Focus on the Role of Klotho Protein in Neuro-Immune Interactions in HT-22 Cells Upon LPS Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture, KLTH Silencing, and LPS Treatment
2.3. MTT Assay
2.4. ATP Level Determination
2.5. Morphological Analysis and Cytoskeleton Staining
2.6. Oxidative and Nitrosative Stress Parameters
2.7. Measurement of Intracellular Calcium and Zinc Levels
2.8. Cell Cycle and Micronuclei Formation Analysis
2.9. Enzyme-Linked Immunosorbent Assays (ELISAs)
2.10. Acridine Orange Staining
2.11. BrdU Incorporation Assay and Immunostaining
2.12. RNA Purification, cDNA Synthesis, and PCRs
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
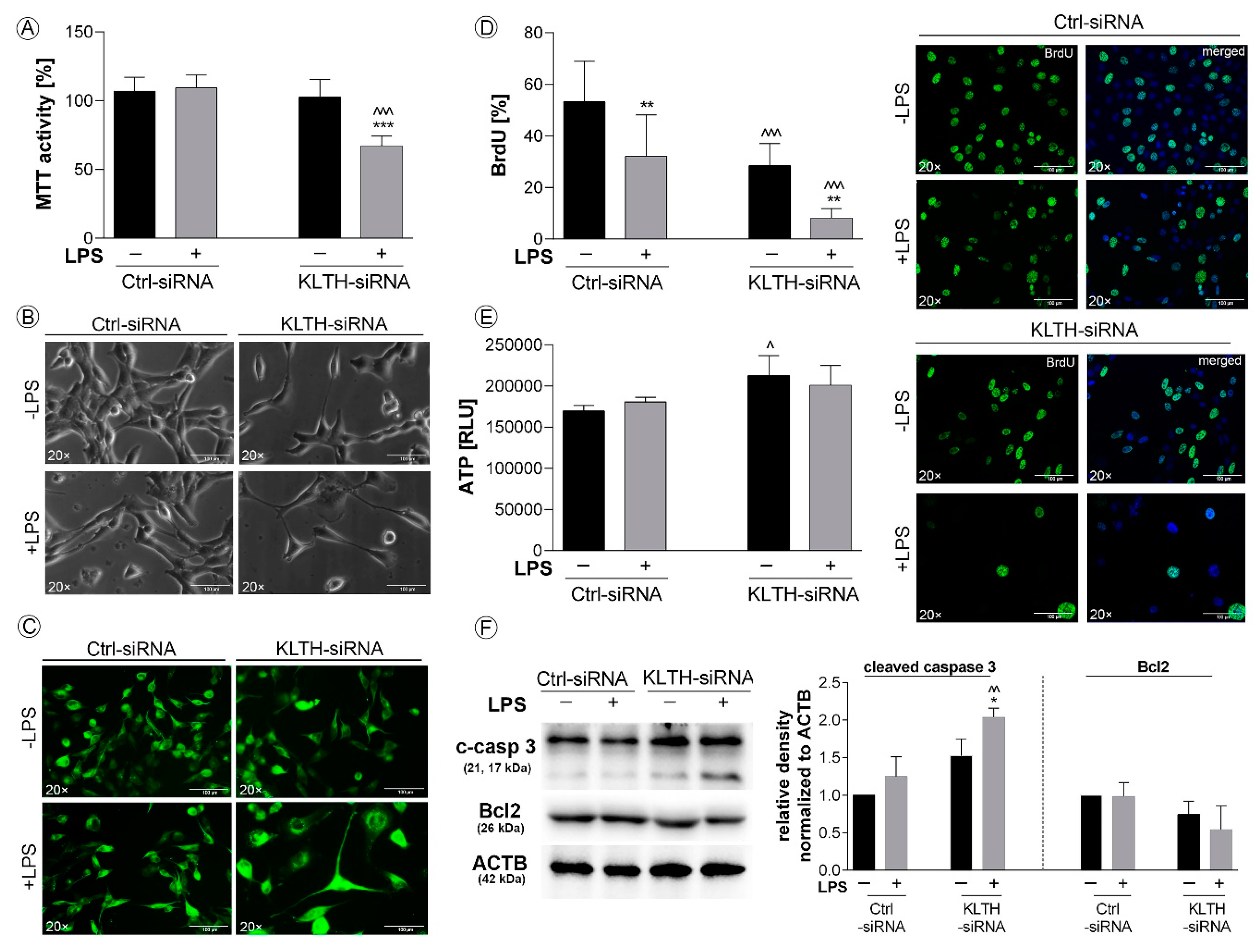
3.1. Klotho-Depleted HT-22 Hippocampal Neuronal Cells are Sensitive to LPS Stimulation
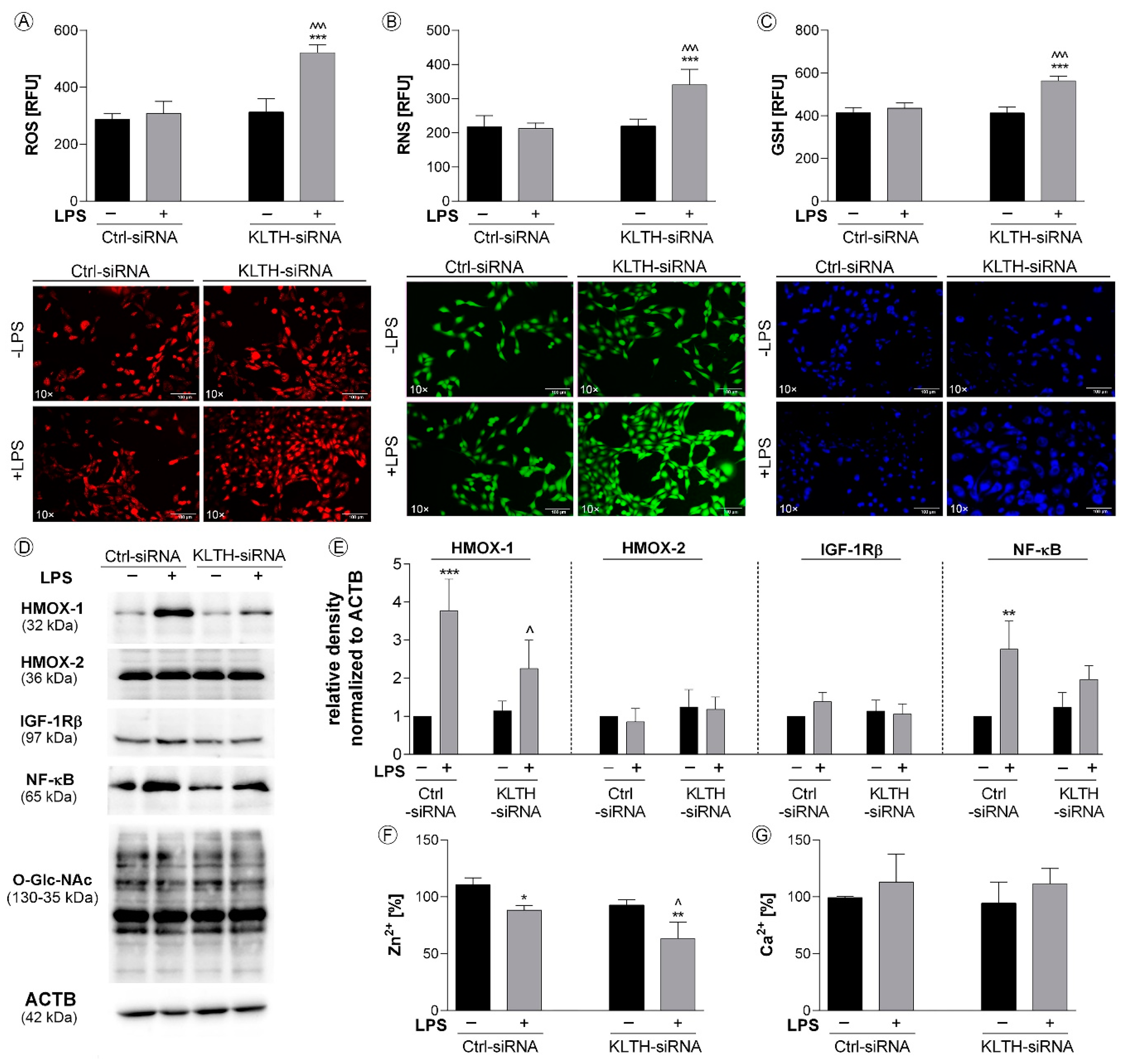
3.2. Klotho-Depletion Affects Intracellular Redox and Mineral Homeostasis
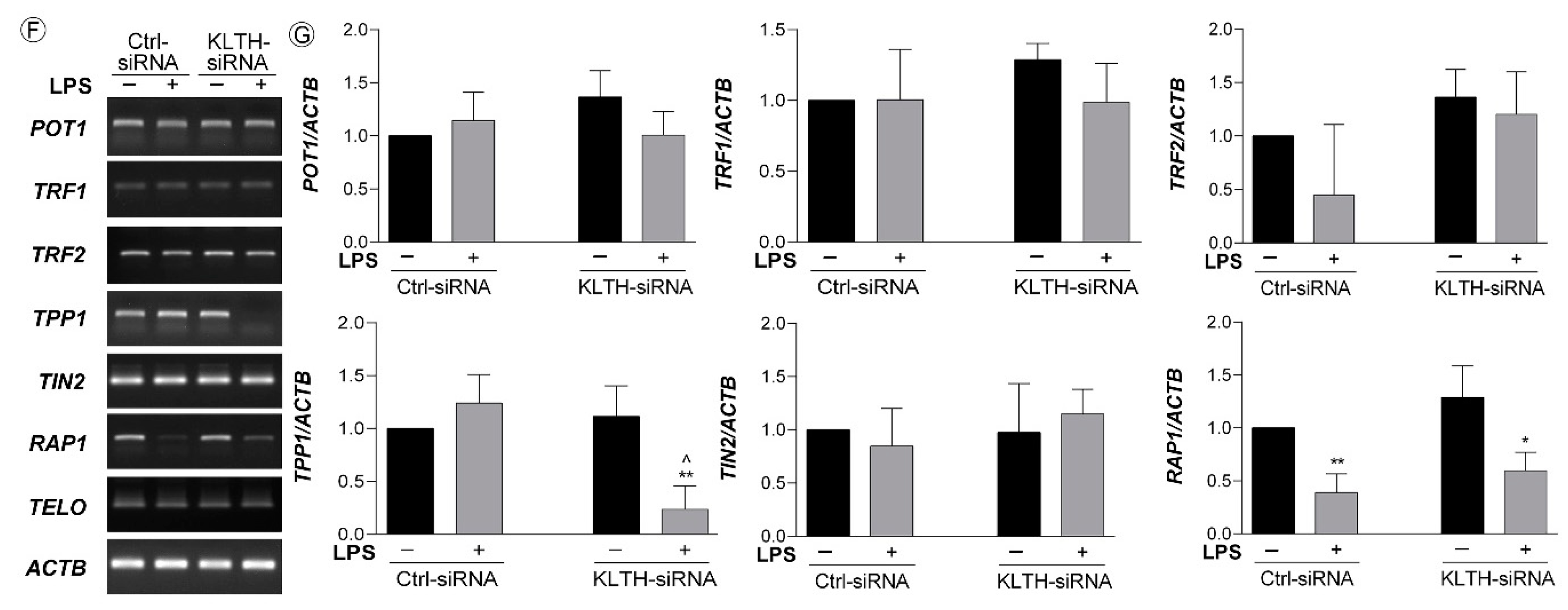
3.3. Klotho-Silencing Results in Oxidative-Mediated DNA and Telomere Instability
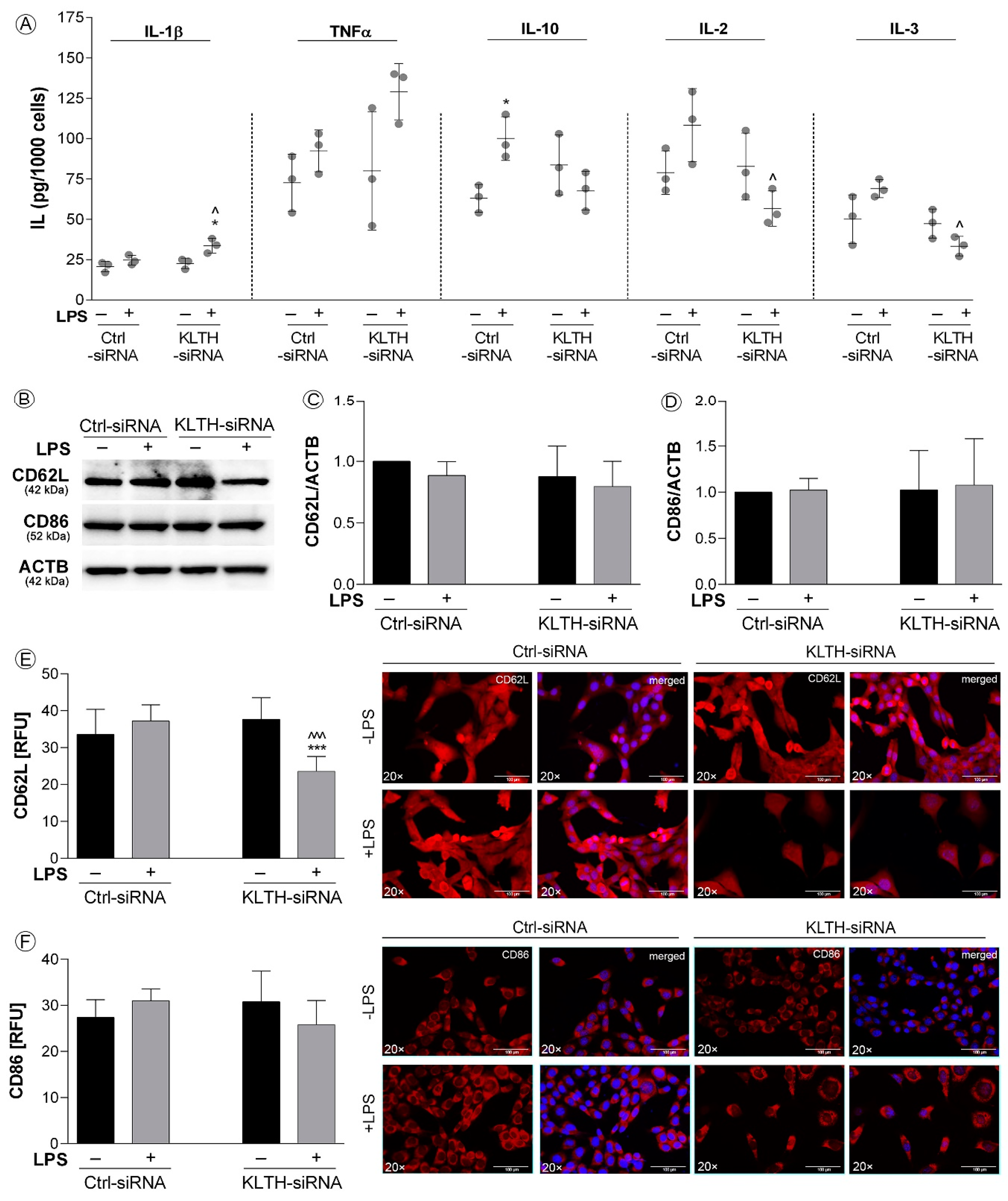
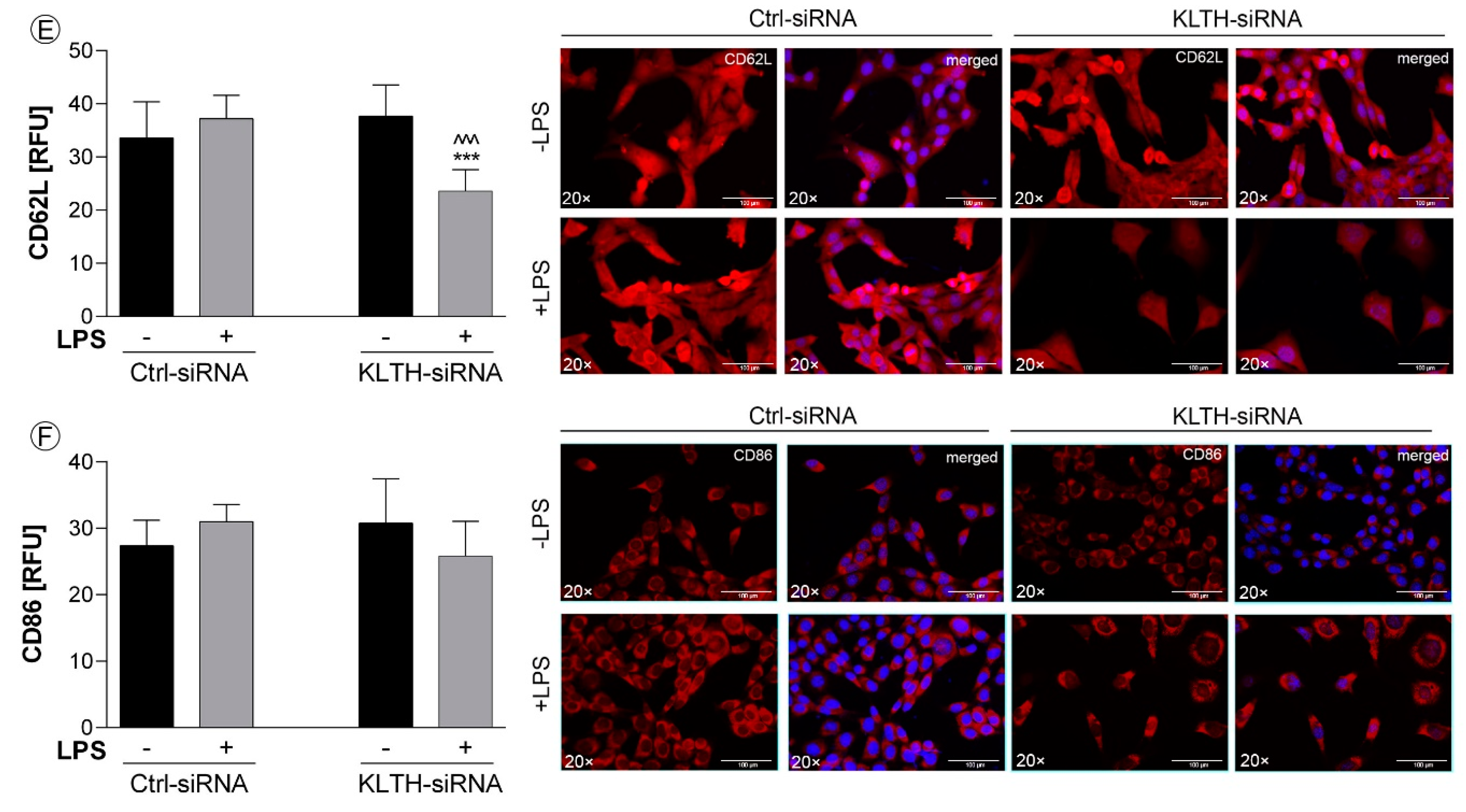
3.4. Klotho-Depletion Affects the Inflammatory Response in HT-22 Hippocampal Neuronal Cells
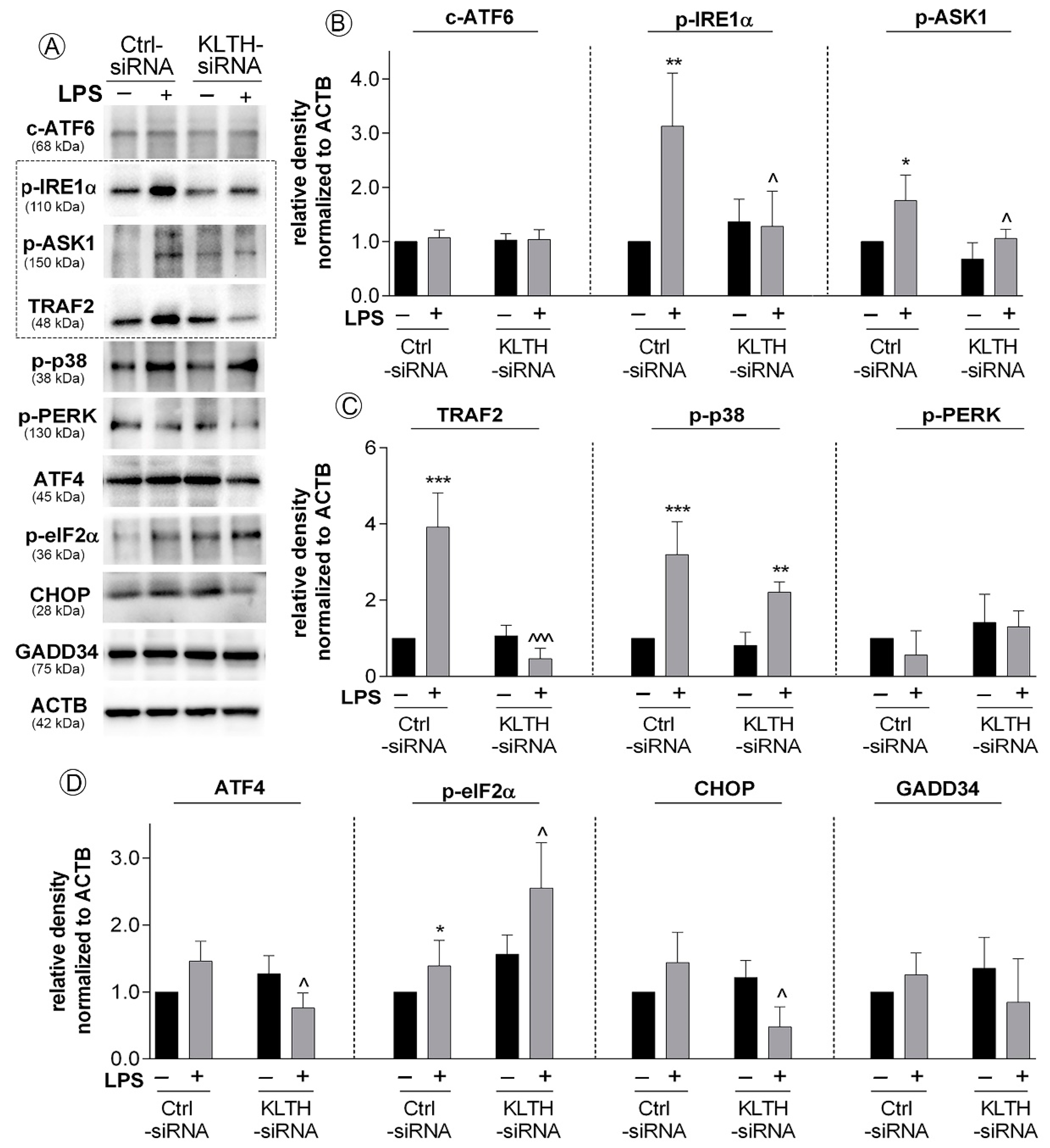
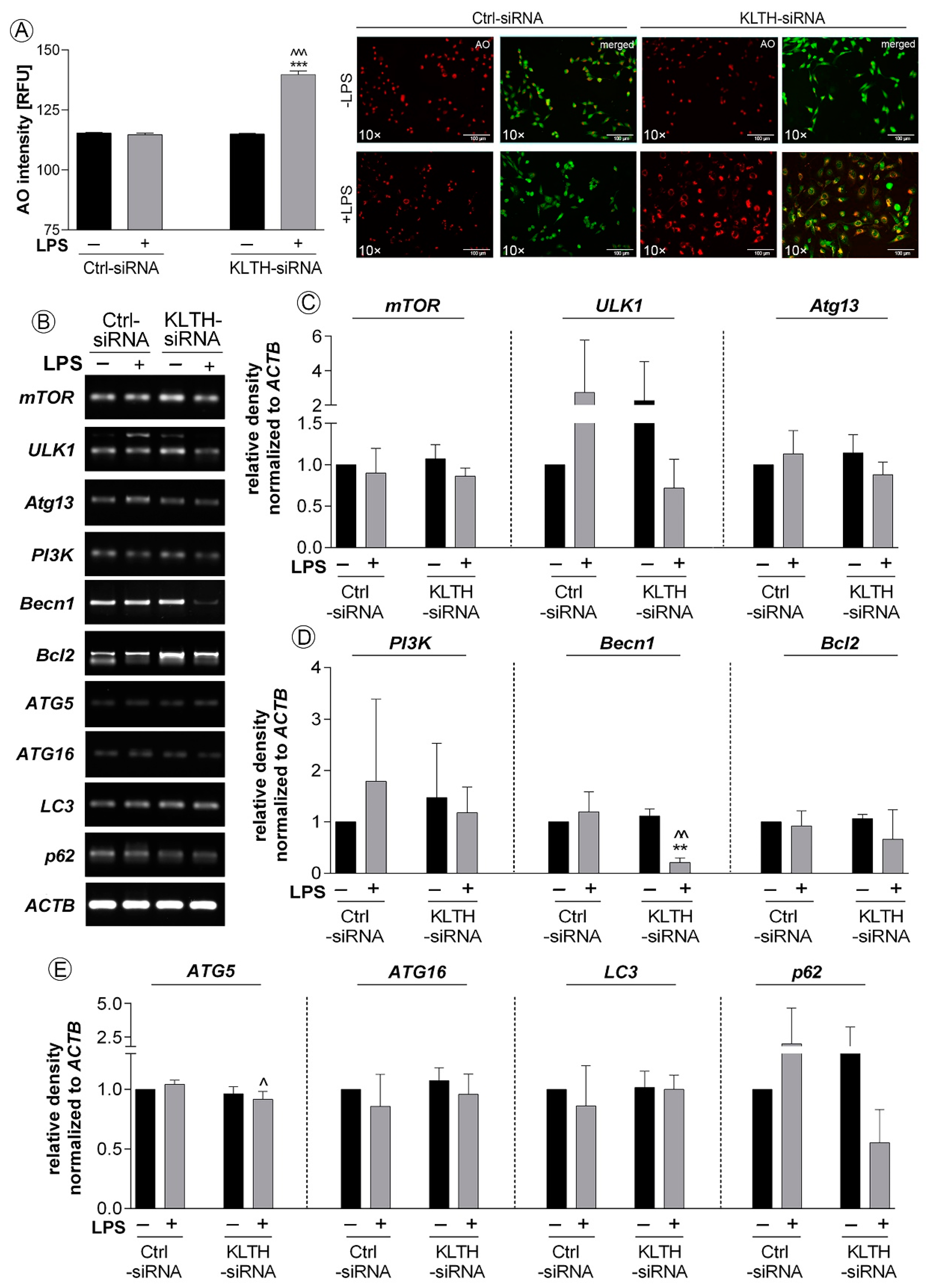
3.5. Klotho-Silencing Promotes Activation of ER Stress Response But Not Autophagy
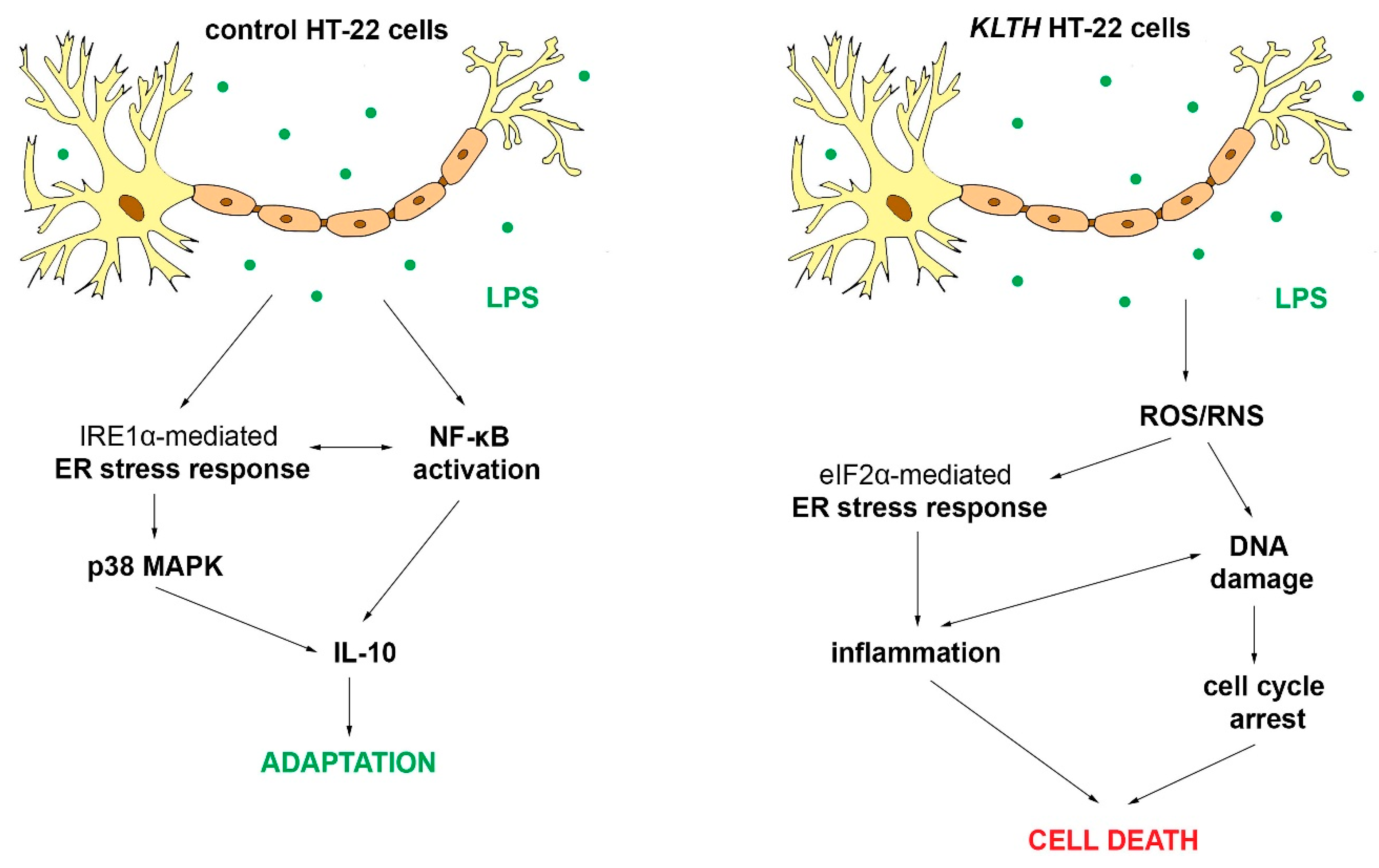
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snow, W.M.; Albensi, B.C. Neuronal Gene Targets of NF-κB and Their Dysregulation in Alzheimer’s Disease. Front Mol Neurosci 2016, 9, 118. [Google Scholar] [CrossRef]
- Liu, Y.; Teige, I.; Birnir, B.; Issazadeh-Navikas, S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006, 12, 518–525. [Google Scholar] [CrossRef]
- Bondy, S.C. Aspects of the immune system that impact brain function. J. Neuroimmunol. 2020, 340, 577167. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Onasanwo, S.A.; Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protective mechanism. Mol. Cell Biochem. 2016, 414, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.G.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun. -Heal. 2020, 2, 100034. [Google Scholar] [CrossRef]
- Rao, J.S.; Kellom, M.; Kim, H.-W.; Rapoport, S.I.; A Reese, E. Neuroinflammation and Synaptic Loss. Neurochem. Res. 2012, 37, 903–910. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Shiozaki, M.; Yoshimura, K.; Shibata, M.; Koike, M.; Matsuura, N.; Uchiyama, Y.; Gotow, T. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience 2008, 152, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.-Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Solek, P.; Koziorowski, M. Klotho modulates ER-mediated signaling crosstalk between prosurvival autophagy and apoptotic cell death during LPS challenge. Apoptosis 2018, 24, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Solek, P.; Tabecka-Lonczynska, A.; Koziorowski, M. Klotho-Mediated Changes in Shelterin Complex Promote Cytotoxic Autophagy and Apoptosis in Amitriptyline-Treated Hippocampal Neuronal Cells. Mol. Neurobiol. 2019, 56, 6952–6963. [Google Scholar] [CrossRef]
- Mytych, J.; Sołek, P.; Będzińska, A.; Rusinek, K.; Warzybok, A.; Tabęcka-Łonczyńska, A.; Koziorowski, M. Klotho-mediated changes in the expression of Atg13 alter formation of ULK1 complex and thus initiation of ER- and Golgi-stress response mediated autophagy. Apoptosis 2019, 25, 57–72. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, X.; Chen, W.; Liu, J. Angelica polysaccharide mitigates lipopolysaccharide-evoked inflammatory injury by regulating microRNA-10a in neuronal cell line HT22. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3194–3201. [Google Scholar] [CrossRef]
- Khan, M.S.; Muhammad, T.; Ikram, M.; Kim, M.O. Dietary supplementation of the antioxidant curcumin halts systemic LPS-induced neuroinflammation-associated neurodegeneration and memory/synaptic impairment via the JNK/NF-κB/Akt signaling pathway in adult rats. Oxid. Med. Cell Longev. 2019, 2019, 7860650. [Google Scholar] [CrossRef]
- Kim, D.-C.; Cho, K.-H.; Ko, W.; Yoon, C.-S.; Sohn, J.H.; Yim, J.H.; Kim, Y.-C.; Oh, H. Anti-Inflammatory and Cytoprotective Effects of TMC-256C1 from Marine-Derived Fungus Aspergillus sp. SF-6354 via up-Regulation of Heme Oxygenase-1 in Murine Hippocampal and Microglial Cell Lines. Int. J. Mol. Sci. 2016, 17, 529. [Google Scholar] [CrossRef]
- Guan, F.; Zhou, X.; Li, P.; Wang, Y.; Liu, M.; Li, F.; Cui, Y.; Huang, T.; Yao, M.; Zhang, Y.; et al. MG53 attenuates lipopolysaccharide-induced neurotoxicity and neuroinflammation via inhibiting TLR4/NF-κB pathway in vitro and in vivo. Prog. Neuropsychopharmacol Biol. Psychiatry 2019, 95, 109684. [Google Scholar] [CrossRef]
- Solek, P.; Majchrowicz, L.; Koziorowski, M. Aloe arborescens juice prevents EMF-induced oxidative stress and thus protects from pathophysiology in the male reproductive system in vitro. Environ. Res. 2018, 166, 141–149. [Google Scholar] [CrossRef]
- Mytych, J.; Wos, I.; Solek, P.; Koziorowski, M. Protective role of klotho protein on epithelial cells upon co-culture with activated or senescent monocytes. Exp. Cell Res. 2017, 350, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Romerowicz-Misielak, M.; Koziorowski, M. Klotho protects human monocytes from LPS-induced immune impairment associated with immunosenescent-like phenotype. Mol. Cell. Endocrinol. 2018, 470, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Solek, P.; Będzińska, A.; Rusinek, K.; Warzybok, A.; Tabecka-Lonczynska, A.; Koziorowski, M. Towards Age-Related Anti-Inflammatory Therapy: Klotho Suppresses Activation of ER and Golgi Stress Response in Senescent Monocytes. Cells 2020, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; De La Fuente, C.; García-Durillo, M.; Garcia-Rodriguez, C.; Villalobos, C.; Núñez, L. Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflammation 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Chen, F.; Li, Y.; Wei, A.; Cao, W. Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J. Mol. Med. 2018, 96, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Sprenkle, N.T.; Sims, S.G.; Sanchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef]
- Svensson, C.; Part, K.; Künnis-Beres, K.; Kaldmäe, M.; Fernaeus, S.Z.; Land, T. Pro-survival effects of JNK and p38 MAPK pathways in LPS-induced activation of BV-2 cells. Biochem. Biophys. Res. Commun. 2011, 406, 488–492. [Google Scholar] [CrossRef]
- Ananieva, O.; Darragh, J.; Johansen, C.; Carr, J.M.; McIlrath, J.; Park, J.M.; Wingate, A.; E Monk, C.; Toth, R.; Santos, S.G.; et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 2008, 9, 1028–1036. [Google Scholar] [CrossRef]
- Dhingra, S.; Sharma, A.K.; Arora, R.C.; Slezak, J.; Singal, P.K. IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc. Res. 2009, 82, 59–66. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Insolera, R.; Fink, D.J.; Mata, M. Interleukin-10 provides direct trophic support to neurons. J. Neurochem. 2009, 110, 1617–1627. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Akha, A.A.S.; Raden, D.; Kaufman, R.J. Adaptation to ER Stress Is Mediated by Differential Stabilities of Pro-Survival and Pro-Apoptotic mRNAs and Proteins. PLoS Boil. 2006, 4, e374. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, J.; Tenkerian, C.; Gupta, J.; Ghaddar, N.; Wang, S.; Darini, C.; Staschke, K.A.; Ghosh, A.; Gandin, V.; Topisirovic, I.; et al. Downregulation of PERK activity and eIF2α serine 51 phosphorylation by mTOR complex 1 elicits pro-oxidant and pro-death effects in tuberous sclerosis-deficient cells. Cell Death Dis. 2018, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Bennett, B.S.; Cullinan, S.B.; Diehl, J.A. PERK and GCN2 Contribute to eIF2α Phosphorylation and Cell Cycle Arrest after Activation of the Unfolded Protein Response Pathway. Mol. Boil. Cell 2005, 16, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Boil. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Zhang, Y.; Liu, C. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Heal. Dis. 2017, 16, 77. [Google Scholar] [CrossRef]
- Xie, B.; Nie, S.; Hu, G.; Xiong, L.; Hu, F.; Li, M.; Peng, T.; Nie, J.; He, Y. The involvement of NF-kappaB/Klotho signaling in colorectal cancer cell survival and invasion. Pathol. Oncol. Res. 2019, 25, 1553–1565. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Zhou, T.B.T.; Harris, D.; Abraham, C.R. The Neuroprotective Effect of Klotho is Mediated via Regulation of Members of the Redox System. J. Boil. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef]
- Omata, Y.; Salvador, G.A.; Supasai, S.; Keenan, A.H.; Oteiza, P.I. Decreased Zinc Availability Affects Glutathione Metabolism in Neuronal Cells and in the Developing Brain. Toxicol. Sci. 2013, 133, 90–100. [Google Scholar] [CrossRef]
- Franco, S.; Blasco, M.A.; Siedlak, S.L.; Harris, P.L.; Moreira, P.I.; Perry, G.; Smith, M.A. Telomeres and telomerase in Alzheimer’s disease: Epiphenomena or a new focus for therapeutic strategy? Alzheimers Dement 2006, 2, 164–168. [Google Scholar] [CrossRef]
- Lehman, S.L.; Cerniglia, G.J.; Johannes, G.J.; Ye, J.; Ryeom, S.; Koumenis, C. Translational Upregulation of an Individual p21Cip1 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress. PLoS Genet. 2015, 11, e1005212. [Google Scholar] [CrossRef]
- Lee, D.; Hokinson, D.; Park, S.; Elvira, R.; Kusuma, F.; Lee, J.M.; Yun, M.; Lee, S.G.; Han, J. ER stress induces cell cycle arrest at the G2/M phase through eIF2alpha phosphorylation and GADD45alpha. Int. J. Mol. Sci. 2019, 20, 6309. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, Q.; Tian, X.; Qian, N.; Chai, P.; Liu, B.; Hu, J.; Blackstone, C.; Zhu, D.; Teng, J.; et al. DNA damage triggers tubular endoplasmic reticulum extension to promote apoptosis by facilitating ER-mitochondria signaling. Cell Res. 2018, 28, 833–854. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Sanz-Rodgriguez, A.; Jimenez-Mateos, E.M.; Concannon, C.G.; Jimenez-Pacheco, A.; Moran, C.; Mesuret, G.; Petit, E.; Delanty, N.; Farrell, M.A.; et al. CHOP regulates the p53–MDM2 axis and is required for neuronal survival after seizures. Brain 2013, 136, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Miyazaki, S.; Matsuyama, S.; Takeda, M.; Kawano, M.; Nakagawa, H.; Nishimura, K.; Matsuo, S. Selection of autophagy or apoptosis in cells exposed to ER-stress depends on ATF4 expression pattern with or without CHOP expression. Boil. Open 2013, 2, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Kong, R.; Ma, Z.-B.; Han, B.; Wang, Y.-W.; Pan, S.-H.; Li, Y.-H.; Sun, B. The activation of c-Jun NH2-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 8. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Boil. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Aguilera, G.; Colín-González, A.L.; Rangel-López, E.; Chavarría, A.; Santamaría, A.; Rangel, E. Redox Signaling, Neuroinflammation, and Neurodegeneration. Antioxid. Redox Signal. 2018, 28, 1626–1651. [Google Scholar] [CrossRef]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Murata, M. Crosstalk between DNA Damage and Inflammation in the Multiple Steps of Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef]
- Grewal, I.S.; Foellmer, H.G.; Grewal, K.D.; Wang, H.; Lee, W.P.; Tumas, D.; Janeway, C.A.; A Flavell, R. CD62L Is Required on Effector Cells for Local Interactions in the CNS to Cause Myelin Damage in Experimental Allergic Encephalomyelitis. Immunity 2001, 14, 291–302. [Google Scholar] [CrossRef]
- Kamimura, D.; Bevan, M.J. Endoplasmic Reticulum Stress Regulator XBP-1 Contributes to Effector CD8+ T Cell Differentiation during Acute Infection1. J. Immunol. 2008, 181, 5433–5441. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Churlaud, G.; Audrain, M.; Michaelsen-Preusse, K.; Fol, R.; Souchet, B.; Braudeau, J.; Korte, M.; Klatzmann, D.; Cartier, N. Faculty Opinions recommendation of Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Fac. Opin. – Post-Publ. Peer Rev. Biomed. Lit. 2017, 140, 826–842. [Google Scholar] [CrossRef]
- Lim, J.C.; Lu, W.; Beckel, J.M.; Mitchell, C.H. Neuronal Release of Cytokine IL-3 Triggered by Mechanosensitive Autostimulation of the P2X7 Receptor Is Neuroprotective. Front. Cell. Neurosci. 2016, 10, 5800. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.; Otth, C.; Mujica, L.; Concha, I.I.; Maccioni, R.B. Interleukin-3 prevents neuronal death induced by amyloid peptide. BMC Neurosci. 2007, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Smith, D.E.; Ibáñez-Sandoval, O.; Sims, J.E.; Friedman, W.J. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J. Neurosci. 2011, 31, 18048–18059. [Google Scholar] [CrossRef]
- Kim, S.; Joe, Y.; Jeong, S.O.; Zheng, M.; Back, S.H.; Park, S.W.; Chung, H.T. Endoplasmic reticulum stress is sufficient for the induction of IL-1beta production via activation of the NF-kappaB and inflammasome pathways. Innate Immun. 2014, 20, 799–815. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Li, Y.; Xu, L.; Yu, X.; Ge, L.; Li, J.; Zhu, Y.; He, S. Necroptosis Mediates TNF-Induced Toxicity of Hippocampal Neurons. BioMed Res. Int. 2014, 2014, 290182. [Google Scholar] [CrossRef]
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine Tumor Necrosis Factor Alpha Links Endoplasmic Reticulum Stress to the Membrane Death Receptor Pathway through IRE1α-Mediated NF-κB Activation and Down-Regulation of TRAF2 Expression. Mol. Cell. Boil. 2006, 26, 3071–3084. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinek, K.; Sołek, P.; Tabęcka-Łonczyńska, A.; Koziorowski, M.; Mytych, J. Focus on the Role of Klotho Protein in Neuro-Immune Interactions in HT-22 Cells Upon LPS Stimulation. Cells 2020, 9, 1231. https://doi.org/10.3390/cells9051231
Rusinek K, Sołek P, Tabęcka-Łonczyńska A, Koziorowski M, Mytych J. Focus on the Role of Klotho Protein in Neuro-Immune Interactions in HT-22 Cells Upon LPS Stimulation. Cells. 2020; 9(5):1231. https://doi.org/10.3390/cells9051231
Chicago/Turabian StyleRusinek, Kinga, Przemysław Sołek, Anna Tabęcka-Łonczyńska, Marek Koziorowski, and Jennifer Mytych. 2020. "Focus on the Role of Klotho Protein in Neuro-Immune Interactions in HT-22 Cells Upon LPS Stimulation" Cells 9, no. 5: 1231. https://doi.org/10.3390/cells9051231
APA StyleRusinek, K., Sołek, P., Tabęcka-Łonczyńska, A., Koziorowski, M., & Mytych, J. (2020). Focus on the Role of Klotho Protein in Neuro-Immune Interactions in HT-22 Cells Upon LPS Stimulation. Cells, 9(5), 1231. https://doi.org/10.3390/cells9051231




