Visfatin Increases VEGF-Dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Clinical Samples
2.4. RT-qPCR of mRNA and miRNA
2.5. Western Blot Analysis
2.6. ELISA Assay
2.7. Transient Transfection
2.8. Plasmid Construction and Luciferase Assays
2.9. EPC Migration and Tube Formation Assays
2.10. In Vivo Matrigel Plug Assay
2.11. Experimental OA Model
2.12. Micro-Computed Tomography (Micro-CT) Imaging
2.13. Statistics
3. Results
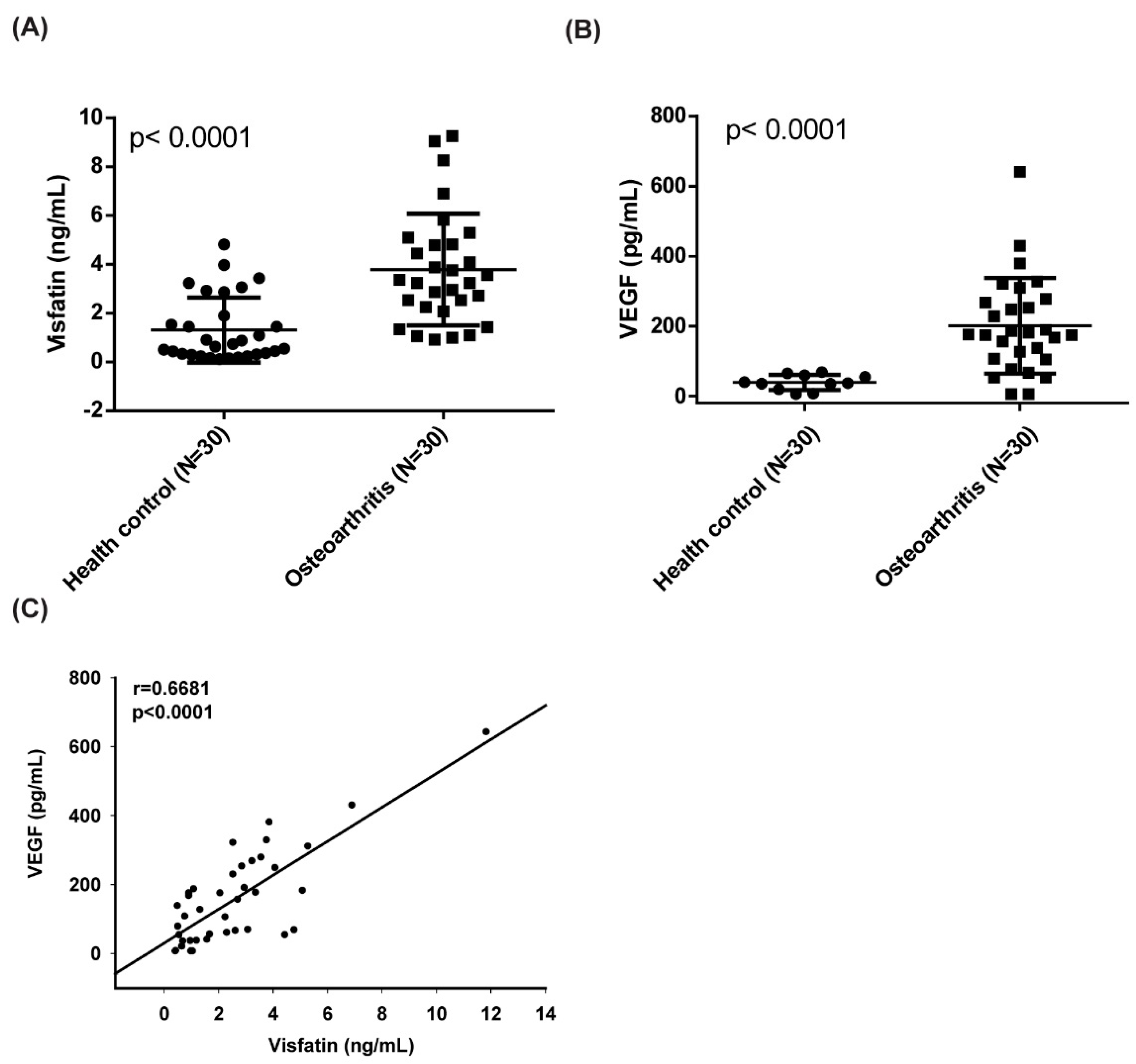
3.1. A Positive Correlation Exists between Visfatin and VEGF Expression in OA
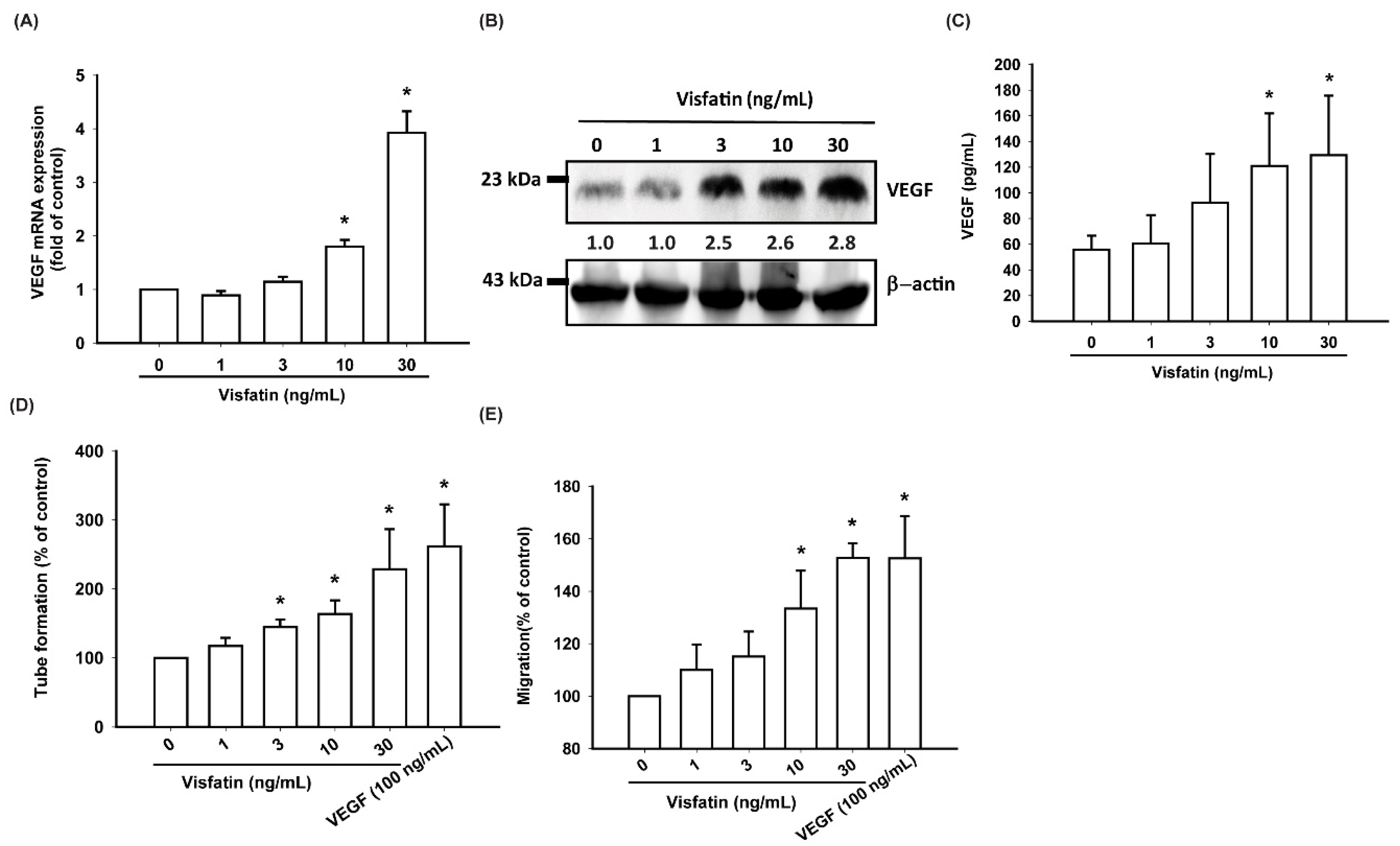
3.2. Visfatin Increases VEGF Expression and EPC Angiogenesis in Human OASFs
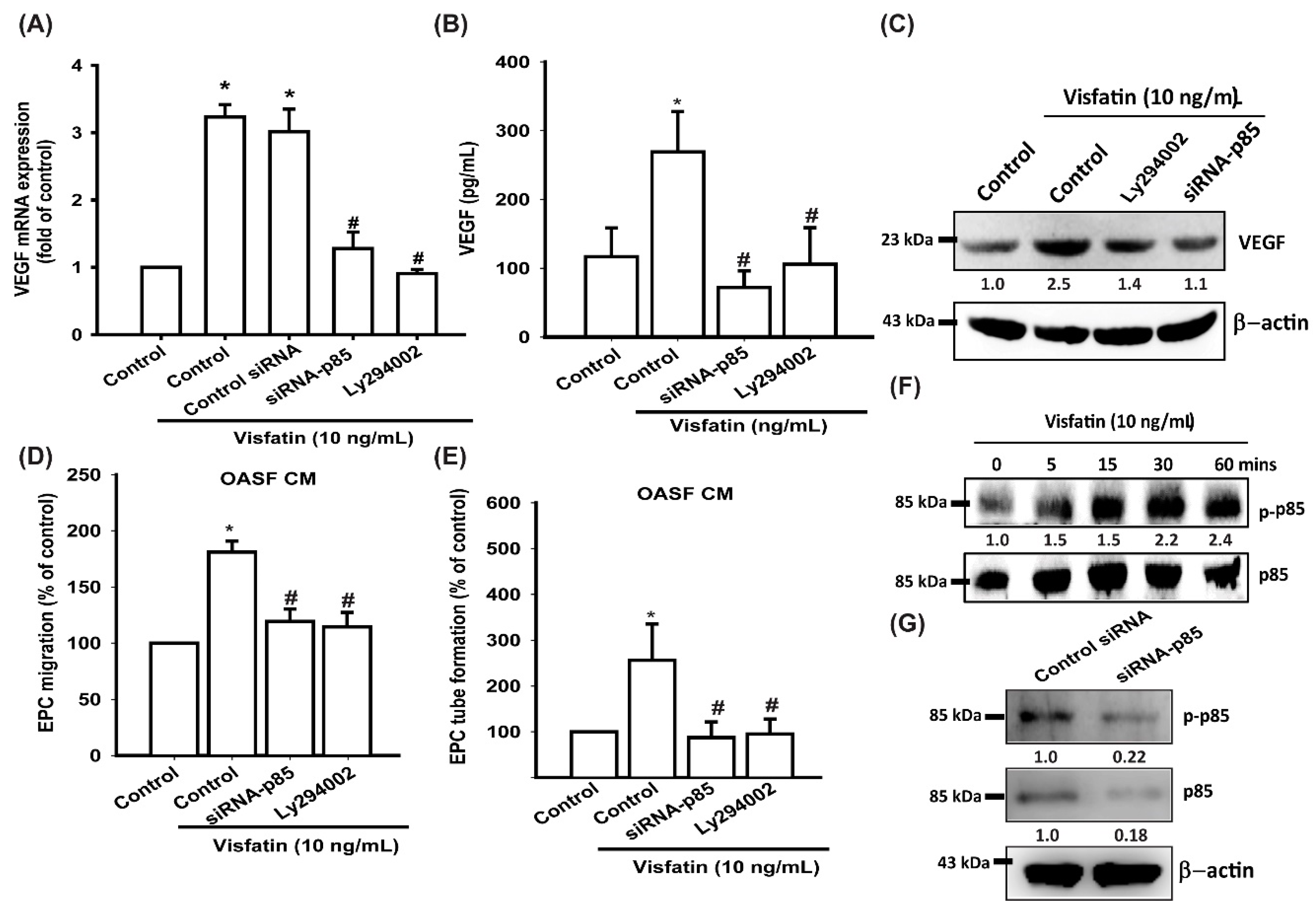
3.3. Visfatin Promotes VEGF Production and EPC Angiogenesis via the PI3K and Akt Signaling Pathways
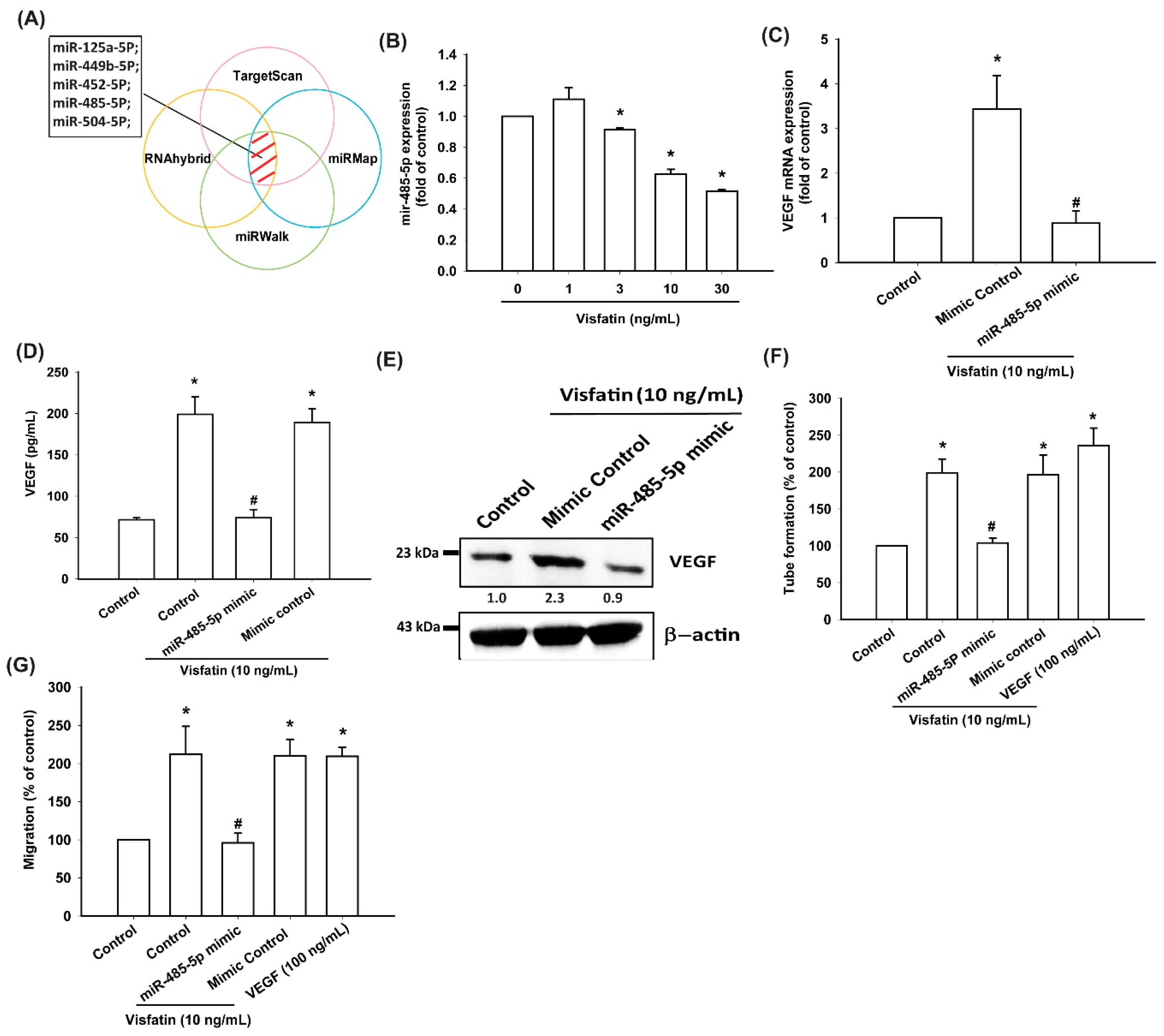
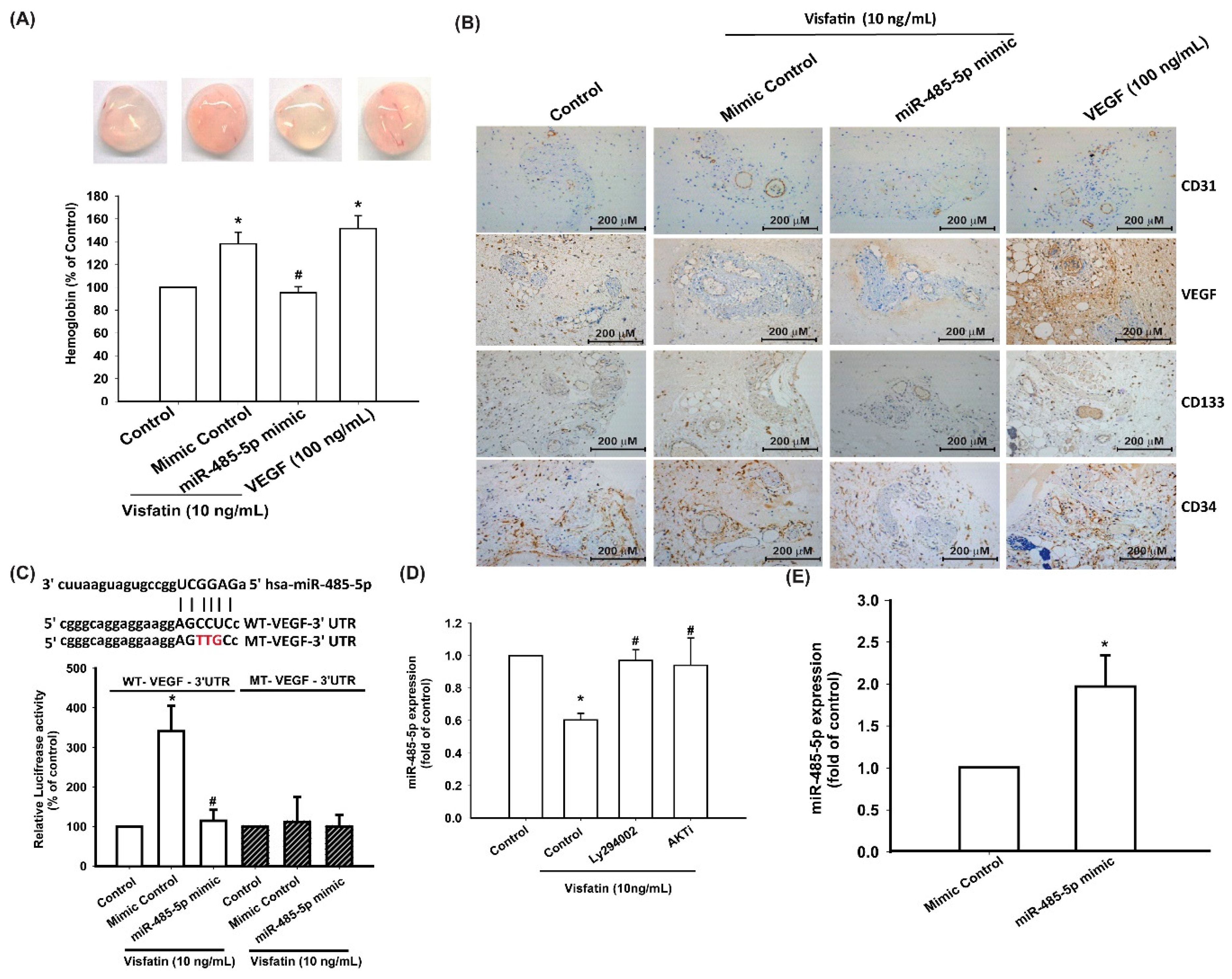
3.4. Visfatin Increases VEGF Production and EPC Angiogenesis via the Inhibition of miR-485-5p Synthesis
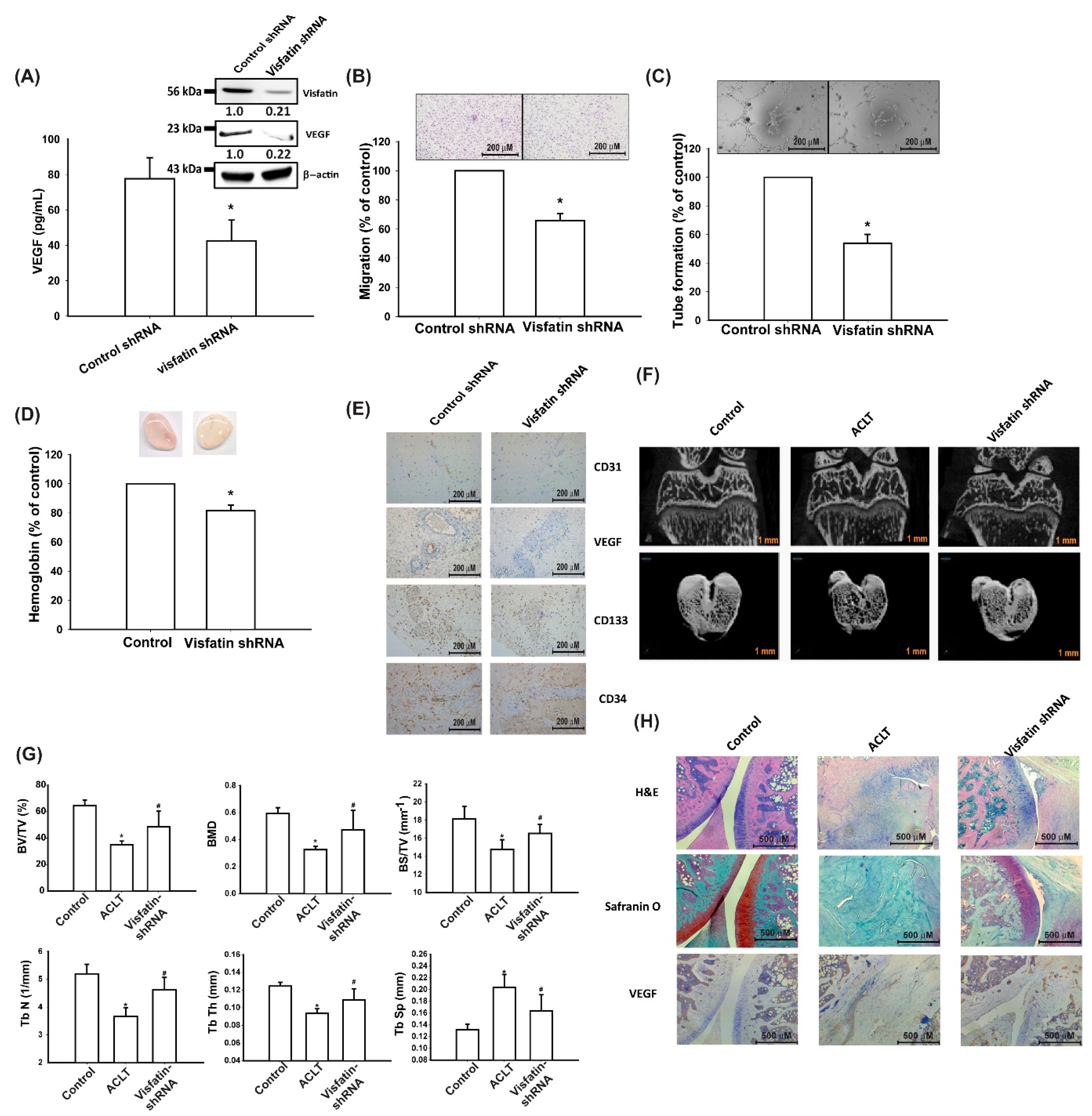
3.5. Visfatin Knockdown Mitigates EPC Angiogenesis and OA Severity In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Meng, H.; Wang, Y.; Peng, J.; Guo, Q.; Wang, A.; Lu, S. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.J.; Yang, W.H.; Liu, S.C.; Tsai, C.H.; Hsu, H.C.; Tang, C.H. Transforming growth factor beta1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microRNA 519b synthesis. PLoS ONE 2017, 12, e0176052. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Tsai, C.-H.; Wu, T.-Y.; Tsai, C.-H.; Tsai, F.-J.; Chung, J.-G.; Huang, C.-Y.; Yang, J.-S.; Hsu, Y.-M.; Yin, M.-C.; et al. Soya-cerebroside reduces IL-1β-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agric. Immunol. 2019, 30, 620–632. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Asgharian, S.; Khalesi, E.; Ahmadi, A.; Lorigooini, Z. Comparative study of the effect of Thymus daenensis gel 5% and diclofenac in patients with knee osteoarthritis. Biomedicine (Taipei) 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Liu, Y.L.; Lin, J.B.; Cheng, K.L.; Wang, Y.G.; Yao, H.L.; Wei, P.; Wu, H.Y.; Su, W.W.; Shaw, P.C.; et al. Preparative expression and purification of a nacreous protein N16 and testing its effect on osteoporosis rat model. Int. J. Biol. Macromol. 2018, 111, 440–445. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Liu, S.C.; Su, C.M.; Wang, Y.H.; Tsai, C.H.; Tang, C.H. Implications of angiogenesis involvement in arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef]
- Patel, J.; Donovan, P.; Khosrotehrani, K. Concise review: Functional definition of endothelial progenitor cells: A molecular perspective. Stem Cells Transl. Med. 2016, 5, 1302–1306. [Google Scholar] [CrossRef]
- Kiewisz, J.; Kaczmarek, M.M.; Pawlowska, A.; Kmiec, Z.; Stompor, T. Endothelial progenitor cells participation in cardiovascular and kidney diseases: A systematic review. Acta Biochim. Pol. 2016, 63, 475–482. [Google Scholar] [CrossRef]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V. Influence of growth factors and cytokines on angiogenic function of endothelial progenitor cells: A review of in vitro human studies. Growth Factors 2014, 32, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Hsu, C.J.; Tsai, C.H.; Huang, C.Y.; Wang, S.W.; Tang, C.H. Resistin promotes angiogenesis in endothelial progenitor cells through inhibition of microRNA206: Potential implications for rheumatoid arthritis. Stem Cells 2015, 33, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Su, C.M.; Hsu, C.J.; Huang, C.C.; Wang, S.W.; Liu, S.C.; Chen, W.C.; Fuh, L.J.; Tang, C.H. CCN1 promotes VEGF production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting miR-126 expression in rheumatoid arthritis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.J.; Liu, S.C.; Huang, C.C.; Kuo, S.J.; Tsai, C.H.; Tang, C.H. Associations between adipokines in arthritic disease and implications for obesity. Int. J. Mol. Sci. 2019, 20, 1505. [Google Scholar] [CrossRef]
- Wu, M.H.; Tsai, C.H.; Huang, Y.L.; Fong, Y.C.; Tang, C.H. Visfatin promotes IL-6 and TNF-alpha production in human synovial fibroblasts by repressing miR-199a-5p through ERK, p38 and JNK signaling pathways. Int. J. Mol. Sci. 2018, 19, 190. [Google Scholar] [CrossRef]
- Tong, K.M.; Chen, C.P.; Huang, K.C.; Shieh, D.C.; Cheng, H.C.; Tzeng, C.Y.; Chen, K.H.; Chiu, Y.C.; Tang, C.H. Adiponectin increases MMP-3 expression in human chondrocytes through AdipoR1 signaling pathway. J. Cell. Biochem. 2011, 112, 1431–1440. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Liao, L.; Chen, Y.; Wang, W. The current progress in understanding the molecular functions and mechanisms of visfatin in osteoarthritis. J. Bone Miner. Metab. 2016, 34, 485–490. [Google Scholar] [CrossRef]
- Fioravanti, A.; Giannitti, C.; Cheleschi, S.; Simpatico, A.; Pascarelli, N.A.; Galeazzi, M. Circulating levels of adiponectin, resistin, and visfatin after mud-bath therapy in patients with bilateral knee osteoarthritis. Int. J. Biometeorol. 2015, 59, 1691–1700. [Google Scholar] [CrossRef]
- Kuo, S.J.; Liu, S.C.; Huang, Y.L.; Tsai, C.H.; Fong, Y.C.; Hsu, H.C.; Tang, C.H. TGF-beta1 enhances FOXO3 expression in human synovial fibroblasts by inhibiting miR-92a through AMPK and p38 pathways. Aging 2019, 11, 4075–4089. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses IL-1beta-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, S.C.; Sun, H.L.; Huang, T.Y.; Chan, C.H.; Yang, C.Y.; Yeh, H.I.; Huang, Y.L.; Chou, W.Y.; Lin, Y.M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, C.Y.; Lin, J.A.; Wang, S.W.; Peng, C.Y.; Cheng, H.C.; Tang, C.H. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene 2014, 33, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chao, C.C.; Chen, P.C.; Liu, P.I.; Yang, Y.C.; Su, C.M.; Huang, W.C.; Tang, C.H. Thrombospondin enhances RANKL-dependent osteoclastogenesis and facilitates lung cancer bone metastasis. Biochem. Pharmacol. 2019, 166, 23–32. [Google Scholar] [CrossRef]
- Liu, J.F.; Lee, C.W.; Tsai, M.H.; Tang, C.H.; Chen, P.C.; Lin, L.W.; Lin, C.Y.; Lu, C.H.; Lin, Y.F.; Yang, S.H.; et al. Thrombospondin 2 promotes tumor metastasis by inducing matrix metalloproteinase-13 production in lung cancer cells. Biochem. Pharmacol. 2018, 155, 537–546. [Google Scholar] [CrossRef]
- Lee, H.P.; Chen, P.C.; Wang, S.W.; Fong, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J. Funct. Foods 2019, 52, 537–544. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Lin, L.W.; Tsai, F.J.; Yang, J.S.; Li, T.M.; Tang, C.H. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agric. Immunol. 2020, 31, 193–204. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef]
- Su, C.M.; Tang, C.H.; Chi, M.J.; Lin, C.Y.; Fong, Y.C.; Liu, Y.C.; Chen, W.C.; Wang, S.W. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem. Pharmacol. 2018, 154, 234–242. [Google Scholar] [CrossRef]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C.; Tsai, F.J.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Cheng, L.Y.; Su, P.H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chiou, P.C.; Chen, P.C.; Liu, P.Y.; Huang, W.C.; Chao, C.C.; Tang, C.H. Melatonin reduces lung cancer stemness through inhibiting of PLC, ERK, p38, beta-catenin, and Twist pathways. Environ. Toxicol. 2019, 34, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chuang, S.M.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 2014, 5, e1485. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Huang, Y.W.; Wang, S.W.; Huang, Y.L.; Tsai, C.H.; Zhao, Y.M.; Huang, B.F.; Xu, G.H.; Fong, Y.C.; Tang, C.H.; et al. Amphiregulin enhances VEGF-A production in human chondrosarcoma cells and promotes angiogenesis by inhibiting miR-206 via FAK/c-Src/PKCdelta pathway. Cancer Lett. 2017, 385, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Chiang, Y.C.; Huang, C.Y.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Osteopontin promotes oncostatin M production in human osteoblasts: Implication of rheumatoid arthritis therapy. J. Immunol. 2015, 195, 3355–3364. [Google Scholar] [CrossRef]
- Wang, C.J.; Cheng, J.H.; Chou, W.Y.; Hsu, S.L.; Chen, J.H.; Huang, C.Y. Changes of articular cartilage and subchondral bone after extracorporeal shockwave therapy in osteoarthritis of the knee. Int. J. Med. Sci. 2017, 14, 213–223. [Google Scholar] [CrossRef]
- Ammendola, M.; Leporini, C.; Luposella, M.; Sacco, R.; Sammarco, G.; Russo, E.; Patruno, R.; De Sarro, G.; Ranieri, G. Targeting endothelial progenitor cells in cancer as a novel biomarker and anti-angiogenic therapy. Curr. Stem Cell Res. Ther. 2015, 10, 181–187. [Google Scholar] [CrossRef]
- Li, T.M.; Liu, S.C.; Huang, Y.H.; Huang, C.C.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. YKL-40-induced inhibition of miR-590-3p promotes interleukin-18 expression and angiogenesis of endothelial progenitor cells. Int. J. Mol. Sci. 2017, 18, 920. [Google Scholar] [CrossRef]
- Chen, S.S.; Tang, C.H.; Chie, M.J.; Tsai, C.H.; Fong, Y.C.; Lu, Y.C.; Chen, W.C.; Lai, C.T.; Wei, C.Y.; Tai, H.C.; et al. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019, 10, 31. [Google Scholar] [CrossRef]
- Su, C.M.; Wang, I.C.; Liu, S.C.; Sun, Y.; Jin, L.; Wang, S.W.; Lee, H.P.; Tseng, W.P.; Tang, C.H. Hypoxia induced mitogenic factor (HIMF) triggers angiogenesis by increasing interleukin-18 production in myoblasts. Sci. Rep. 2017, 7, 7393. [Google Scholar] [CrossRef][Green Version]
- Taipaleenmaki, H. Regulation of bone metabolism by microRNAs. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gao, W.; Wu, Z.; Zhou, T.; Qiu, X.; Wang, X.; Lian, C.; Peng, Y.; Liang, A.; Qiu, J.; et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 162. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Liu, J.F.; Fong, Y.C.; Huang, Y.L.; Chao, C.C.; Tang, C.H. CCN3 facilitates Runx2 and osterix expression by inhibiting miR-608 through PI3K/Akt signaling in osteoblasts. Int. J. Mol. Sci. 2019, 20, 3300. [Google Scholar] [CrossRef]
- Tzeng, H.E.; Tang, C.H.; Wu, S.H.; Chen, H.T.; Fong, Y.C.; Lu, Y.C.; Chen, W.C.; Huang, H.D.; Lin, C.Y.; Wang, S.W.; et al. CCN6-mediated MMP-9 activation enhances metastatic potential of human chondrosarcoma. Cell Death Dis. 2018, 9, 955. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Samakova, A.; Gazova, A.; Sabova, N.; Valaskova, S.; Jurikova, M.; Kyselovic, J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol. Res. 2019, 68, S131–S138. [Google Scholar] [CrossRef]
- Nugent, M. MicroRNAs: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway protective effects of MicroRNAs on human chondrocytes in an in vitro model of osteoarthritis. Mol. Ther. Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Liu, S.-C.; Chung, W.-H.; Wang, S.-W.; Wu, M.-H.; Tang, C.-H. Visfatin Increases VEGF-Dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression. Cells 2020, 9, 1315. https://doi.org/10.3390/cells9051315
Tsai C-H, Liu S-C, Chung W-H, Wang S-W, Wu M-H, Tang C-H. Visfatin Increases VEGF-Dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression. Cells. 2020; 9(5):1315. https://doi.org/10.3390/cells9051315
Chicago/Turabian StyleTsai, Chun-Hao, Shan-Chi Liu, Wen-Hui Chung, Shih-Wei Wang, Min-Huan Wu, and Chih-Hsin Tang. 2020. "Visfatin Increases VEGF-Dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression" Cells 9, no. 5: 1315. https://doi.org/10.3390/cells9051315
APA StyleTsai, C.-H., Liu, S.-C., Chung, W.-H., Wang, S.-W., Wu, M.-H., & Tang, C.-H. (2020). Visfatin Increases VEGF-Dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression. Cells, 9(5), 1315. https://doi.org/10.3390/cells9051315




