Symmetry Breaking and Emergence of Directional Flows in Minimal Actomyosin Cortices
Abstract
1. Introduction
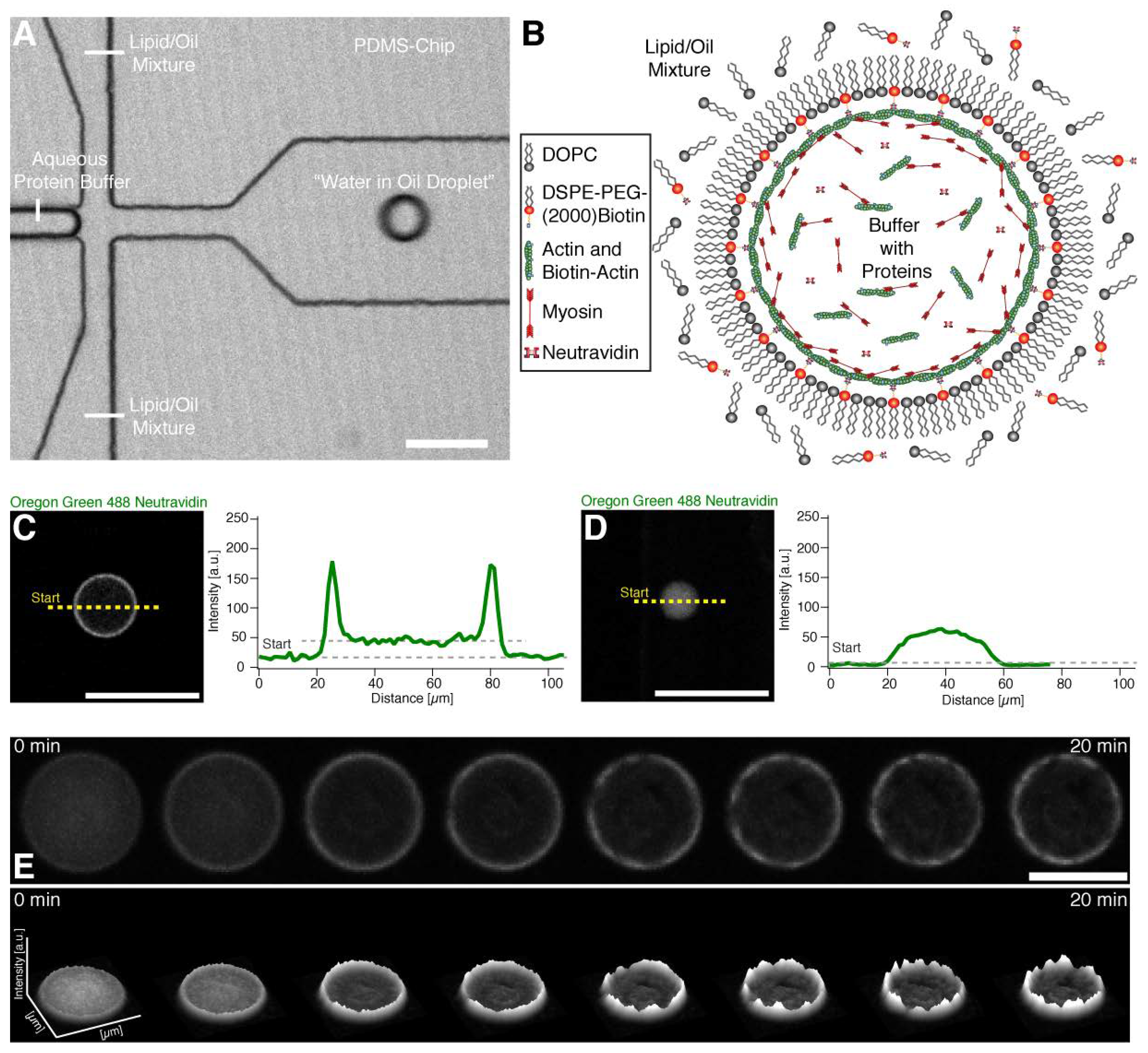
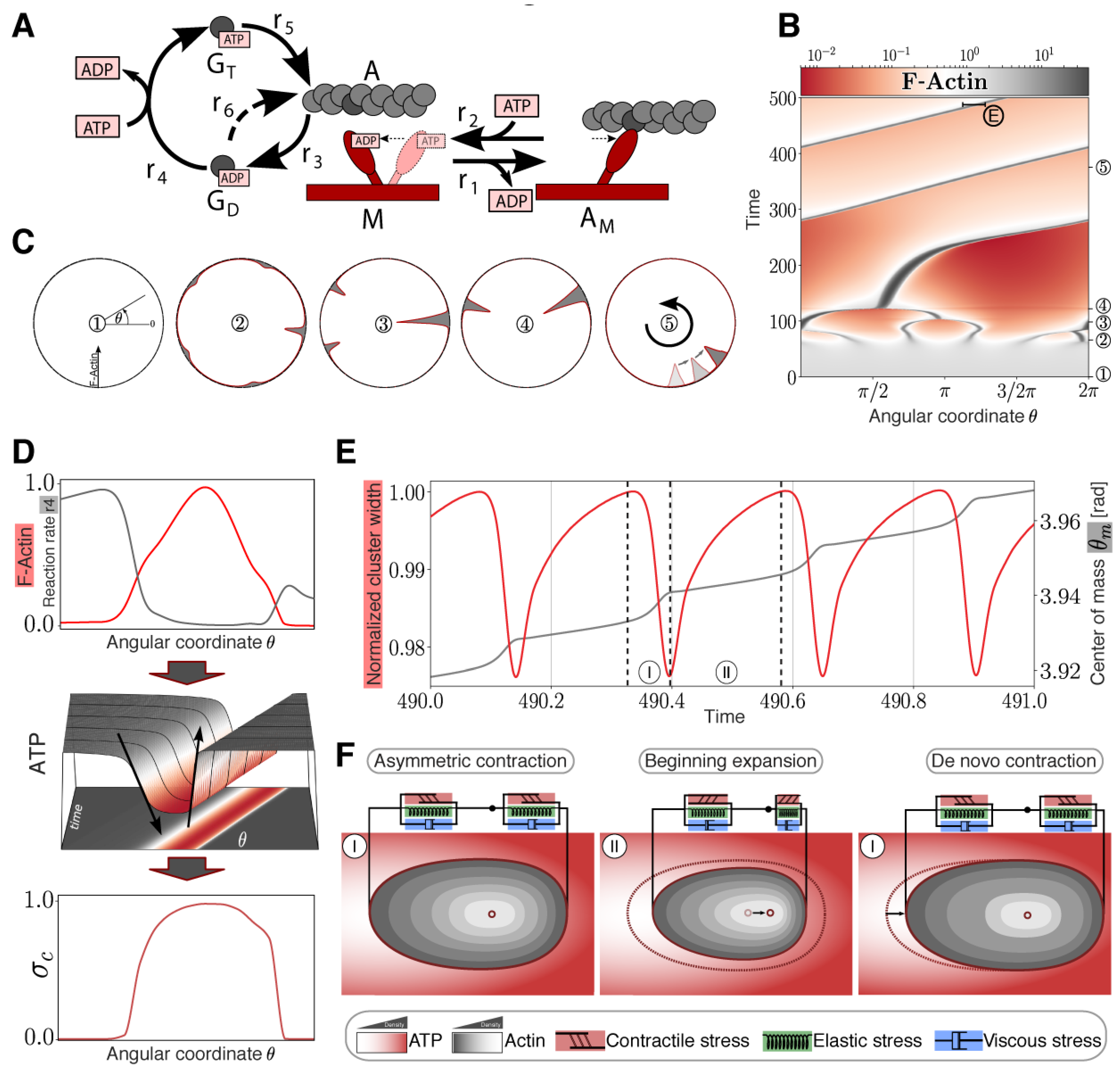
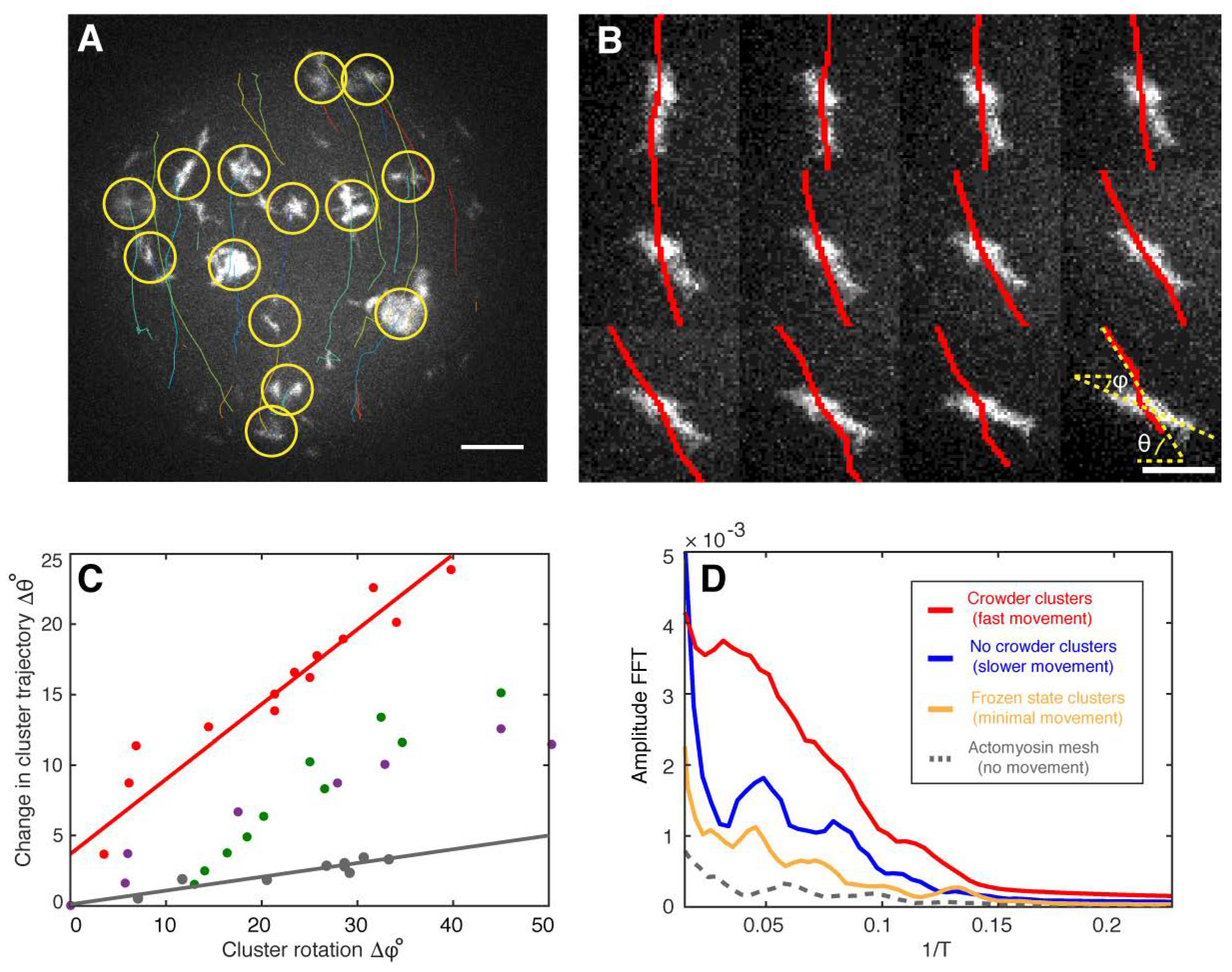
2. Results
Theoretical Model of the Cortical Actomyosin Motions
3. Discussion
4. Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, D.; White, J.G. Cortical flow in animal cells. Science 1988, 239, 883–888. [Google Scholar] [CrossRef]
- Sedzinski, J.; Biro, M.; Oswald, A.; Tinevez, J.Y.; Salbreux, G.; Paluch, E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 2011, 476, 462–466. [Google Scholar] [CrossRef]
- Khaliullin, R.N.; Green, R.A.; Shi, L.Z.; Gomez-Cavazos, J.S.; Berns, M.W.; Desai, A.; Oegema, K. A positive-feedback-based mechanism for constriction rate acceleration during cytokinesis in Caenorhabditis elegans. Elife 2018, 7, e36073. [Google Scholar] [CrossRef] [PubMed]
- Naganathan, S.R.; Middelkoop, T.C.; Furthauer, S.; Grill, S.W. Actomyosin-driven left-right asymmetry: From molecular torques to chiral self organization. Curr. Opin. Cell Biol. 2016, 38, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wollrab, V.; Thiagarajan, R.; Wald, A.; Kruse, K.; Riveline, D. Still and rotating myosin clusters determine cytokinetic ring constriction. Nat. Commun. 2016, 7, 11860. [Google Scholar] [CrossRef] [PubMed]
- Callan-Jones, A.C.; Voituriez, R. Actin flows in cell migration: From locomotion and polarity to trajectories. Curr. Opin. Cell Biol. 2016, 38, 12–17. [Google Scholar] [CrossRef]
- Klughammer, N.; Bischof, J.; Schnellbacher, N.D.; Callegari, A.; Lenart, P.; Schwarz, U.S. Cytoplasmic flows in starfish oocytes are fully determined by cortical contractions. PLoS Comput. Biol. 2018, 14, e1006588. [Google Scholar] [CrossRef] [PubMed]
- Munro, E.; Nance, J.; Priess, J.R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 2004, 7, 413–424. [Google Scholar] [CrossRef]
- Naganathan, S.R.; Furthauer, S.; Nishikawa, M.; Julicher, F.; Grill, S.W. Active torque generation by the actomyosin cell cortex drives left-right symmetry breaking. Elife 2014, 3, e04165. [Google Scholar] [CrossRef]
- Nishikawa, M.; Naganathan, S.R.; Julicher, F.; Grill, S.W. Controlling contractile instabilities in the actomyosin cortex. Elife 2017, 6, e19595. [Google Scholar] [CrossRef]
- Fletcher, D. Which biological systems should be engineered? Nature 2018, 563, 177–179. [Google Scholar] [CrossRef]
- Schwille, P. Bottom-up synthetic biology: Engineering in a tinkerer’s world. Science 2011, 333, 1252–1254. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyazaki, M.; Takagi, J.; Itabashi, T.; Ishiwata, S. Spatial confinement of active microtubule networks induces large-scale rotational cytoplasmic flow. Proc. Natl. Acad. Sci. USA 2017, 114, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Malik-Garbi, M.; Abu-Shah, E.; Li, J.; Sharma, A.; MacKintosh, F.C.; Keren, K.; Schmidt, C.F.; Fakhri, N. Self-organized stress patterns drive state transitions in actin cortices. Sci. Adv. 2018, 4, eaar2847. [Google Scholar] [CrossRef] [PubMed]
- Schaller, V.; Weber, C.; Semmrich, C.; Frey, E.; Bausch, A.R. Polar patterns of driven filaments. Nature 2010, 467, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Keber, F.C.; Loiseau, E.; Sanchez, T.; DeCamp, S.J.; Giomi, L.; Bowick, M.J.; Marchetti, M.C.; Dogic, Z.; Bausch, A.R. Topology and dynamics of active nematic vesicles. Science 2014, 345, 1135–1139. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Borisy, G.G. Correlative light and electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 1998, 298, 570–592. [Google Scholar]
- Stachowiak, M.R.; Laplante, C.; Chin, H.F.; Guirao, B.; Karatekin, E.; Pollard, T.D.; O’Shaughnessy, B. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev. Cell 2014, 29, 547–561. [Google Scholar] [CrossRef]
- Vogel, S.K.; Heinemann, F.; Chwastek, G.; Schwille, P. The design of MACs (minimal actin cortices). Cytoskeleton 2013, 70, 706–717. [Google Scholar] [CrossRef]
- Vogel, S.K.; Petrasek, Z.; Heinemann, F.; Schwille, P. Myosin Motors Fragment and Compact Membrane-Bound Actin Filaments. eLife 2013, 2013, e00116. [Google Scholar] [CrossRef]
- Thielicke, W. PIVlab—Towards User-friendly, Affordable and Accurate Digital Particle Image Velocimetry in MATLAB. J. Open Res. Softw. 2014, 2, e30. [Google Scholar] [CrossRef]
- Huxley, H.E. The mechanism of muscular contraction. Science 1969, 164, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Lymn, R.W.; Taylor, E.W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 1971, 10, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Spudich, J.A. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell Biol. 2001, 2, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Howard, J. (Ed.) Mechanics of Motor Proteins and the Cytoskeleton; Sinauer Associates Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef]
- Pollard, T.D.; Goldberg, I.; Schwarz, W.H. Nucleotide exchange, structure, and mechanical properties of filaments assembled from ATP-actin and ADP-actin. J. Biol. Chem. 1992, 267, 20339–20345. [Google Scholar]
- Mogilner, A. Mathematics of cell motility: Have we got its number? J. Math. Biol. 2009, 58, 105–134. [Google Scholar] [CrossRef]
- Lewis, O.L.; Guy, R.D.; Allard, J.F. Actin-myosin spatial patterns from a simplified isotropic viscoelastic model. Biophys. J. 2014, 107, 863–870. [Google Scholar] [CrossRef]
- Wolfer, C.; Vogel, S.K.; Mangold, M. A curvilinear Model Approach: Actin Cortex Clustering Due to ATP-induced Myosin Pulls. IFAC Pap. 2016, 49, 103–108. [Google Scholar] [CrossRef]
- Kruse, K.; Camalet, S.; Julicher, F. Self-propagating patterns in active filament bundles. Phys. Rev. Lett. 2001, 87, 138101. [Google Scholar] [CrossRef] [PubMed]
- Kreten, F.H.; Hoffmann, C.; Riveline, D.; Kruse, K. Active bundles of polar and bipolar filaments. Phys. Rev. E 2018, 98, 012413. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, S.K.; Wölfer, C.; Ramirez-Diaz, D.A.; Flassig, R.J.; Sundmacher, K.; Schwille, P. Symmetry Breaking and Emergence of Directional Flows in Minimal Actomyosin Cortices. Cells 2020, 9, 1432. https://doi.org/10.3390/cells9061432
Vogel SK, Wölfer C, Ramirez-Diaz DA, Flassig RJ, Sundmacher K, Schwille P. Symmetry Breaking and Emergence of Directional Flows in Minimal Actomyosin Cortices. Cells. 2020; 9(6):1432. https://doi.org/10.3390/cells9061432
Chicago/Turabian StyleVogel, Sven K., Christian Wölfer, Diego A. Ramirez-Diaz, Robert J. Flassig, Kai Sundmacher, and Petra Schwille. 2020. "Symmetry Breaking and Emergence of Directional Flows in Minimal Actomyosin Cortices" Cells 9, no. 6: 1432. https://doi.org/10.3390/cells9061432
APA StyleVogel, S. K., Wölfer, C., Ramirez-Diaz, D. A., Flassig, R. J., Sundmacher, K., & Schwille, P. (2020). Symmetry Breaking and Emergence of Directional Flows in Minimal Actomyosin Cortices. Cells, 9(6), 1432. https://doi.org/10.3390/cells9061432




