Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role
Abstract
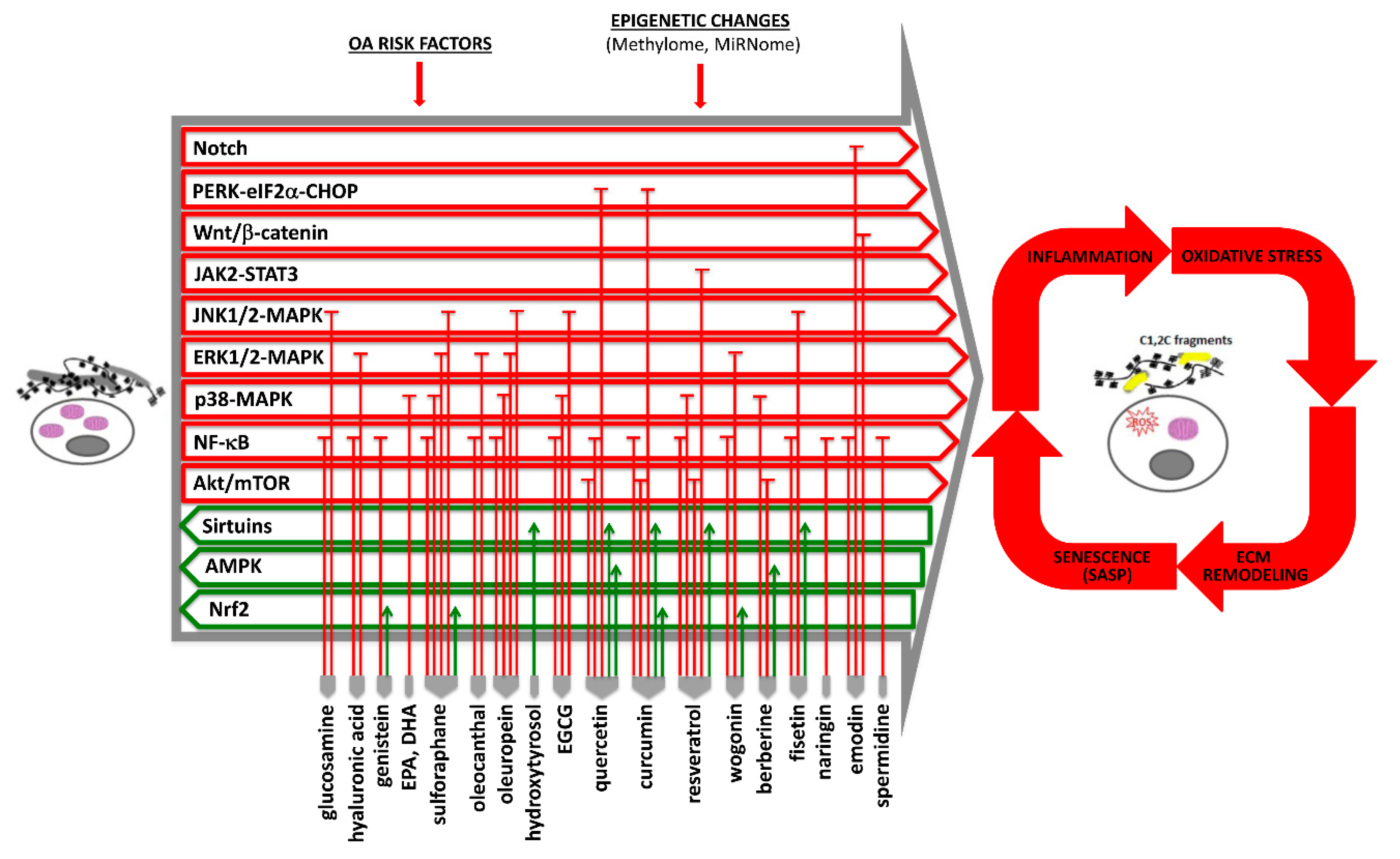
1. Introduction
2. Osteoarthritis Pathophysiology
3. Efficacy of Current Nutraceuticals in Osteoarthritis and Their Nutrigenomic Role
3.1. Glucosamine, Chondroitin Sulfate, and Hyaluronic Acid
3.2. Avocado/Soybean Unsaponifiables
3.3. Omega-3 Polyunsaturated Fatty Acids
3.4. Sulforaphane and Organosulfur Compounds
3.5. Olive-Derived Compounds
3.6. Green Tea Polyphenols
3.7. Quercetin
3.8. Curcumin
3.9. Resveratrol
3.10. Wogonin
3.11. Berberine
3.12. Other Emerging Compounds
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyere, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.Y. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019, 36, 15–24. [Google Scholar] [CrossRef]
- Nakata, K.; Hanai, T.; Take, Y.; Osada, T.; Tsuchiya, T.; Shima, D.; Fujimoto, Y. Disease-modifying effects of cox-2 selective inhibitors and non-selective nsaids in osteoarthritis: A systematic review. Osteoarthritis Cartilage 2018, 26, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Hirst-Jones, K.L.; Davidson, R.K.; Jupp, O.; Bao, Y.; MacGregor, A.J.; Donell, S.T.; Cassidy, A.; Clark, I.M. The potential for dietary factors to prevent or treat osteoarthritis. Proc. Nutr. Soc. 2014, 73, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse effects of nutraceuticals and dietary supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Spencer, A.C.; Topolski, T.D.; Belza, B.; Patrick, D.L. Use of alternative therapies by older adults with osteoarthritis. Arthritis Rheum. 2001, 45, 222–227. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Muhammad, N.; Steele, R.; Kornbluth, J.; Ray, R.B. Bitter melon enhances natural killer-mediated toxicity against head and neck cancer cells. Cancer Prev. Res. (Phila) 2017, 10, 337–344. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Muhammad, N.; Steele, R.; Peng, G.; Ray, R.B. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget 2016, 7, 33202–33209. [Google Scholar] [CrossRef]
- Muhammad, N.; Steele, R.; Isbell, T.S.; Philips, N.; Ray, R.B. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget 2017, 8, 66226–66236. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzi, R.M. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxid. Med. Cell. Longev. 2018, 2018, 3075293. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; van den Berg, W.B. Osteoarthritis in the context of ageing and evolution. Loss of chondrocyte differentiation block during ageing. Ageing Res. Rev. 2008, 7, 106–113. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, J.; Verzijl, N.; Wenting-van Wijk, M.J.; Jacobs, K.M.; Van El, B.; Van Roermund, P.M.; Bank, R.A.; Bijlsma, J.W.; TeKoppele, J.M.; Lafeber, F.P. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004, 50, 1207–1215. [Google Scholar] [CrossRef]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 48–56. [Google Scholar]
- Nefla, M.; Holzinger, D.; Berenbaum, F.; Jacques, C. The danger from within: Alarmins in arthritis. Nat. Rev. Rheumatol. 2016, 12, 669–683. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (dmm) model of osteoarthritis in the 129/svev mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Glasson, S.S. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr. Drug Targets 2007, 8, 367–376. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon mmp-13 regulation in osteoarthritis. Eur. Cell Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Kobayashi, M.; Yasuda, T.; Meijers, T.; Pidoux, I.; Poole, A.R. Chondrocyte hypertrophy can be induced by a cryptic sequence of type ii collagen and is accompanied by the induction of mmp-13 and collagenase activity: Implications for development and arthritis. Matrix Biol. 2007, 26, 247–258. [Google Scholar] [CrossRef]
- Yasuda, T.; Tchetina, E.; Ohsawa, K.; Roughley, P.J.; Wu, W.; Mousa, A.; Ionescu, M.; Pidoux, I.; Poole, A.R. Peptides of type ii collagen can induce the cleavage of type ii collagen and aggrecan in articular cartilage. Matrix Biol. 2006, 25, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Platano, D.; Olivotto, E.; Filardo, G.; Trisolino, G.; Facchini, A.; Borzi, R.M.; Meliconi, R. Human osteoarthritic cartilage shows reduced in vivo expression of il-4, a chondroprotective cytokine that differentially modulates il-1beta-stimulated production of chemokines and matrix-degrading enzymes in vitro. PLoS ONE 2014, 9, e96925. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Dalal, K. Adamts-4 and adamts-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Akagi, R.; Yamaguchi, S.; Muramatsu, Y.; Akatsu, Y.; Yamamoto, Y.; Sasaki, T.; Takahashi, K.; Sasho, T. Effect of inhibiting mmp13 and adamts5 by intra-articular injection of small interfering rna in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017, 368, 379–387. [Google Scholar] [CrossRef]
- Miller, R.E.; Tran, P.B.; Ishihara, S.; Larkin, J.; Malfait, A.M. Therapeutic effects of an anti-adamts-5 antibody on joint damage and mechanical allodynia in a murine model of osteoarthritis. Osteoarthr. Cartil. 2016, 24, 299–306. [Google Scholar] [CrossRef]
- Barksby, H.E.; Milner, J.M.; Patterson, A.M.; Peake, N.J.; Hui, W.; Robson, T.; Lakey, R.; Middleton, J.; Cawston, T.E.; Richards, C.D.; et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: Implications for cartilage degradation in arthritis. Arthritis Rheum. 2006, 54, 3244–3253. [Google Scholar] [CrossRef]
- Milner, J.M.; Elliott, S.F.; Cawston, T.E. Activation of procollagenases is a key control point in cartilage collagen degradation: Interaction of serine and metalloproteinase pathways. Arthritis Rheum. 2001, 44, 2084–2096. [Google Scholar] [CrossRef]
- Eyre, D.R.; Weis, M.A.; Wu, J.J. Articular cartilage collagen: An irreplaceable framework? Eur. Cell Mater. 2006, 12, 57–63. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. Nf-kappab signaling: Multiple angles to target oa. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1beta signaling in osteoarthritis - chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Minguzzi, M.; Silvestri, Y.; Borzi, R.M.; Flamigni, F. Micrornas and autophagy: Fine players in the control of chondrocyte homeostatic activities in osteoarthritis. Oxid. Med. Cell. Longev. 2017, 2017, 3720128. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. Microrna-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Araldi, E.; Schipani, E. Microrna-140 and the silencing of osteoarthritis. Genes Dev. 2010, 24, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, M.A.; Donica, M.; Baker, L.W.; Stevenson, M.E.; Annan, A.C.; Humphrey, M.B.; James, J.A.; Sawalha, A.H. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheumatol. 2014, 66, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microrna-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Lotz, M.K.; Carames, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and oa. Nat. Rev. Rheumatol. 2011, 7, 579–587. [Google Scholar] [CrossRef]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef]
- Helton, E.S.; Chen, X. P53 modulation of the DNA damage response. J. Cell. Biochem. 2007, 100, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Philipot, D.; Guerit, D.; Platano, D.; Chuchana, P.; Olivotto, E.; Espinoza, F.; Dorandeu, A.; Pers, Y.M.; Piette, J.; Borzi, R.M.; et al. P16ink4a and its regulator mir-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 2014, 16, R58. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Recalde, U.; Lorenzo-Gomez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Dominguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef]
- Scudellari, M. To stay young, kill zombie cells. Nature 2017, 550, 448–450. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef]
- Fan, P.; Xie, X.H.; Chen, C.H.; Peng, X.; Zhang, P.; Yang, C.; Wang, Y.T. Molecular regulation mechanisms and interactions between reactive oxygen species and mitophagy. DNA Cell Biol. 2019, 38, 10–22. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Malaise, O.; Tachikart, Y.; Constantinides, M.; Mumme, M.; Ferreira-Lopez, R.; Noack, S.; Krettek, C.; Noel, D.; Wang, J.; Jorgensen, C.; et al. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging (Albany NY) 2019, 11, 9128–9146. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Smith, T.O.; Cooper, C.; Guglielmi, G.; Reginster, J.Y.; Rizzoli, R.; et al. Adherence to a mediterranean diet is associated with lower prevalence of osteoarthritis: Data from the osteoarthritis initiative. Clin. Nutr. 2017, 36, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L. Therapeutic effects of olive and its derivatives on osteoarthritis: From bench to bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharm. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Jerosch, J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in oa: Outlook on other nutrient partners especially omega-3 fatty acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am. J. Vet. Res. 2005, 66, 1870–1876. [Google Scholar] [CrossRef]
- Neil, K.M.; Orth, M.W.; Coussens, P.M.; Chan, P.S.; Caron, J.P. Effects of glucosamine and chondroitin sulfate on mediators of osteoarthritis in cultured equine chondrocytes stimulated by use of recombinant equine interleukin-1beta. Am. J. Vet. Res. 2005, 66, 1861–1869. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andres, M.C.; Hashimoto, K.; Pitt, D.; Itoi, E.; Goldring, M.B.; Roach, H.I.; Oreffo, R.O. The epigenetic effect of glucosamine and a nuclear factor-kappa b (nf-kb) inhibitor on primary human chondrocytes--implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011, 405, 362–367. [Google Scholar] [CrossRef]
- Ricci, M.; Micheloni, G.M.; Berti, M.; Perusi, F.; Sambugaro, E.; Vecchini, E.; Magnan, B. Clinical comparison of oral administration and viscosupplementation of hyaluronic acid (ha) in early knee osteoarthritis. Musculoskelet. Surg. 2017, 101, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, T.; Mochizuki, S.; Takizawa, M.; Chijiiwa, M.; Okada, A.; Kimura, T.; Fujita, Y.; Matsumoto, H.; Toyama, Y.; Okada, Y. Hyaluronan inhibits expression of adamts4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis. 2009, 68, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, K.; Felisaz, N.; Bogdanowicz, P.; Galera, P.; Guillou, G.B.; Pujol, J.P. Avocado/soya unsaponifiables enhance the expression of transforming growth factor beta1 and beta2 in cultured articular chondrocytes. Arthritis Rheum. 1999, 42, 148–156. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Deberg, M.A.; Crielaard, J.M.; Piccardi, N.; Msika, P.; Sanchez, C. Avocado/soybean unsaponifiables prevent the inhibitory effect of osteoarthritic subchondral osteoblasts on aggrecan and type ii collagen synthesis by chondrocytes. J. Rheumatol. 2006, 33, 1668–1678. [Google Scholar] [PubMed]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Smith, B.J.; Sinichi, N.; Hodges, S.B.; Juma, S.; Munson, M.E.; Payton, M.E.; Tivis, R.D.; et al. Soy protein may alleviate osteoarthritis symptoms. Phytomedicine 2004, 11, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Soung do, Y.; Lucas, E.A.; Madihally, S.V.; Levenson, C.W.; Arjmandi, B.H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem. 2007, 18, 609–614. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, W.; Wu, N.; Jiang, S.; Li, W. Protective effect of genistein on condylar cartilage through downregulating nf-kappab expression in experimentally created osteoarthritis rats. BioMed Res. Int. 2019, 2019, 2629791. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids epa and dha: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M.; et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, A.; Ma, L.; Yu, H.; Zhang, L.; Meng, H.; Cui, Y.; Yu, F.; Yang, B. Docosahexenoic acid treatment ameliorates cartilage degeneration via a p38 mapk-dependent mechanism. Int. J. Mol. Med. 2016, 37, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Facchini, A.; Stanic, I.; Cetrullo, S.; Borzi, R.M.; Filardo, G.; Flamigni, F. Sulforaphane protects human chondrocytes against cell death induced by various stimuli. J. Cell. Physiol. 2011, 226, 1771–1779. [Google Scholar] [CrossRef]
- Ko, J.Y.; Choi, Y.J.; Jeong, G.J.; Im, G.I. Sulforaphane-plga microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 2013, 34, 5359–5368. [Google Scholar] [CrossRef]
- Davidson, R.K.; Jupp, O.; de Ferrars, R.; Kay, C.D.; Culley, K.L.; Norton, R.; Driscoll, C.; Vincent, T.L.; Donell, S.T.; Bao, Y.; et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013, 65, 3130–3140. [Google Scholar] [CrossRef]
- Pal, S.; Konkimalla, V.B. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int. Immunopharmacol. 2016, 35, 85–98. [Google Scholar] [CrossRef]
- Davidson, R.; Gardner, S.; Jupp, O.; Bullough, A.; Butters, S.; Watts, L.; Donell, S.; Traka, M.; Saha, S.; Mithen, R.; et al. Isothiocyanates are detected in human synovial fluid following broccoli consumption and can affect the tissues of the knee joint. Sci. Rep. 2017, 7, 3398. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Feng, Y.; Liu, N.; Fu, Z.; Wu, J.; Li, T.; Chen, H.; Chen, J.; Chen, C.; et al. Natural ingredients-derived antioxidants attenuate h2o2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via keap1/nrf2 pathway. Free Radic. Biol. Med. 2020, in press. [Google Scholar] [CrossRef]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gomez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gomez-Reino, J.J.; Smith, A.B., 3rd; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of mip-1alpha and il-6 in j774 macrophages and in atdc5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal inhibits catabolic and inflammatory mediators in lps-activated human primary osteoarthritis (oa) chondrocytes through mapks/nf-kappab pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the il-1beta-induced expression of inflammatory mediators by suppressing the activation of nf-kappab and mapks in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Facchini, A.; Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Minguzzi, M.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene alpha. PLoS ONE 2014, 9, e109724. [Google Scholar] [CrossRef]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via sirt6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Borzi, R.M.; Flamigni, F. Effect of oxidative stress and 3-hydroxytyrosol on DNA methylation levels of mir-9 promoters. J. Cell. Mol. Med. 2019, 23, 7885–7889. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the il-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappab activation by degradation of the inhibitor of nuclear factor kappab. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-o-gallate up-regulates microrna-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell. Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Zhang, M.; Ying, H. Green tea polyphenols attenuate lps-induced inflammation through upregulating microrna-9 in murine chondrogenic atdc5 cells. J. Cell. Physiol. 2019, 234, 22604–22612. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Sakanashi, Y.; Oyama, K.; Matsui, H.; Oyama, T.B.; Oyama, T.M.; Nishimura, Y.; Sakai, H.; Oyama, Y. Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular ca2+: A model experiment. Life Sci. 2008, 83, 164–169. [Google Scholar] [CrossRef]
- Miodini, P.; Fioravanti, L.; Di Fronzo, G.; Cappelletti, V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. Cancer 1999, 80, 1150–1155. [Google Scholar] [CrossRef]
- Rao, C.N.; Rao, V.H.; Steinmann, B. Influence of bioflavonoids on the metabolism and crosslinking of collagen. Ital. J. Biochem. 1981, 30, 259–270. [Google Scholar]
- Mok, S.W.; Fu, S.C.; Cheuk, Y.C.; Chu, I.M.; Chan, K.M.; Qin, L.; Yung, S.H.; Kevin Ho, K.W. Intra-articular delivery of quercetin using thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage 2018, 1947603518796550. [Google Scholar] [CrossRef]
- Permatasari, D.A.; Karliana, D.; Iskandarsyah, I.; Arsianti, A.; Bahtiar, A. Quercetin prevent proteoglycan destruction by inhibits matrix metalloproteinase-9, matrix metalloproteinase-13, a disintegrin and metalloproteinase with thrombospondin motifs-5 expressions on osteoarthritis model rats. J. Adv. Pharm. Technol. Res. 2019, 10, 2–8. [Google Scholar]
- Martini, F.M.; Brandstetter de Bellesini, A.; Miolo, A.; Del Coco, L.; Fanizzi, F.P.; Crovace, A. Combining a joint health supplement with tibial plateau leveling osteotomy in dogs with cranial cruciate ligament rupture. An exploratory controlled trial. Int. J. Vet. Sci. Med. 2017, 5, 105–112. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Tang, L.; Ji, Y.; Yan, C.; Zhang, X. Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev. Res. 2019, 80, 360–367. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated ampk/sirt1 signaling pathway in oa rats. Biomed. Pharm. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via sirt1/ampk-mediated inhibition of er stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage m1 polarisation exacerbates experimental osteoarthritis partially through r-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to m2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, S.; Mao, X.; Wang, W.; Tai, H. Inhibition effect of curcumin on tnf-alpha and mmp-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 2013, 91, 77–85. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Gu, J.H.; Wang, F.Y.; Shang, X.S.; Tao, H.R.; Wang, X. Regulation of type ii collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of nf-kappab signaling in rat chondrocytes. Mol. Med. Rep. 2017, 16, 1837–1845. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of tlr4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/are is a key pathway for curcumin-mediated protection of tmj chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin inhibits the perk-eif2alpha-chop pathway through promoting sirt1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid. Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018, 38, BSR20171691. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V.; et al. Exploiting curcumin synergy with natural products using quantitative analysis of dose-effect relationships in an experimental in vitro model of osteoarthritis. Front. Pharm. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by il-1beta via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2019, 28, 547–553. [Google Scholar] [CrossRef]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signaling by resveratrol in human chondrocytes in vitro. Biochem. Pharm. 2008, 75, 677–687. [Google Scholar] [CrossRef]
- Gusman, J.; Malonne, H.; Atassi, G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 2001, 22, 1111–1117. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Elmali, N.; Esenkaya, I.; Harma, A.; Ertem, K.; Turkoz, Y.; Mizrak, B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 2005, 54, 158–162. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits il-1 beta-induced stimulation of caspase-3 and cleavage of parp in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharm. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and atp production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Hung, L.F.; Wu, W.L.; Chang, D.M.; Huang, C.Y.; Lai, J.H.; Ho, L.J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.G.; Xiao, D.M.; Fan, M.; Wang, D.P.; Xiong, J.Y.; Chen, Y.; Ding, Y.; Liu, S.L. Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating sirt1 and thereby suppressing nuclear factor-kappab activity. Eur. J. Pharm. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating sirt1 and thereby silencing hif-2alpha. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective effect of resveratrol against il-1beta-induced inflammatory response on human osteoarthritic chondrocytes partly via the tlr4/myd88/nf-kappab signaling pathway: An “in vitro study”. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef]
- Gu, H.; Jiao, Y.; Yu, X.; Li, X.; Wang, W.; Ding, L.; Liu, L. Resveratrol inhibits the il-1beta-induced expression of mmp-13 and il-6 in human articular chondrocytes via tlr4/myd88-dependent and -independent signaling cascades. Int. J. Mol. Med. 2017, 39, 734–740. [Google Scholar] [CrossRef]
- Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral resveratrol prevents osteoarthritis progression in c57bl/6j mice fed a high-fat diet. Nutrients 2016, 8, 233. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral administration of resveratrol alleviates osteoarthritis pathology in c57bl/6j mice model induced by a high-fat diet. Mediat. Inflamm. 2017, 2017, 7659023. [Google Scholar] [CrossRef]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in c57bl/6 mice by inducing autophagy via ampk/mtor pathway. J. Pharm. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; He, J.; Gu, H.; Yang, Y.; Huang, Y.; Xu, X.; Liu, L. Protective effect of resveratrol on obesity-related osteoarthritis via alleviating jak2/stat3 signaling pathway is independent of socs3. Toxicol. Appl. Pharm. 2020, 388, 114871. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, H.; Ma, T.; Lan, H.; Feng, S.; Zhu, H.; Ji, Y. Resveratrol protects murine chondrogenic atdc5 cells against lps-induced inflammatory injury through up-regulating mir-146b. Cell. Physiol. Biochem. 2018, 47, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol supplementation reduces pain and inflammation in knee osteoarthritis patients treated with meloxicam: A randomized placebo-controlled study. J. Med. Food 2018. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, H.J.; Lee, D.Y.; Jo, H.S.; Jeong, J.H.; Kim, D.H.; Nam, D.C.; Lee, C.J.; Hwang, S.C. Chondroprotective effects of wogonin in experimental models of osteoarthritis in vitro and in vivo. Biomol. Ther. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A wogonin-rich-fraction of scutellaria baicalensis root extract exerts chondroprotective effects by suppressing il-1beta-induced activation of ap-1 in human oa chondrocytes. Sci. Rep. 2017, 7, 43789. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ros/erk/nrf2 signaling pathways in human osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Ansari, M.Y.; Haqqi, T.M. Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in il-1beta stimulated osteoarthritis chondrocytes. Chem. Biol. Interact. 2017, 274, 13–23. [Google Scholar] [CrossRef]
- Smith, J.F.; Starr, E.G.; Goodman, M.A.; Hanson, R.B.; Palmer, T.A.; Woolstenhulme, J.B.; Weyand, J.A.; Marchant, A.D.; Bueckers, S.L.; Nelson, T.K.; et al. Topical application of wogonin provides a novel treatment of knee osteoarthritis. Front. Physiol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M. A review on the chemical and pharmacological aspects of genus berberis. Planta Med. 1975, 28, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Jeong, H.S.; Chun, C.S.; Kim, H.M. Baekjeolyusin-tang and its active component berberine block the release of collagen and proteoglycan from il-1beta-stimulated rabbit cartilage and down-regulate matrix metalloproteinases in rabbit chondrocytes. Phytother. Res. 2011, 25, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.F.; Chen, W.P.; Tang, J.L.; Bao, J.P.; Wu, L.D. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother. Res. 2011, 25, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, T.; Xia, C.; Shi, L.; Wang, S.; Zheng, X.; Hu, T.; Zhang, B. Berberine ameliorates cartilage degeneration in interleukin-1beta-stimulated rat chondrocytes and in a rat model of osteoarthritis via akt signaling. J. Cell. Mol. Med. 2014, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.Q.; Yu, L.; He, B.; Wu, S.H.; Zhao, Q.; Xia, S.Q.; Mei, H.J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via ampk and p38 mapk signaling. Apoptosis 2015, 20, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, H.; Li, Y.; Deng, M.; He, B.; Xia, S.; Zhang, C.; Liu, S. Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via wnt/beta-catenin pathway. Eur. J. Pharm. 2016, 789, 109–118. [Google Scholar] [CrossRef]
- Liu, S.C.; Lee, H.P.; Hung, C.Y.; Tsai, C.H.; Li, T.M.; Tang, C.H. Berberine attenuates ccn2-induced il-1beta expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharm. 2015, 289, 20–29. [Google Scholar] [CrossRef]
- Yu, S.M.; Cho, H.; Kim, G.H.; Chung, K.W.; Seo, S.Y.; Kim, S.J. Berberine induces dedifferentiation by actin cytoskeleton reorganization via phosphoinositide 3-kinase/akt and p38 kinase pathways in rabbit articular chondrocytes. Exp. Biol. Med. (Maywood) 2016, 241, 800–807. [Google Scholar] [CrossRef]
- Li, X.; He, P.; Hou, Y.; Chen, S.; Xiao, Z.; Zhan, J.; Luo, D.; Gu, M.; Lin, D. Berberine inhibits the interleukin-1 beta-induced inflammatory response via mapk downregulation in rat articular chondrocytes. Drug Dev. Res. 2019, 80, 637–645. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.Q.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxids Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kang, S.H.; Jeong, S.J.; Kim, S.H.; Ko, H.S.; Kim, S.H. Inhibition of c-jun n-terminal kinase and nuclear factor kappa b pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated raw264.7 cells. Immunopharmacol. Immunotoxicol. 2012, 34, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits il-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating sirt1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Wang, W.; Zhang, H.; Chen, J.; Su, P.; Liu, L.; Li, W. Naringin protects against cartilage destruction in osteoarthritis through repression of nf-kappab signaling pathway. Inflammation 2016, 39, 385–392. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.F.; Sun, W.X. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med. Sci. Monit. 2017, 23, 3746–3751. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, C. Emodin attenuates apoptosis and inflammation induced by lps through up-regulating lncrna tug1 in murine chondrogenic atdc5 cells. Biomed. Pharm. 2018, 103, 897–902. [Google Scholar] [CrossRef]
- Ding, Q.H.; Ye, C.Y.; Chen, E.M.; Zhang, W.; Wang, X.H. Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting nf-kappab and wnt/beta-catenin signaling in-vitro and in-vivo. Int. Immunopharmacol. 2018, 61, 222–230. [Google Scholar] [CrossRef]
- Sacitharan, P.K.; Lwin, S.; Gharios, G.B.; Edwards, J.R. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via ep300. Exp. Mol. Med. 2018, 50, 123. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzi, R.M. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic. Biol. Med. 2020, 153, 159–172. [Google Scholar] [CrossRef]
- Davidson, R.K.; Green, J.; Gardner, S.; Bao, Y.; Cassidy, A.; Clark, I.M. Identifying chondroprotective diet-derived bioactives and investigating their synergism. Sci. Rep. 2018, 8, 17173. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. https://doi.org/10.3390/cells9051232
D’Adamo S, Cetrullo S, Panichi V, Mariani E, Flamigni F, Borzì RM. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells. 2020; 9(5):1232. https://doi.org/10.3390/cells9051232
Chicago/Turabian StyleD’Adamo, Stefania, Silvia Cetrullo, Veronica Panichi, Erminia Mariani, Flavio Flamigni, and Rosa Maria Borzì. 2020. "Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role" Cells 9, no. 5: 1232. https://doi.org/10.3390/cells9051232
APA StyleD’Adamo, S., Cetrullo, S., Panichi, V., Mariani, E., Flamigni, F., & Borzì, R. M. (2020). Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells, 9(5), 1232. https://doi.org/10.3390/cells9051232




