Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Adipose Tissue Handling
2.3. Adipocyte Cultures
2.4. Subcellular Fractionation Studies
2.5. AQP11 Knockdown by siRNA Transfection
2.6. Real-Time PCR
2.7. Western-Blot
2.8. Immunohistochemistry of AQP11
2.9. Confocal Immunofluorescence Microscopy of AQP11
2.10. Statistical Analysis
3. Results
3.1. Obesity and Obesity-Associated Type 2 Diabetes Upregulated AQP11 Expression in Human Visceral Fat
3.2. AQP11 is Increased during Adipocyte Differentiation and Lipolysis
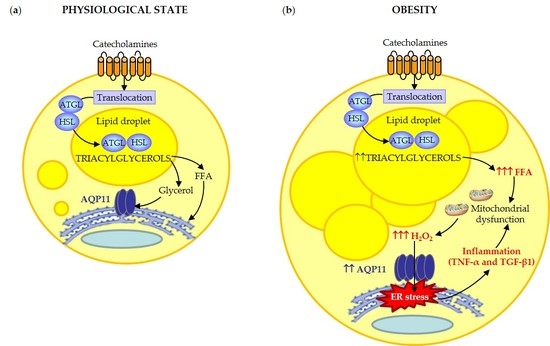
3.3. AQP11 Participates in TGF-β1-Induced Endoplasmic Reticulum Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agre, P. The 2009 lindau nobel laureate meeting: Peter agre, chemistry 2003. J. Vis. Exp. JoVE 2009, e1565. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Wang, W. Molecular aspects of aquaporins. Vitam. Horm. 2020, 113, 129–181. [Google Scholar] [CrossRef]
- Tesse, A.; Grossini, E.; Tamma, G.; Brenner, C.; Portincasa, P.; Marinelli, R.A.; Calamita, G. Aquaporins as targets of dietary bioactive phytocompounds. Front. Mol. Biosci. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Rodríguez, A.; Balaguer, I.; Frühbeck, G. Role of aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, L.; Pellegrini-Calace, M.; Oliva, R. In silico study of human aquaporin AQP11 and AQP12 channels. Protein Sci. 2013, 22, 455–466. [Google Scholar] [CrossRef]
- Ishibashi, K.; Koike, S.; Kondo, S.; Hara, S.; Tanaka, Y. The role of a group III AQP, AQP11 in intracellular organelle homeostasis. J. Med. Investig. 2009, 56, 312–317. [Google Scholar] [CrossRef][Green Version]
- Yakata, K.; Hiroaki, Y.; Ishibashi, K.; Sohara, E.; Sasaki, S.; Mitsuoka, K.; Fujiyoshi, Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim. Biophys. Acta 2007, 1768, 688–693. [Google Scholar] [CrossRef]
- Madeira, A.; Fernandez-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafre, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef]
- Bestetti, S.; Galli, M.; Sorrentino, I.; Pinton, P.; Rimessi, A.; Sitia, R.; Medrano-Fernandez, I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020, 28, 101326. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpao, C.; Mosca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human aquaporin-5 facilitates hydrogen peroxide permeation affecting adaption to oxidative stress and cancer cell migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Marchissio, M.J.; Frances, D.E.; Carnovale, C.E.; Marinelli, R.A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 2012, 264, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef][Green Version]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef]
- Rützler, M.; Rojek, A.; Damgaard, M.V.; Andreasen, A.; Fenton, R.A.; Nielsen, S. Temporal deletion of Aqp11 in mice is linked to the severity of cyst-like disease. Am. J. Physiol. Renal. Physiol. 2017, 312, F343–F351. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sonoda, H.; Nishimura, R.; Mori, K.; Ishibashi, K.; Ikeda, M. Involvement of the NADPH oxidase 2 pathway in renal oxidative stress in Aqp11 (-/-) mice. Biochem. Biophys. Rep. 2019, 17, 169–176. [Google Scholar] [CrossRef]
- Rojek, A.; Fuchtbauer, E.M.; Fuchtbauer, A.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after feeding amino acids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G501–G515. [Google Scholar] [CrossRef]
- Ezquerro, S.; Becerril, S.; Tuero, C.; Méndez-Giménez, L.; Mocha, F.; Moncada, R.; Valentí, V.; Cienfuegos, J.A.; Catalán, V.; Gómez-Ambrosi, J.; et al. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int. J. Obes. 2020, 44, 475–487. [Google Scholar] [CrossRef]
- Kawasaki, N.; Asada, R.; Saito, A.; Kanemoto, S.; Imaizumi, K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012, 2, 799. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Gorgun, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef]
- Guzmán-Ruiz, R.; Tercero-Alcázar, C.; Rabanal-Ruiz, Y.; Díaz-Ruiz, A.; El Bekay, R.; Rangel-Zuñiga, O.A.; Navarro-Ruiz, M.C.; Molero, L.; Membrives, A.; Ruiz-Rabelo, J.F.; et al. Adipose tissue depot-specific intracellular and extracellular cues contributing to insulin resistance in obese individuals. FASEB J. 2020. [Google Scholar] [CrossRef]
- Pulido, M.R.; Díaz-Ruiz, A.; Jiménez-Gómez, Y.; García-Navarro, S.; Gracia-Navarro, F.; Tinahones, F.; López-Miranda, J.; Frühbeck, G.; Vázquez-Martínez, R.; Malagón, M.M. Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS ONE 2011, 6, e22931. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Pastor, C.; Rotellar, F.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Vendrell, J.; et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008, 18, 695–701. [Google Scholar] [CrossRef]
- Díaz-Ruiz, A.; Guzmán-Ruiz, R.; Moreno, N.R.; García-Ríos, A.; Delgado-Casado, N.; Membrives, A.; Tunez, I.; El Bekay, R.; Fernández-Real, J.M.; Tovar, S.; et al. Proteasome dysfunction associated to oxidative stress and proteotoxicity in adipocytes compromises insulin sensitivity in human obesity. Antioxid. Redox Signal. 2015, 23, 597–612. [Google Scholar] [CrossRef]
- Heid, H.; Rickelt, S.; Zimbelmann, R.; Winter, S.; Schumacher, H.; Dorflinger, Y.; Kuhn, C.; Franke, W.W. On the formation of lipid droplets in human adipocytes: The organization of the perilipin-vimentin cortex. PLoS ONE 2014, 9, e90386. [Google Scholar] [CrossRef]
- Skinner, J.R.; Harris, L.A.; Shew, T.M.; Abumrad, N.A.; Wolins, N.E. Perilipin 1 moves between the fat droplet and the endoplasmic reticulum. Adipocyte 2013, 2, 80–86. [Google Scholar] [CrossRef]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.A.; Fernández, S.; Rodríguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Krintel, C.; Hernebring, M.; Haataja, T.J.; de Marè, S.; Wasserstrom, S.; Kosinska-Eriksson, U.; Palmgren, M.; Holm, C.; Stenkula, K.G.; et al. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase A mediated phosphorylation. Metabolism 2016, 65, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef]
- Paar, M.; Jungst, C.; Steiner, N.A.; Magnes, C.; Sinner, F.; Kolb, D.; Lass, A.; Zimmermann, R.; Zumbusch, A.; Kohlwein, S.D.; et al. Remodeling of lipid droplets during lipolysis and growth in adipocytes. J. Biol. Chem. 2012, 287, 11164–11173. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Hernández-Pardos, A.W.; Frühbeck, G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Heinonen, S.; Muniandy, M.; Buzkova, J.; Mardinoglu, A.; Rodríguez, A.; Frühbeck, G.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Kaprio, J.; et al. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: A study of young healthy MZ twins. Diabetologia 2017, 60, 169–181. [Google Scholar] [CrossRef]
- Baldini, F.; Portincasa, P.; Grasselli, E.; Damonte, G.; Salis, A.; Bonomo, M.; Florio, M.; Serale, N.; Voci, A.; Gena, P.; et al. Aquaporin-9 is involved in the lipid-lowering activity of the nutraceutical silybin on hepatocytes through modulation of autophagy and lipid droplets composition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158586. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Z.; Zhao, S.; Xiang, R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016, 6, 27486. [Google Scholar] [CrossRef] [PubMed]
- Chiadak, J.D.; Arsenijevic, T.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Delporte, C. Involvement of JNK/NFkB signaling pathways in the lipopolysaccharide-induced modulation of aquaglyceroporin expression in 3T3-L1 Cells differentiated into adipocytes. Int. J. Mol. Sci. 2016, 17, 1742. [Google Scholar] [CrossRef] [PubMed]





| Lean | Obese NG | Obese IGT/T2D | p | |
|---|---|---|---|---|
| n | 14 | 24 | 29 | - |
| Sex (male/female) | 6/8 | 10/14 | 13/16 | 0.973 |
| Age (years) | 48 ± 3 | 41 ± 3 | 45 ± 2 | 0.140 |
| BMI (kg/m2) | 23.1 ± 0.8 | 47.2 ± 1.5 a | 48.6 ± 1.6 a | <0.0001 |
| Body fat (%) | 24.8 ± 2.8 | 51.7 ± 1.4 a | 52.1 ± 1.3 a | <0.0001 |
| Glucose (mg/dL) | 85 ± 3 | 91 ± 2 | 120 ± 7 a,b | 0.001 |
| Glucose 2-h OGTT (mg/dL) | - | 119 ± 6 | 194 ± 14 b | <0.0001 |
| Insulin (µU/mL) | 7.8 ± 1.4 | 21.1 ± 2.8 a | 23.9 ± 4.9 a | 0.004 |
| Insulin 2-h OGTT (µU/mL) | - | 93.2 ± 13.6 | 90.6 ± 7.3 | 0.862 |
| HOMA | 1.7 ± 0.3 | 4.8 ± 0.7 a | 7.8 ± 2.0 a | 0.007 |
| QUICKI | 0.36 ± 0.01 | 0.31 ± 0.01 a | 0.31 ± 0.01 a | 0.014 |
| FFA (mmol/L) | 13.3 ± 1.6 | 17.0 ± 1.3 | 18.6 ± 1.9 a | 0.040 |
| Glycerol (mg/dL) | 22.5 ± 3.5 | 34.1 ± 3.4 | 44.4 ± 5.0 a | 0.008 |
| Adipo-IR index | 21.1 ± 3.1 | 83.6 ± 11.6 | 108.6 ± 19.8 a | 0.003 |
| Triacylglycerol (mg/dL) | 68 ± 9 | 135 ± 19 a | 165 ± 33 a | 0.011 |
| Total cholesterol (mg/dL) | 191 ± 8 | 196 ± 8 | 200 ± 6 | 0.800 |
| LDL-cholesterol (mg/dL) | 117 ± 8 | 119 ± 8 | 130 ± 6 | 0.448 |
| HDL-cholesterol (mg/dL) | 59 ± 2 | 49 ± 5 a | 44 ± 2 a | 0.013 |
| CRP (mg/L) | 2.3 ± 0.6 | 8.8 ± 1.4 a | 11.5 ± 2.8 a | 0.001 |
| Uric acid (mg/dL) | 4.2 ± 0.4 | 9.2 ± 2.8 a | 6.5 ± 0.2 a | 0.007 |
| Leptin (ng/mL) | 7.2 ± 1.4 | 46.9 ± 5.8 a | 53.5 ± 6.5 a | 0.004 |
| TNF-α (ng/mL) | 0.87 ± 0.15 | 1.89 ± 0.12 a | 2.02 ± 0.41 a | 0.003 |
| Fibrinogen (mg/dL) | 251 ± 42 | 358 ± 16 a | 372 ± 15 a | 0.003 |
| von Willebrand factor (%) | 87 ± 11 | 126 ± 9 a | 154 ± 14 a | 0.025 |
| Antihypertensive therapy, n (%) | 0 (0%) | 7 (29%) | 8 (28%) | 0.092 |
| Antidiabetic therapy, n (%) | 0 (0%) | 0 (0%) | 4 (14%) | 0.041 |
| Lipid-lowering therapy, n (%) | 0 (0%) | 4 (17%) | 2 (22%) | 0.384 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frühbeck, G.; Balaguer, I.; Méndez-Giménez, L.; Valentí, V.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; Salvador, J.; Calamita, G.; et al. Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation. Cells 2020, 9, 1403. https://doi.org/10.3390/cells9061403
Frühbeck G, Balaguer I, Méndez-Giménez L, Valentí V, Becerril S, Catalán V, Gómez-Ambrosi J, Silva C, Salvador J, Calamita G, et al. Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation. Cells. 2020; 9(6):1403. https://doi.org/10.3390/cells9061403
Chicago/Turabian StyleFrühbeck, Gema, Inmaculada Balaguer, Leire Méndez-Giménez, Víctor Valentí, Sara Becerril, Victoria Catalán, Javier Gómez-Ambrosi, Camilo Silva, Javier Salvador, Giuseppe Calamita, and et al. 2020. "Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation" Cells 9, no. 6: 1403. https://doi.org/10.3390/cells9061403
APA StyleFrühbeck, G., Balaguer, I., Méndez-Giménez, L., Valentí, V., Becerril, S., Catalán, V., Gómez-Ambrosi, J., Silva, C., Salvador, J., Calamita, G., Malagón, M. M., & Rodríguez, A. (2020). Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation. Cells, 9(6), 1403. https://doi.org/10.3390/cells9061403