Preliminary Trichinella spiralis Infection Ameliorates Subsequent RSV Infection-Induced Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell, Parasite, and Virus Preparation
2.2. In Vivo Experiment and Animal Ethics
2.3. Serum Collection and RSV-Specific Antibody Response Detection
2.4. Lung Viral Load Reduction
2.5. Inflammatory Cytokine and Bronchoalveolar Lavage Fluid Cell Influx Detection
2.6. Histopathological Assessment of Murine Lung Tissues
2.7. Protein Expression Using Western Blot
2.8. Statistical Analyses
3. Results
3.1. T. spiralis Infection Contributed to Mounting an Enhanced RSV-Specific Antibody Response
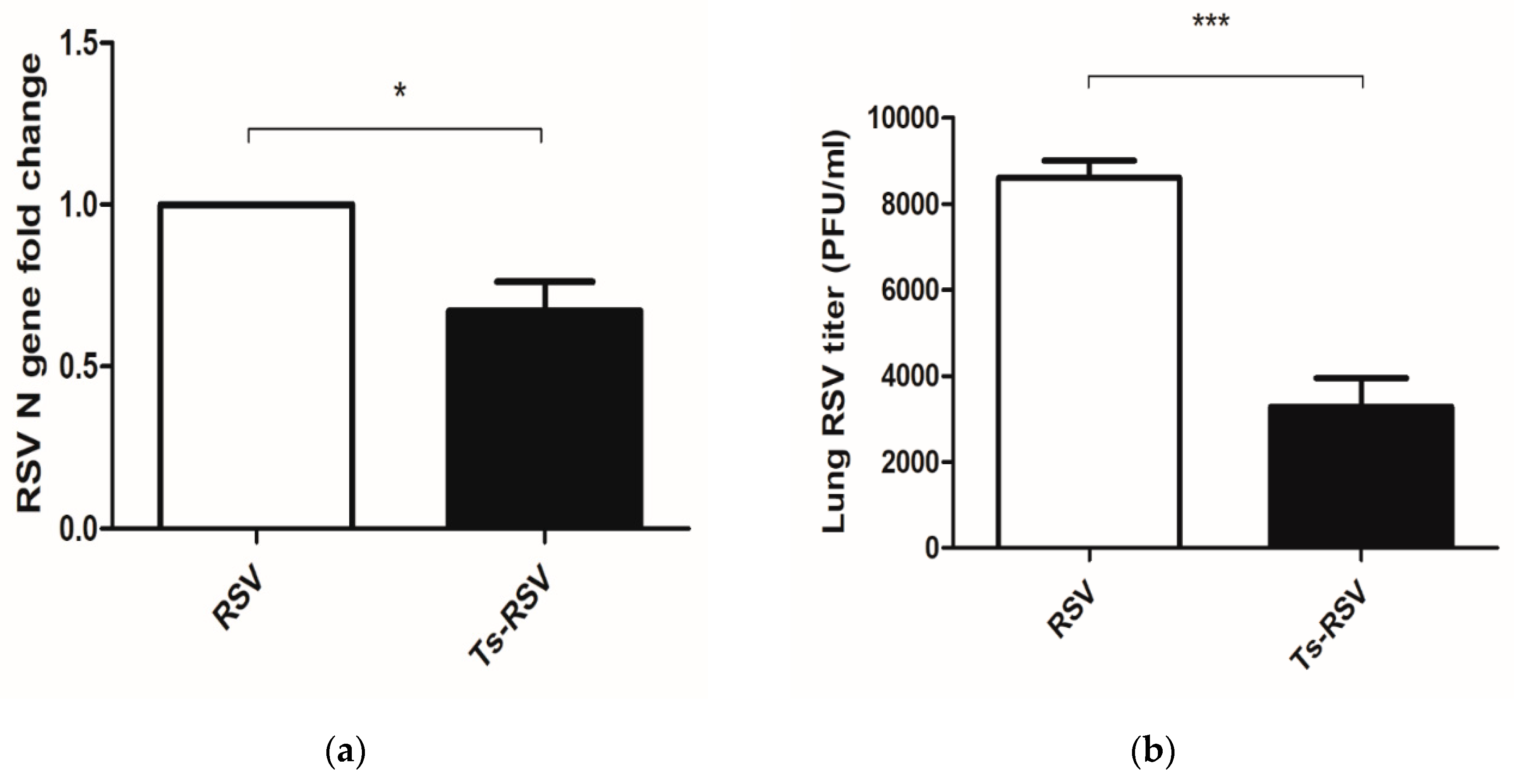
3.2. Preliminary T. spiralis Infection Impedes Viral Replication of RSV in the Lungs
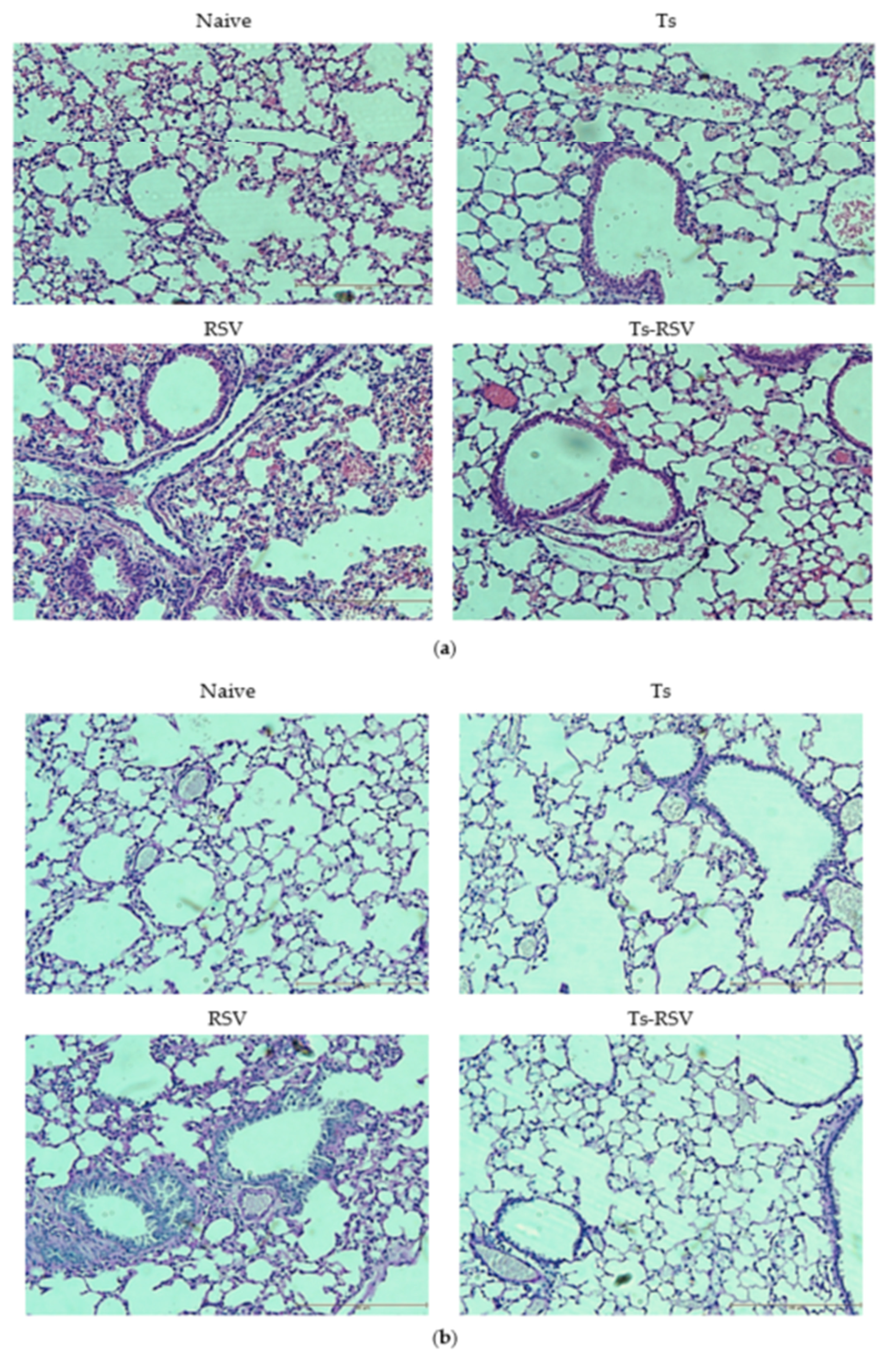
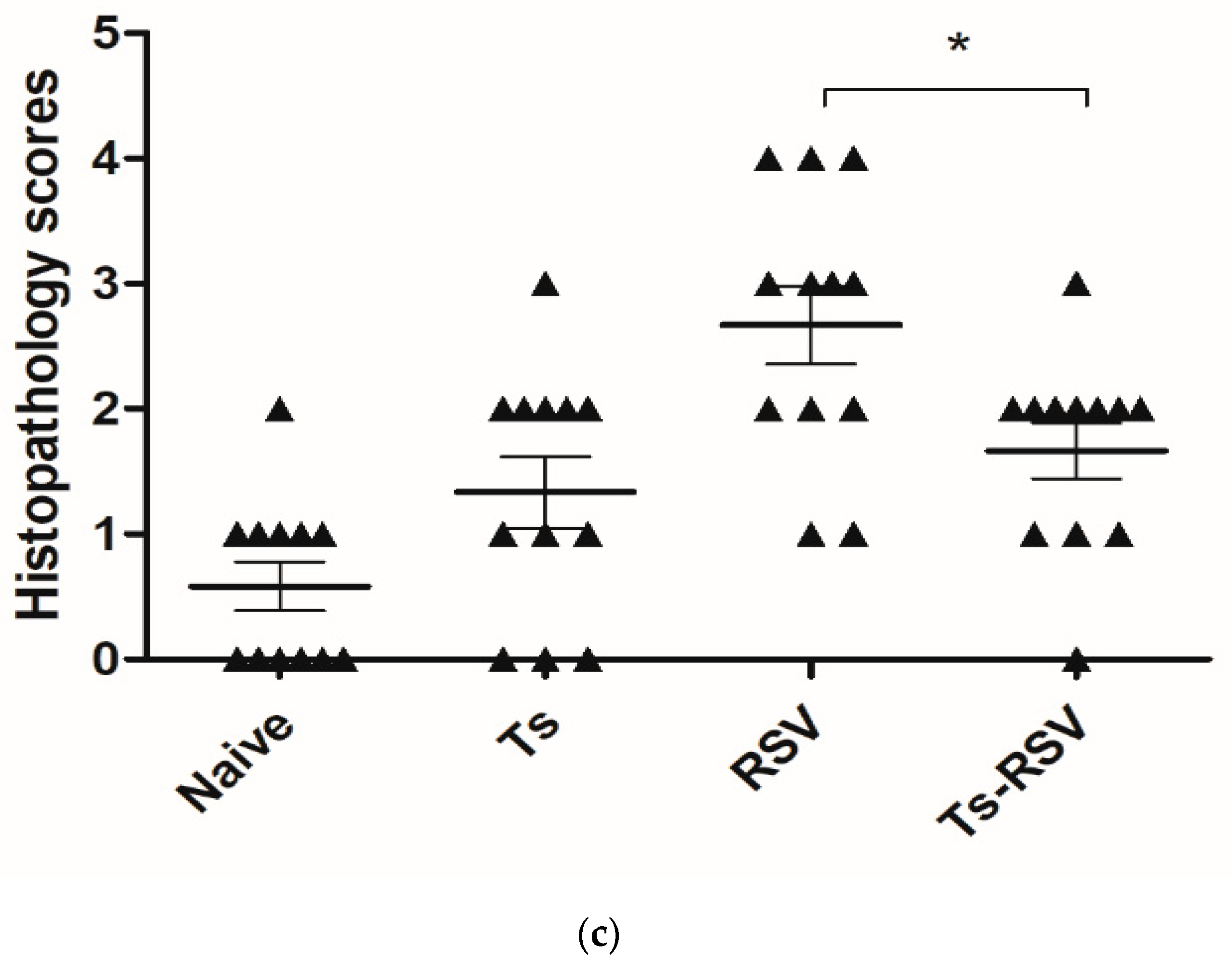
3.3. T. spiralis Curtails Pulmonary Inflammation Triggered by RSV Infection
3.4. The Presence of T. spiralis Contributes to Alleviating Inflammatory Response Induced upon Subsequent RSV Infection
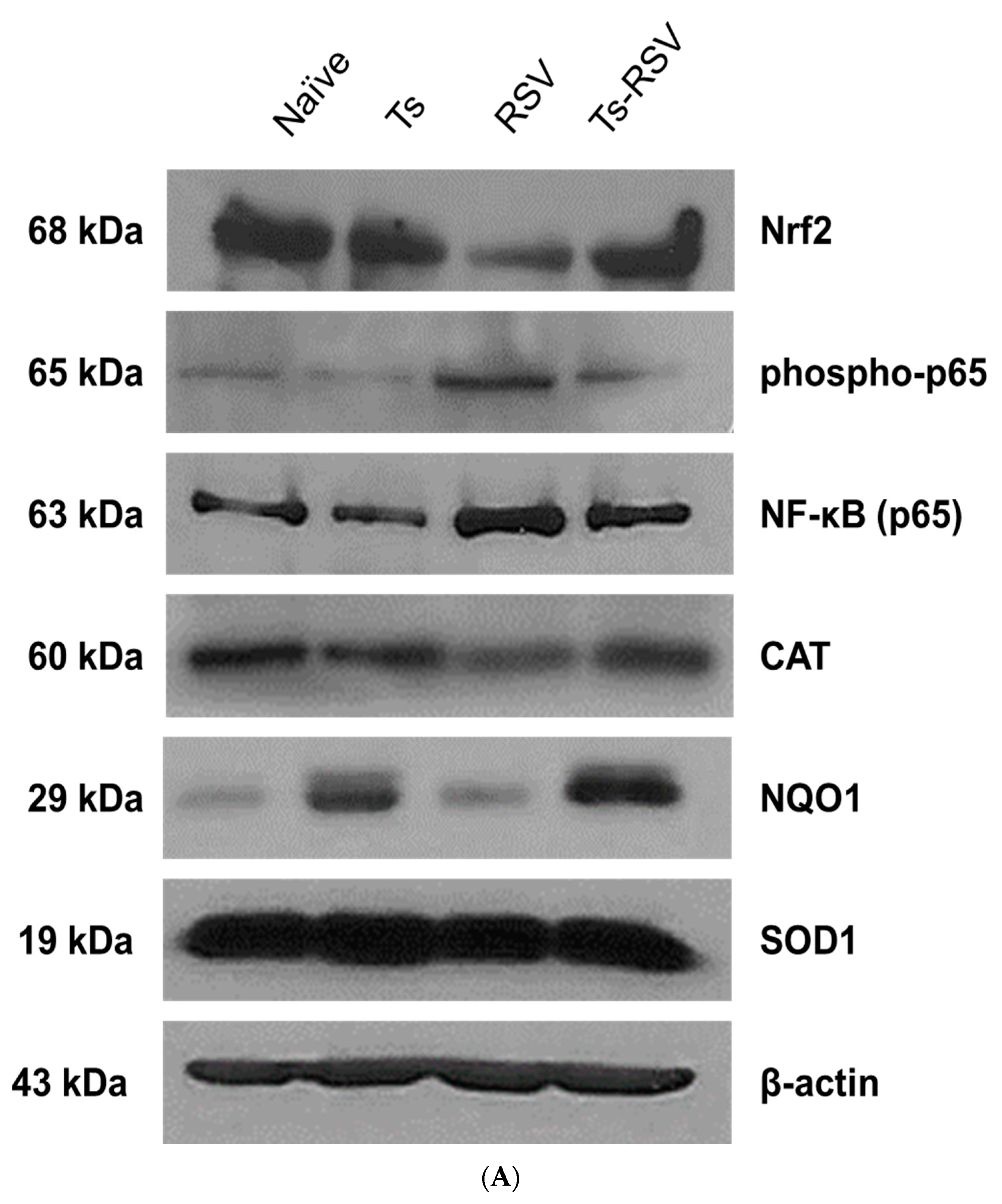
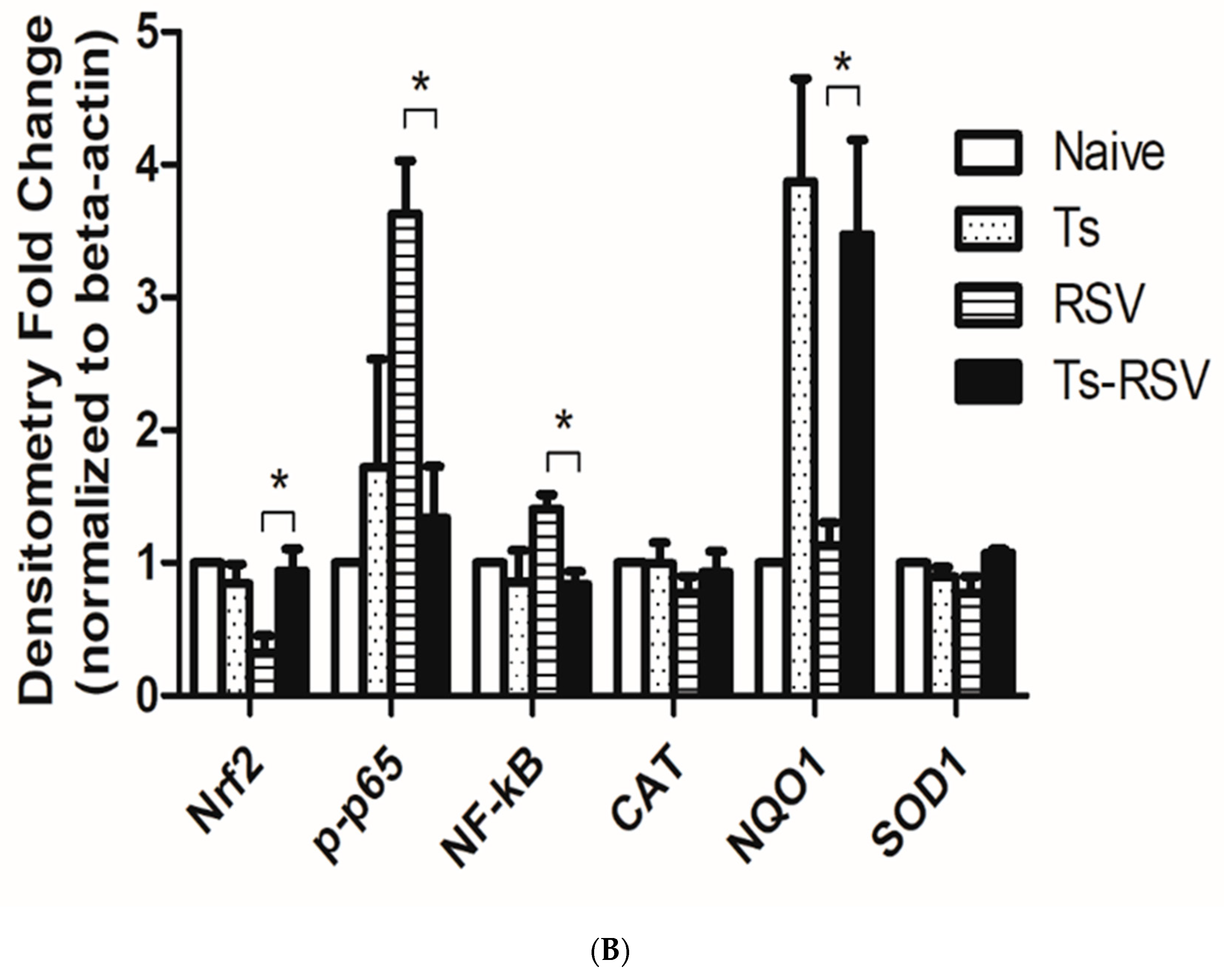
3.5. Antioxidant Enzyme Expressions were Enriched by T. spiralis Infection
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Guvenel, A.K.; Chiu, C.; Openshaw, P.J. Current concepts and progress in RSV vaccine development. Expert Rev. Vaccines 2014, 13, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, M.A.; Gori-Savellini, G.; Gandolfo, C.; Papa, G.; Kaufmann, C.; Felder, E.; Ginori, A.; Disanto, M.G.; Spina, D.; Cusi, M.G. A Respiratory Syncytial Virus Vaccine Vectored by a Stable Chimeric and Replication-Deficient Sendai Virus Protects Mice without Inducing Enhanced Disease. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Chiu, C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 2013, 3, 468–474. [Google Scholar] [CrossRef]
- Komaravelli, N.; Tian, B.; Ivanciuc, T.; Mautemps, N.; Brasier, A.R.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 2015, 88, 391–403. [Google Scholar] [CrossRef]
- Castro, S.M.; Guerrero-Plata, A.; Suarez-Real, G.; Adegboyega, P.A.; Colasurdo, G.N.; Khan, A.M.; Garofalo, R.P.; Casola, A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am. J. Respir. Crit. Care Med. 2006, 174, 1361–1369. [Google Scholar] [CrossRef]
- Shor, D.B.; Shoenfeld, Y. Autoimmunity: Will worms cure rheumatoid arthritis? Nat. Rev. Rheumatol. 2013, 9, 138–140. [Google Scholar] [CrossRef]
- Weinstock, J.V. Autoimmunity: The worm returns. Nature 2012, 491, 183–185. [Google Scholar] [CrossRef]
- Syverton, J.T.; McCoy, O.R.; Koomen, J. THE TRANSMISSION OF THE VIRUS OF LYMPHOCYTIC CHORIOMENINGITIS BY TRICHINELLA SPIRALIS. J. Exp. Med. 1947, 85, 759–769. [Google Scholar] [CrossRef]
- Zimmermann, W.J.; Schwarte, L.H. The transmission of hog cholera virus by Trichinella spiralis larvae. Can. J. Comp. Med. Vet. Sci. 1966, 30, 84–86. [Google Scholar]
- Cypess, R.H.; Lubiniecki, A.S.; Hammon, W.M. Immunosuppression and increased susceptibility to Japaneses B encephalitis virus in Trichinella spiralis-infected mice. Proc. Soc. Exp. Biol. Med. 1973, 143, 469–473. [Google Scholar] [CrossRef]
- Lubiniecki, A.S.; Cypess, R.H.; Lucas, J.P. Synergistic interaction of two agents in mice: Japanese B encephalitis virus and Trichinella spiralis. Am. J. Trop. Med. Hyg. 1974, 23, 235–241. [Google Scholar] [CrossRef]
- Kang, S.A.; Park, M.K.; Cho, M.K.; Park, S.K.; Jang, M.S.; Yang, B.G.; Jang, M.H.; Kim, D.H.; Yu, H.S. Parasitic nematode-induced CD4+Foxp3+T cells can ameliorate allergic airway inflammation. PLoS Negl. Trop. Dis. 2014, 8, e3410. [Google Scholar] [CrossRef]
- Park, H.K.; Cho, M.K.; Choi, S.H.; Kim, Y.S.; Yu, H.S. Trichinella spiralis: Infection reduces airway allergic inflammation in mice. Exp. Parasitol. 2011, 127, 539–544. [Google Scholar] [CrossRef]
- Aranzamendi, C.; de Bruin, A.; Kuiper, R.; Boog, C.J.; van Eden, W.; Rutten, V.; Pinelli, E. Protection against allergic airway inflammation during the chronic and acute phases of Trichinella spiralis infection. Clin. Exp. Allergy 2013, 43, 103–115. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Yuan, Y.; Wang, L.; He, W.; Xie, H.; Gao, S.; Cheng, R.; Qian, H.; Jiang, H.; et al. Preventive and therapeutic effects of Trichinella spiralis adult extracts on allergic inflammation in an experimental asthma mouse model. Parasites Vectors 2019, 12, 326. [Google Scholar] [CrossRef]
- Furze, R.C.; Hussell, T.; Selkirk, M.E. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect. Immun. 2006, 74, 1924–1932. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, X.; Wang, X.; Zhuang, Q.; Huyan, X.; Sun, X.; Huang, J.; Zhan, B.; Zhu, X. Trichinella spiralis Infection Mitigates Collagen-Induced Arthritis via Programmed Death 1-Mediated Immunomodulation. Front. Immunol. 2018, 9, 1566. [Google Scholar] [CrossRef]
- Xu, J.; Yu, P.; Wu, L.; Liu, M.; Lu, Y. Effect of Trichinella spiralis intervention on TNBS-induced experimental colitis in mice. Immunobiology 2019, 224, 147–153. [Google Scholar] [CrossRef]
- Eissa, M.M.; Mostafa, D.K.; Ghazy, A.A.; El Azzouni, M.Z.; Boulos, L.M.; Younis, L.K. Anti-Arthritic Activity of Schistosoma mansoni and Trichinella spiralis Derived-Antigens in Adjuvant Arthritis in Rats: Role of FOXP3+ Treg Cells. PLoS ONE 2016, 11, e0165916. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, D.H.; Lee, S.H.; Rubino, I.; Choi, H.J.; Quan, F.S. Protection induced by virus-like particle vaccine containing tandem repeat gene of respiratory syncytial virus G protein. PLoS ONE 2018, 13, e0191277. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, A.R.; Piao, Y.; Lee, J.H.; Quan, F.S. A rapid, simple, and accurate plaque assay for human respiratory syncytial virus (HRSV). J. Immunol. Methods 2017, 446, 15–20. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.Q.; Xie, Y.Y.; Zhao, X.D.; Jiang, L.P.; Wang, L.J.; Cui, Y.X. Inhibition of respiratory syncytial virus of subgroups A and B using deoxyribozyme DZ1133 in mice. Virus Res. 2007, 130, 241–248. [Google Scholar] [CrossRef]
- Tasker, L.; Lindsay, R.W.; Clarke, B.T.; Cochrane, D.W.; Hou, S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin. Exp. Immunol. 2008, 153, 277–288. [Google Scholar] [CrossRef]
- Jensen, I.P.; Thisted, E.; Glikmann, G.; Obel, N.; Kofoed, P.E.; Sambo, M.; Valerius, N.H.; Mordhorst, C.H. Secretory IgM and IgA antibodies to respiratory syncytial virus in nasopharyngeal aspirates: A diagnostic supplement to antigen detection. Clin. Diagn. Virol. 1997, 8, 219–226. [Google Scholar] [CrossRef]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef]
- Jiang, J.; Fisher, E.M.; Concannon, M.; Lustigman, S.; Shen, H.; Murasko, D.M. Enhanced humoral response to influenza vaccine in aged mice with a novel adjuvant, rOv-ASP-1. Vaccine 2016, 34, 887–892. [Google Scholar] [CrossRef]
- Lal, R.B.; Rudolph, D.; Alpers, M.P.; Sulzer, A.J.; Shi, Y.P.; Lal, A.A. Immunologic cross-reactivity between structural proteins of human T-cell lymphotropic virus type I and the blood stage of Plasmodium falciparum. Clin. Diagn. Lab. Immunol. 1994, 1, 5–10. [Google Scholar] [CrossRef]
- Porter, K.R.; Anthony, R.L.; Solihin, A.; Hayes, C.G. Mapping of a human T-lymphotropic virus type I gag protein epitope that cross-reacts with anti-Plasmodium falciparum antibodies. J. Med. Virol. 1995, 45, 469–474. [Google Scholar] [CrossRef]
- Chu, K.B.; Lee, D.H.; Kang, H.J.; Quan, F.S. The resistance against Trichinella spiralis infection induced by primary infection with respiratory syncytial virus. Parasitology 2019, 146, 634–642. [Google Scholar] [CrossRef]
- Fink, K.; Duval, A.; Martel, A.; Soucy-Faulkner, A.; Grandvaux, N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J. Immunol. 2008, 180, 6911–6922. [Google Scholar] [CrossRef]
- Yoboua, F.; Martel, A.; Duval, A.; Mukawera, E.; Grandvaux, N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J. Virol. 2010, 84, 7267–7277. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Salinas, G.; Fernandez, V.; Fernandez, C.; Selkirk, M.E. Echinococcus granulosus: Cloning of a thioredoxin peroxidase. Exp. Parasitol. 1998, 90, 298–301. [Google Scholar] [CrossRef]
- Robinson, M.W.; Hutchinson, A.T.; Dalton, J.P.; Donnelly, S. Peroxiredoxin: A central player in immune modulation. Parasite Immunol. 2010, 32, 305–313. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, N.Z.; Li, W.H.; Li, L.; Yan, H.B.; Qu, Z.G.; Li, T.T.; Cui, J.M.; Yang, Y.; Jia, W.Z.; et al. Proteomic analysis of differentially expressed proteins in the three developmental stages of Trichinella spiralis. Vet. Parasitol. 2016, 231, 32–38. [Google Scholar] [CrossRef]
- Ivanciuc, T.; Sbrana, E.; Casola, A.; Garofalo, R.P. Protective Role of Nuclear Factor Erythroid 2-Related Factor 2 Against Respiratory Syncytial Virus and Human Metapneumovirus Infections. Front. Immunol. 2018, 9, 854. [Google Scholar] [CrossRef]
- Cho, H.Y.; Imani, F.; Miller-DeGraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M.; Polack, F.P.; Kleeberger, S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009, 179, 138–150. [Google Scholar] [CrossRef]
- Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Increased Lung Catalase Activity Confers Protection Against Experimental RSV Infection. Sci. Rep. 2020, 10, 3653. [Google Scholar] [CrossRef]
- Kwon, J.; Han, E.; Bui, C.B.; Shin, W.; Lee, J.; Lee, S.; Choi, Y.B.; Lee, A.H.; Lee, K.H.; Park, C.; et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012, 13, 150–156. [Google Scholar] [CrossRef]
- Siegel, D.; Gustafson, D.L.; Dehn, D.L.; Han, J.Y.; Boonchoong, P.; Berliner, L.J.; Ross, D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004, 65, 1238–1247. [Google Scholar] [CrossRef]
- McFarlane, A.J.; McSorley, H.J.; Davidson, D.J.; Fitch, P.M.; Errington, C.; Mackenzie, K.J.; Gollwitzer, E.S.; Johnston, C.J.C.; MacDonald, A.S.; Edwards, M.R.; et al. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J. Allergy Clin. Immunol. 2017, 140, 1068–1078. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Hartmann, S.; Selkirk, M.E.; Roberts, L.B.; Openshaw, P.J.; Schnoeller, C. The Helminth-Derived Immunomodulator AvCystatin Reduces Virus Enhanced Inflammation by Induction of Regulatory IL-10+ T Cells. PLoS ONE 2016, 11, e0161885. [Google Scholar] [CrossRef]
- Gottstein, B.; Pozio, E.; Nockler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Venturiello, S.M.; Verzoletti, M.L.; Costantino, S.N.; Forastiero, M.A.; Roux, M.E. Early pulmonary response in rats infected with Trichinella spiralis. Parasitology 2007, 134, 281–288. [Google Scholar] [CrossRef]
- Falduto, G.H.; Vila, C.C.; Saracino, M.P.; Calcagno, M.A.; Venturiello, S.M. Trichinella spiralis: Killing of newborn larvae by lung cells. Parasitol. Res. 2015, 114, 679–685. [Google Scholar] [CrossRef]
- Harley, J.P.; Gallicchio, V. Trichinella spiralis: Migration of larvae in the rat. Exp. Parasitol. 1971, 30, 11–21. [Google Scholar] [CrossRef]
- Kosanovic, M.; Cvetkovic, J.; Gruden-Movsesijan, A.; Vasilev, S.; Svetlana, M.; Ilic, N.; Sofronic-Milosavljevic, L. Trichinella spiralis muscle larvae release extracellular vesicles with immunomodulatory properties. Parasite Immunol. 2019, 41, e12665. [Google Scholar] [CrossRef]
- Cardoso, L.S.; Oliveira, S.C.; Góes, A.M.; Oliveira, R.R.; Pacífico, L.G.; Marinho, F.V.; Fonseca, C.T.; Cardoso, F.C.; Carvalho, E.M.; Araujo, M.I. Schistosoma mansoni antigens modulate the allergic response in a murine model of ovalbumin-induced airway inflammation. Clin. Exp. Immunol. 2010, 160, 266–274. [Google Scholar] [CrossRef]
- Finlay, C.M.; Stefanska, A.M.; Coleman, M.M.; Jahns, H.; Cassidy, J.P.; McLoughlin, R.M.; Mills, K.H.G. Secreted products of Fasciola hepatica inhibit the induction of T cell responses that mediate allergy. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef]
- Pitrez, P.M.; Gualdi, L.P.; Barbosa, G.L.; Sudbrack, S.; Ponzi, D.; Cao, R.G.; Silva, A.C.; Machado, D.C.; Jones, M.H.; Stein, R.T.; et al. Effect of different helminth extracts on the development of asthma in mice: The influence of early-life exposure and the role of IL-10 response. Exp. Parasitol. 2015, 156, 95–103. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Werner-Klein, M.; Wohlleben, G.; Polte, T.; Hansen, G.; Ehlers, S.; Erb, K.J. Helminth-derived products inhibit the development of allergic responses in mice. Am. J. Respir. Crit. Care Med. 2007, 175, 336–344. [Google Scholar] [CrossRef]
- Callejas, B.E.; Mendoza-Rodriguez, M.G.; Villamar-Cruz, O.; Reyes-Martinez, S.; Sanchez-Barrera, C.A.; Rodriguez-Sosa, M.; Delgado-Buenrostro, N.L.; Martinez-Saucedo, D.; Chirino, Y.I.; Leon-Cabrera, S.A.; et al. Helminth-derived molecules inhibit colitis-associated colon cancer development through NF-kappaB and STAT3 regulation. Int. J. Cancer 2019, 145, 3126–3139. [Google Scholar] [CrossRef]
- Cvetkovic, J.; Ilic, N.; Sofronic-Milosavljevic, L.; Gruden-Movsesijan, A. Glycans expressed on Trichinella spiralis excretory-secretory antigens are important for anti-inflamatory immune response polarization. Comp. Immunol. Microbiol. Infect. Dis 2014, 37, 355–367. [Google Scholar] [CrossRef]
- Bai, X.; Wu, X.; Wang, X.; Guan, Z.; Gao, F.; Yu, J.; Yu, L.; Tang, B.; Liu, X.; Song, Y.; et al. Regulation of cytokine expression in murine macrophages stimulated by excretory/secretory products from Trichinella spiralis in vitro. Mol. Cell. Biochem. 2012, 360, 79–88. [Google Scholar] [CrossRef]
- Sofronic-Milosavljevic, L.J.; Radovic, I.; Ilic, N.; Majstorovic, I.; Cvetkovic, J.; Gruden-Movsesijan, A. Application of dendritic cells stimulated with Trichinella spiralis excretory-secretory antigens alleviates experimental autoimmune encephalomyelitis. Med. Microbiol. Immunol. 2013, 202, 239–249. [Google Scholar] [CrossRef]
- Kobpornchai, P.; Flynn, R.J.; Reamtong, O.; Rittisoonthorn, N.; Kosoltanapiwat, N.; Boonnak, K.; Boonyuen, U.; Ampawong, S.; Jiratanh, M.; Tattiyapong, M.; et al. A novel cystatin derived from Trichinella spiralis suppresses macrophage-mediated inflammatory responses. PLoS Negl. Trop. Dis. 2020, 14, e0008192. [Google Scholar] [CrossRef]
- Al-Riyami, L.; Pineda, M.A.; Rzepecka, J.; Huggan, J.K.; Khalaf, A.I.; Suckling, C.J.; Scott, F.J.; Rodgers, D.T.; Harnett, M.M.; Harnett, W. Designing anti-inflammatory drugs from parasitic worms: A synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. J. Med. Chem. 2013, 56, 9982–10002. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, K.-B.; Lee, H.-A.; Kang, H.-J.; Moon, E.-K.; Quan, F.-S. Preliminary Trichinella spiralis Infection Ameliorates Subsequent RSV Infection-Induced Inflammatory Response. Cells 2020, 9, 1314. https://doi.org/10.3390/cells9051314
Chu K-B, Lee H-A, Kang H-J, Moon E-K, Quan F-S. Preliminary Trichinella spiralis Infection Ameliorates Subsequent RSV Infection-Induced Inflammatory Response. Cells. 2020; 9(5):1314. https://doi.org/10.3390/cells9051314
Chicago/Turabian StyleChu, Ki-Back, Hae-Ahm Lee, Hae-Ji Kang, Eun-Kyung Moon, and Fu-Shi Quan. 2020. "Preliminary Trichinella spiralis Infection Ameliorates Subsequent RSV Infection-Induced Inflammatory Response" Cells 9, no. 5: 1314. https://doi.org/10.3390/cells9051314
APA StyleChu, K.-B., Lee, H.-A., Kang, H.-J., Moon, E.-K., & Quan, F.-S. (2020). Preliminary Trichinella spiralis Infection Ameliorates Subsequent RSV Infection-Induced Inflammatory Response. Cells, 9(5), 1314. https://doi.org/10.3390/cells9051314



