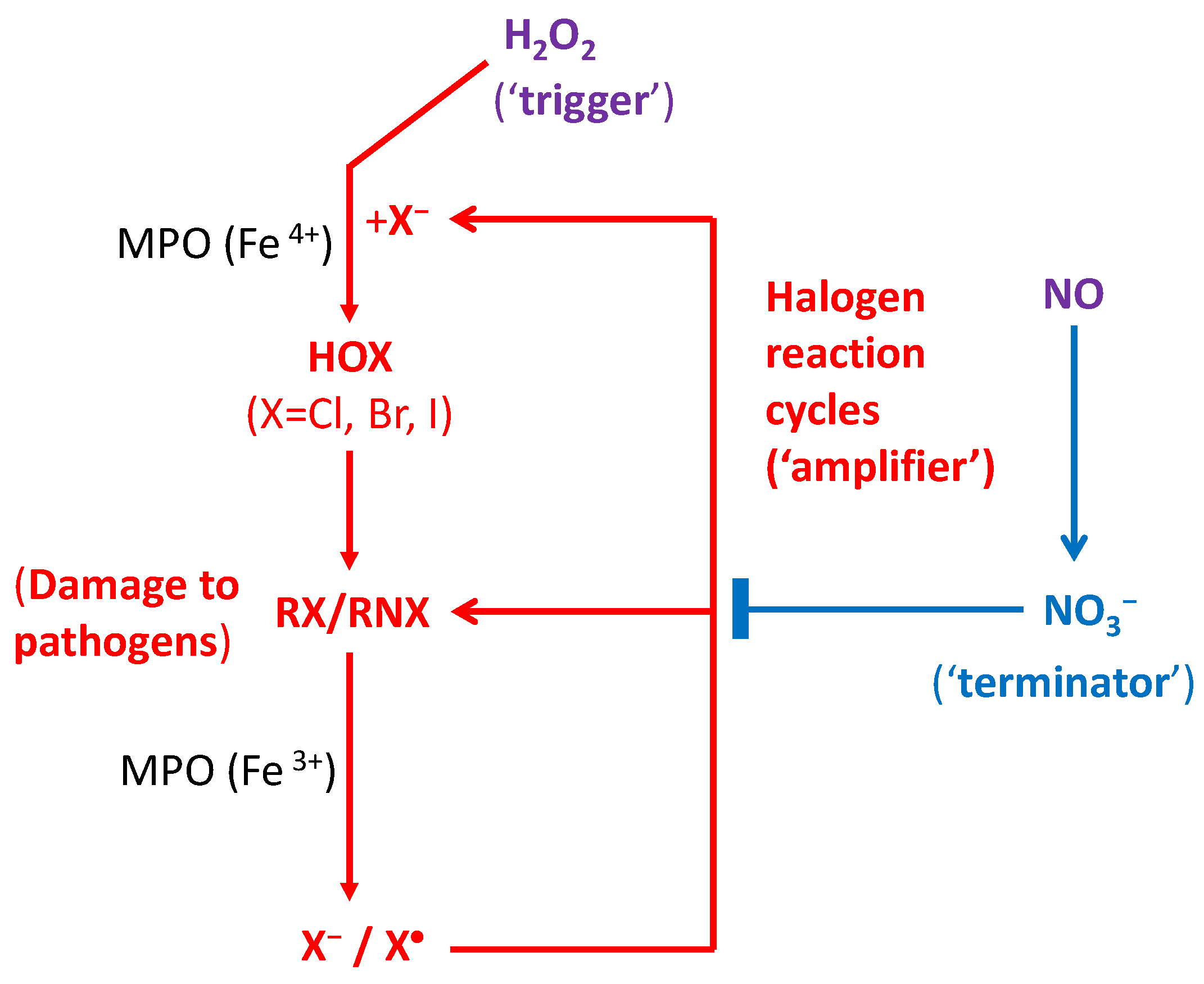
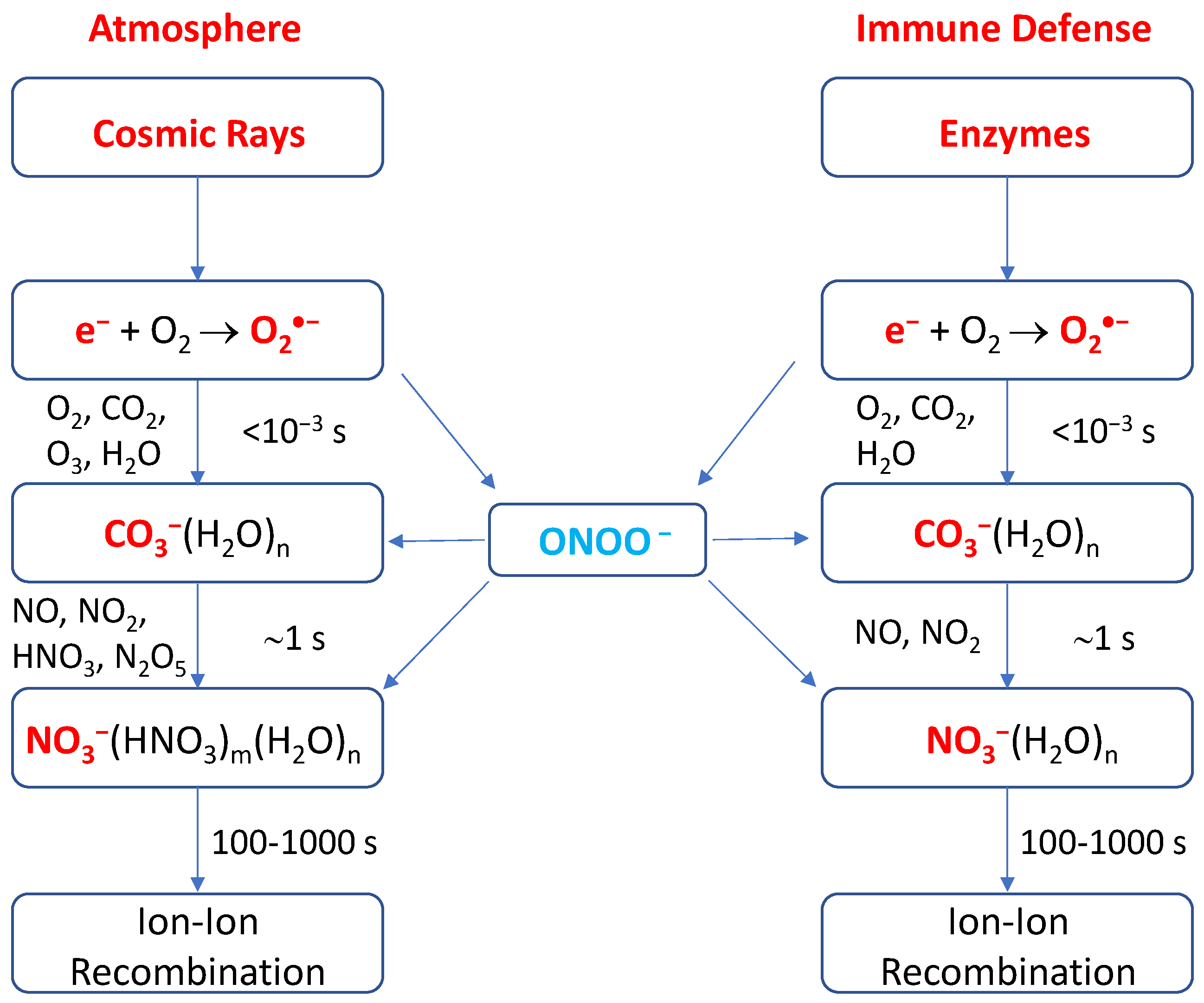
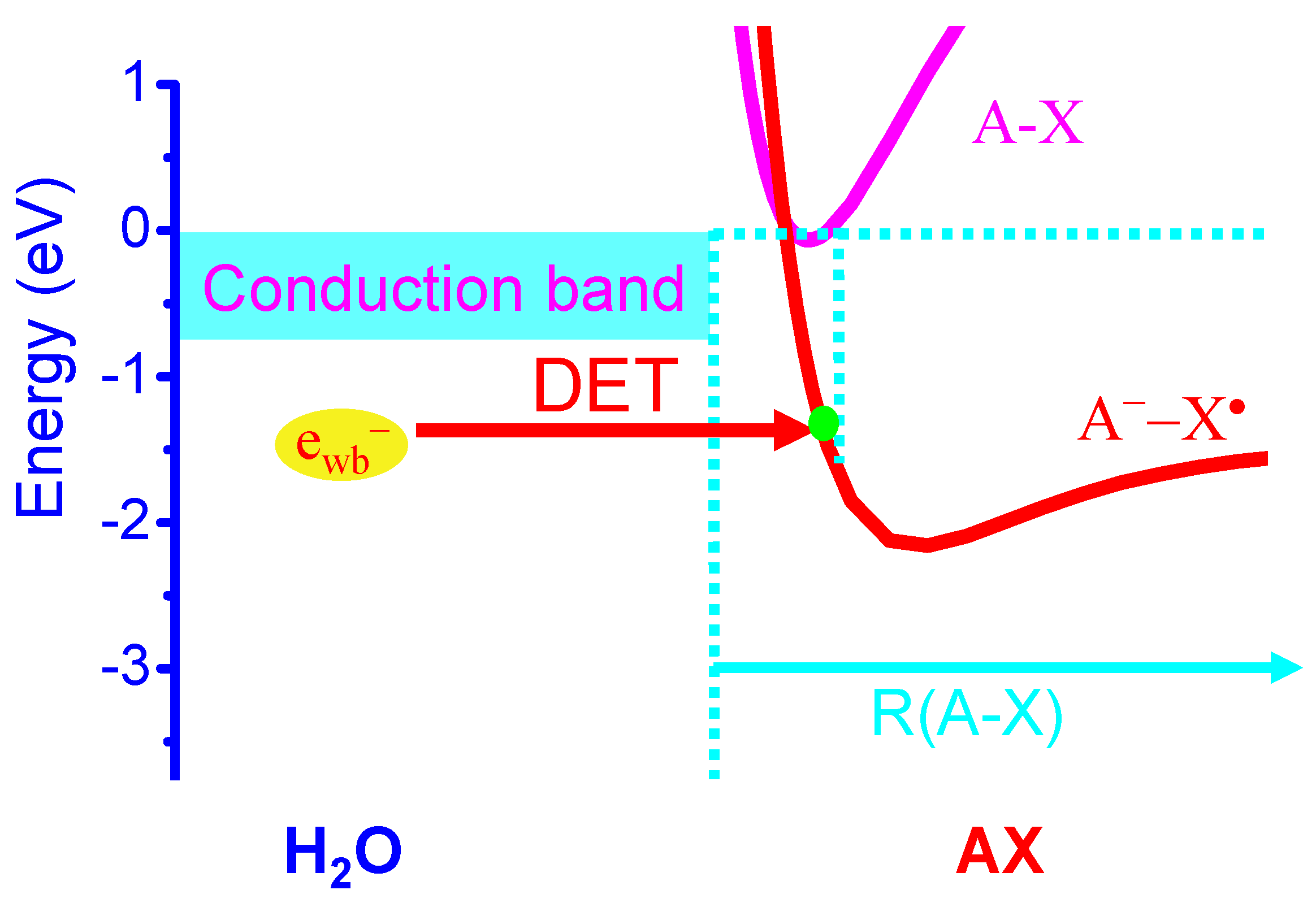
2.1. Halogen Cyclic Reactions
2.2. Halogen Cyclic Reaction Rates
2.3. Significance of Halogen Cyclic Reactions
- (1)
- The H2O2-initiated halogen cyclic reactions should greatly amplify the pathogen-killing effects of RHS in phagocytosis for innate and adaptive immune defenses. The proposed mechanism might lead to discoveries of highly effective antiviral drugs for effective treatment of various viruses such as HIV and COVID-19.
- (2)
- It also provides a long-sought amplification mechanism for reactive radicals produced by IR to generate the clinically required radiobiological effects in human body, as compared with background ROS.
- (3)
- It reveals that H2O2 as a signaling molecule plays the key protective (pathogen-killing) role in immunological defenses in many organisms from plants and animals to humans.
- (4)
2.4. Implications for the Pathology of COVID-19
3. Halogenated Aromatic Drugs as Repurposed Drugs
3.1. New Molecular Mechanism of Action (MOA) of Halogenated Aromatic Drugs
3.2. Potential New Therapeutics
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rhee, S.G. CELL SIGNALING: H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Neish, A.S. Nox Enzymes and New Thinking on Reactive Oxygen: A Double-Edged Sword Revisited. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dang, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Li, Y.; Huang, T.T.; Carlson, E.J.; Melov, S.; Ursell, P.C.; Olson, J.L.; Noble, L.J.; Yoshimura, M.P.; Berger, C.; Chan, P.H.; et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995, 11, 376–381. [Google Scholar] [CrossRef]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Jackson, R.L.; Van Remmen, H.; Epstein, C.J.; Huang, T.-T. Cu/Zn-SOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005, 24, 367–380. [Google Scholar] [CrossRef]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.-T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of Cu/Zn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free. Rad. Bio. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef]
- Williams, M.D.; van Remrnen, H.; Conrad, C.C.; Huang, T.T.; Epstein, C.J.; Richardson, A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998, 273, 28510–28515. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.W.; Lindgren, A.L.; Baker, S.A.; Stevens, R.H. Superoxide ion as the cause of the oxygen effect. Radiat. Res. 1976, 68, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Petkau, A.; Chelack, W.S.; Pleskach, S.D. Protection of post irradiated mice by superoxide dismutase. Int. J. Radiat. Biol. 1976, 29, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Azzarn, E.I.; Li, J.L.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Lehnert, S. Biomolecular Action of Ionizing Radiation; CRC Press: Boca Raton, FL, USA, 2008; pp. 97–120. [Google Scholar]
- Lu, Q.B.; Madey, T.E. Giant enhancement of electron-induced dissociation of chlorofluorocarbons coadsorbed with water or ammonia ices: Implications for the atmospheric ozone depletion. J. Chem. Phys. 1999, 111, 2861–2864. [Google Scholar] [CrossRef]
- Lu, Q.B.; Sanche, L. Effects of Cosmic Rays on Atmospheric Chlorofluorocarbon Dissociation and Ozone Depletion. Phys. Rev. Lett. 2001, 87, 078501. [Google Scholar] [CrossRef]
- Lu, Q.B. Cosmic-Ray-Driven Electron-Induced Reactions of Halogenated Molecules Adsorbed on Ice Surfaces: Implications for Atmospheric Ozone Depletion and Global Climate Change. Phys. Rep. 2010, 487, 141–167. [Google Scholar] [CrossRef]
- Lu, Q.B. New Theories and Predictions of the Ozone Hole and Climate Change; World Scientific: Hackensack, NJ, USA, 2015; Chapters 1, 4 & 5. [Google Scholar]
- Peterhans, E.; Grob, M.; Burge, T.H.; Zanoni, R. Virus-Induced Formation of Reactive Oxygen Intermediates in Phagocytic Cells. Free Rad. Res. Comms. 1987, 3, 39–46. [Google Scholar] [CrossRef]
- Chase, M.J.; Klebanoff, S.J. Viricidal effect of stimulated human mononuclear phagocytes on human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1992, 89, 5582–5585. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Coombs, R.W. Viricidal effect of polymorphonuclear leukocytes on HIV-1: Role of the myeloperoxidase system. J. Clin. Investig. 1992, 89, 2014–2017. [Google Scholar] [CrossRef]
- Kramer, M.; Schulte, B.M.; Toonen, L.W.J.; Barral, P.M.; Fisher, P.B.; Lanke, K.H.W.; Galama, J.M.D.; van Kuppeveld, F.J.M.; Adema, G.J. Phagocytosis of Picornavirus-Infected Cells Induces an RNA-Dependent Antiviral State in Human Dendritic Cells. J. Virol. 2008, 82, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Vangeti, S.; Yu, M.; Smed-Sörensen, A. Respiratory Mononuclear Phagocytes in Human Influenza A Virus Infection: Their Role in Immune Protection and As Targets of the Virus. Front. Immunol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.J.; Maury, W. The role of mononuclear phagocytes in Ebola virus infection. J. Leukoc. Biol. 2018, 104, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Van Strijp, J.A.G.; Kok, P.M.; Kessel, V.; van der Tol, M.E.; Verhoef, J. Complement-mediated Phagocytosis of Herpes Simplex Virus by Granulocytes Binding or Ingestion. J. Clin. Investig. 1989, 84, 107–112. [Google Scholar] [CrossRef]
- Powell, L.R.R.; Fox, A.; Itri, V.; Zolla-Pazner, S. Primary Human Neutrophils Exhibit a Unique HIV-Directed Antibody-Dependent Phagocytosis Profile. J. Innate Immun. 2019, 11, 181–190. [Google Scholar] [CrossRef]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Zhang, G.; Cowled, C.; Shi, Z.; Huang, Z.; Bishop-Lilly, K.A.; Fang, X.; Wynne, J.W.; Xiong, Z.; Baker, M.L.; Zhao, W.; et al. Comparative Analysis of Bat Genomes Provides Insight into the Evolution of Flight and Immunity. Science 2013, 339, 456–460. [Google Scholar] [CrossRef]
- Albrich, J.M.; McCarthy, C.A.; Hurst, J.K. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA 1981, 78, 210–214. [Google Scholar] [CrossRef]
- Arnhold, J.; Monzani, E.; Furtmüller, P.G.; Zederbauer, M.; Casella, L.; Obinger, C. Kinetics and Thermodynamics of Halide and Nitrite Oxidation by Mammalian Heme Peroxidases. Eur. J. Inorg. Chem. 2006, 2006, 3801–3811. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L.; Pattison, D.I.; Rees, M.D. Mammalian Heme Peroxidases: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arc. Biochem. Biophys 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, D.M. Peroxynitrite and inflammatory bowel disease. Gut 2000, 46, 436–439. [Google Scholar] [CrossRef]
- Radia, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Rowland, F.S. Nobel Lecture in Chemistry (1995).
- Brasseur, G.P.; Orlando, J.J.; Tyndall, G.S. (Eds.) Atmospheric Chemistry and Global Change; Chapters 8 & 14; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atomic-atalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Ward, J.F. DNA Damage as the Cause of Ionizing Radiation-Induced Gene Activation. Radiat. Res. 1994, 138, S85–S88. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Xin, W.; Zhao, B. Production and interaction of oxygen and nitric oxide free radicals in PMA stimulated macrophages during the respiratory burst. Redox. Rep. 2000, 5, 353–358. [Google Scholar] [CrossRef]
- Hazen, S.L.; Hsu, F.F.; Duffin, K.; Heinecke, J.W. Molecular Chlorine Generated by the Myeloperoxidase-Hydrogen Peroxide-Chloride System of Phagocytes Converts Low Density Lipoprotein Cholesterol into a Family of Chlorinated Sterols. J. Biol. Chem. 1996, 271, 23080–23088. [Google Scholar] [CrossRef]
- Armesto, X.L.; Canle, L.M.; García, M.V.; Santaballa, J.A. Aqueous chemistry of N-halo-compounds. Chem. Soc. Rev. 1998, 27, 453–460. [Google Scholar] [CrossRef]
- Dixon-Warren, S.J.; Jensen, E.T.; Polanyi, J.C. Photochemistry of adsorbed molecules. XI. Charge-transfer photodissociation and photoreaction in chloromethanes on Ag(111). J. Chem. Phys. 1993, 98, 5938–5953. [Google Scholar] [CrossRef]
- Dixon-Warren, S.J.; Heyd, D.V.; Jensen, E.T.; Polanyi, J.C. Photochemistry of Adsorbed Molecules. XII. Photoinduced Ion-Molecule Reactions at a Metal Surface for CH3X/RCl/Ag(111) (X=Br, I). J. Chem. Phys. 1993, 98, 5954–5960. [Google Scholar] [CrossRef]
- Lu, Q.B.; Madey, T.E. Negative-ion enhancements in electron-stimulated desorption of CF2Cl2 coadsorbed with nonpolar and polar gases on Ru(0001). Phys. Rev. Lett. 1999, 82, 4122–4125. [Google Scholar] [CrossRef]
- Lu, Q.B.; Sanche, L. Enhanced Dissociative Electron Attachment to CF2Cl2 by Transfer of Electrons Localized in Preexisting Traps of Water and Ammonia Ice. Phys. Rev. B 2001, 63, 153403. [Google Scholar] [CrossRef]
- Wang, C.R.; Lu, Q.B. Real-time observation of a molecular reaction mechanism of aqueous 5-halo-2’-deoxyuridines under UV/ionizing radiation. Angew. Chem. Intl. Ed. 2007, 46, 6316–6321. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B. Effects of Ultrashort-Lived Prehydrated Electrons in Radiation Biology and Their Applications for Radiotherapy of Cancer. Mutat. Res. Rev. Mutat. Res. 2010, 704, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Stähler, J.; Gahl, C.; Wolf, M. Trapped Electrons on Supported Ice Crystallites. Acc. Chem. Res. 2012, 45, 131–138. [Google Scholar] [CrossRef][Green Version]
- Lind, J.; Jonsson, M.; Xinhua, S.; Eriksen, T.E.; Merenyi, G.; Eberson, L. Kinetics of radical-initiated chain bromination of 2-methyl-2-propanol by N-bromosuccinimide in water. J. Am. Chem. Soc. 1993, 115, 3503–3510. [Google Scholar] [CrossRef]
- Pattison, D.I.; O’Reilly, R.J.; Skaff, O.; Radom, L.; Anderson, R.F.; Davies, M.J. One-Electron Reduction of N-Chlorinated and N-Brominated Species Is a Source of Radicals and Bromine Atom Formation. Chem. Res. Toxicol. 2011, 24, 371–382. [Google Scholar] [CrossRef]
- Vlasova, I.I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef]
- Kee, T.K.; Son, D.H.; Kambhampati, P.; Barbara, P.F. A unified electron transfer model for the different precursors and excited states of the hydrated electron. J. Phys. Chem. A 2001, 105, 8434–8439. [Google Scholar] [CrossRef]
- Nguyen, J.; Ma, Y.; Luo, T.; Bristow, R.G.; Jaffray, D.A.; Lu, Q.B. Direct observation of ultrafast electron transfer reactions unravels high effectiveness of reductive DNA damage. Proc. Natl. Acad. Sci. USA 2011, 108, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.S.; Chen, E.C.; Sane, N. The electron affinities of the radicals formed by the loss of an aromatic hydrogen atom from adenine, guanine, cytosine, uracil, and thymine. Biochem. Biophys Res. Commun. 1998, 246, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Bankura, A.; Bankura, A.; Santra, B.; DiStasio, R.A., Jr.; Swartz, C.W.; Klein, M.L.; Wu, X. A systematic study of chloride ion solvation in water using van derWaals inclusive hybrid density functional theory. Mol. Phys. 2015, 113, 2842–2854. [Google Scholar] [CrossRef]
- Adam, F.; Gosselain, P.A.; Goldfinger, P. Laws of Addition and Substitution in Atomic Reactions of Halogens. Nature 1953, 171, 704–705. [Google Scholar] [CrossRef]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.Y.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; et al. Peroxisomes Are Signaling Platforms for Antiviral Innate Immunity. Cell 2010, 141, 668–681. [Google Scholar] [CrossRef]
- Viggiano, A.A.; Arnold, F. Ion Chemistry and Composition of the Atmosphere. In Handbook of Atmospheric Electrodynamics; Volland, H., Ed.; Chapter 1; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Viggiano, A.A. In situ mass spectrometry and ion chemistry in the stratosphere and troposphere. Mass. Spectr. Rev. 1993, 12, 115–137. [Google Scholar] [CrossRef]
- Leach, J.K.; Tuyle, G.V.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing Radiation-induced, Mitochondria-dependent Generation of Reactive Oxygen/Nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar]
- Furtmüller, P.G.; Burner, U.; Obinger, C. Reaction of Myeloperoxidase Compound I with Chloride, Bromide, Iodide, and Thiocyanate. Biochemistry 1998, 37, 17923–17930. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Regelsberger, G.; Obinger, C. Spectral and Kinetic Studies on the Formation of Eosinophil Peroxidase Compound I and Its Reaction with Halides and Thiocyanate. Biochemistry 2000, 39, 15578–15584. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trend 2020, 14, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; de Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B.; Zhang, Q.R.; Ou, N.; Wang, C.R.; Warrington, J. In vitro and in vivo Studies of Non-Platinum-Based Halogenated Compounds as a New Class of Potent Antitumor Agents for Natural Targeted Chemotherapy of Cancers. EBioMedicine 2015, 2, 544–553. [Google Scholar] [CrossRef][Green Version]
- Wang, C.R.; Mahmood, J.; Zhang, Q.R.; Vedadi, A.; Warrington, J.; Ou, N.; Bristow, R.G.; Jaffray, D.A.; Lu, Q.-B. In Vitro and In Vivo Studies of a New Class of Anticancer Molecules for Targeted Radiotherapy of Cancer. Mol. Cancer Ther. 2016, 15, 640–650. [Google Scholar] [CrossRef]
- Goetze, R.G.; Buchholz, S.M.; Ou, N.; Zhang, Q.; Patil, S.; Schirmer, M.; Singh, S.K.; Ellenrieder, V.; Hessmann, E.; Lu, Q.-B.; et al. Preclinical evaluation of 1,2-Diamino-4,5-dibromobenzene in genetically engineered mouse models of pancreatic cancer. Cells 2019, 8, 563. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Krise, J.P. Lysosomal Sequestration of Amine-containing Drugs: Analysis and Therapeutic Implications. J. Pharm. Sci. 2007, 96, 729–746. [Google Scholar] [CrossRef]
- Ohkuma, S.; Poole, B. Fluorescence Probe Measurement of the Intralysosomal pH in Living Cells and the Perturbation of pH by Various Agents. Proc. Natl. Acad. Sci. USA 1978, 75, 3327–3331. [Google Scholar] [CrossRef]
- Oda, K.; Koriyama, Y.; Yamada, E.; Ikehara, Y. Effects of Weakly Basic Amines on Proteolytic Processing and Terminal Glycosylation of Secretory Proteins in Cultured Rat Hepatocytes. Biochem. J. 1986, 240, 739–745. [Google Scholar] [CrossRef]
- Hurst, N.P.; French, J.K.; Gorjatschko, L.; Betts, W.H. Chloroquine and Hydroxychloroquine Inhibit Multiple Sites in Metabolic Pathways Leading to Neutrophil Superoxide Release. J. Rheumatol. 1988, 15, 23–27. [Google Scholar] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-Like Receptors. Ann. Rev. Immun. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Lu, Q.B. Real-time observation of an ultrafast dissocaitive electron transfer reaction of a dihalogen-diamino-benzene molecule with the prehydrated electron. Unpublished.
- Jorge, A.; Ung, C.; Young, L.H.; Melles, R.B.; Choi, H.K. Hydroxychloroquine retinopathy –implications of research advances for rheumatology care. Nat. Rev. Rheumatol. 2018, 14, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral. Res. 2020. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
| Reaction | Products | k (M–1 s–1) | Refs |
|---|---|---|---|
| X− + H2O2 + MPO + H+ | HOX/OX−/X2 + H2O | 2.5 × 104, 1.1 × 106, 7.2 × 106 (X = Cl, Br, I) | [31] |
| X− + H2O2 + EPO + H+ | 3.1 × 103, 1.9 × 107, 9.3 × 107 (X = Cl, Br, I) | ||
| Cl− + OH• + H+ | Cl• + H2O | 1.5 × 1010 | [39] |
| R2NH + HOX/XO− | R2NX | 107–108 | [44] |
| Cl2+ RNH2 | RNHX + Cl− + H+ | (>)109 | [44] |
| NH2Cl + eaq− | Cl− + NH2 | 2.2 × 1010 | [53] |
| R2NCl + eaq− | Cl− + R2N• | 1.5–1.9 × 1010 | [52,53] |
| RNHCl + eaq− | Cl− + RNH• | 6.1–9.3 × 109 | [52,53] |
| RR’NBr + eaq− | Br• + RR’N− | 2.9 × 1010 (1.1 × 1011) | [52,53] |
| RR’NBr+ O2− | Br• + RR’N− | 2–9 × 108 | [52,53] |
| NO3− + epre− | NO32− | 1.2 × 1013 | [55,56] |
| CO3− + O2•− | CO32−+ O2 | 4 × 108 | [39] |
| NO2 + OH• | NO3− + H+ | 1.3 × 109 | [39] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.-B. Reaction Cycles of Halogen Species in the Immune Defense: Implications for Human Health and Diseases and the Pathology and Treatment of COVID-19. Cells 2020, 9, 1461. https://doi.org/10.3390/cells9061461
Lu Q-B. Reaction Cycles of Halogen Species in the Immune Defense: Implications for Human Health and Diseases and the Pathology and Treatment of COVID-19. Cells. 2020; 9(6):1461. https://doi.org/10.3390/cells9061461
Chicago/Turabian StyleLu, Qing-Bin. 2020. "Reaction Cycles of Halogen Species in the Immune Defense: Implications for Human Health and Diseases and the Pathology and Treatment of COVID-19" Cells 9, no. 6: 1461. https://doi.org/10.3390/cells9061461
APA StyleLu, Q.-B. (2020). Reaction Cycles of Halogen Species in the Immune Defense: Implications for Human Health and Diseases and the Pathology and Treatment of COVID-19. Cells, 9(6), 1461. https://doi.org/10.3390/cells9061461