Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Findings and Histologic Features
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
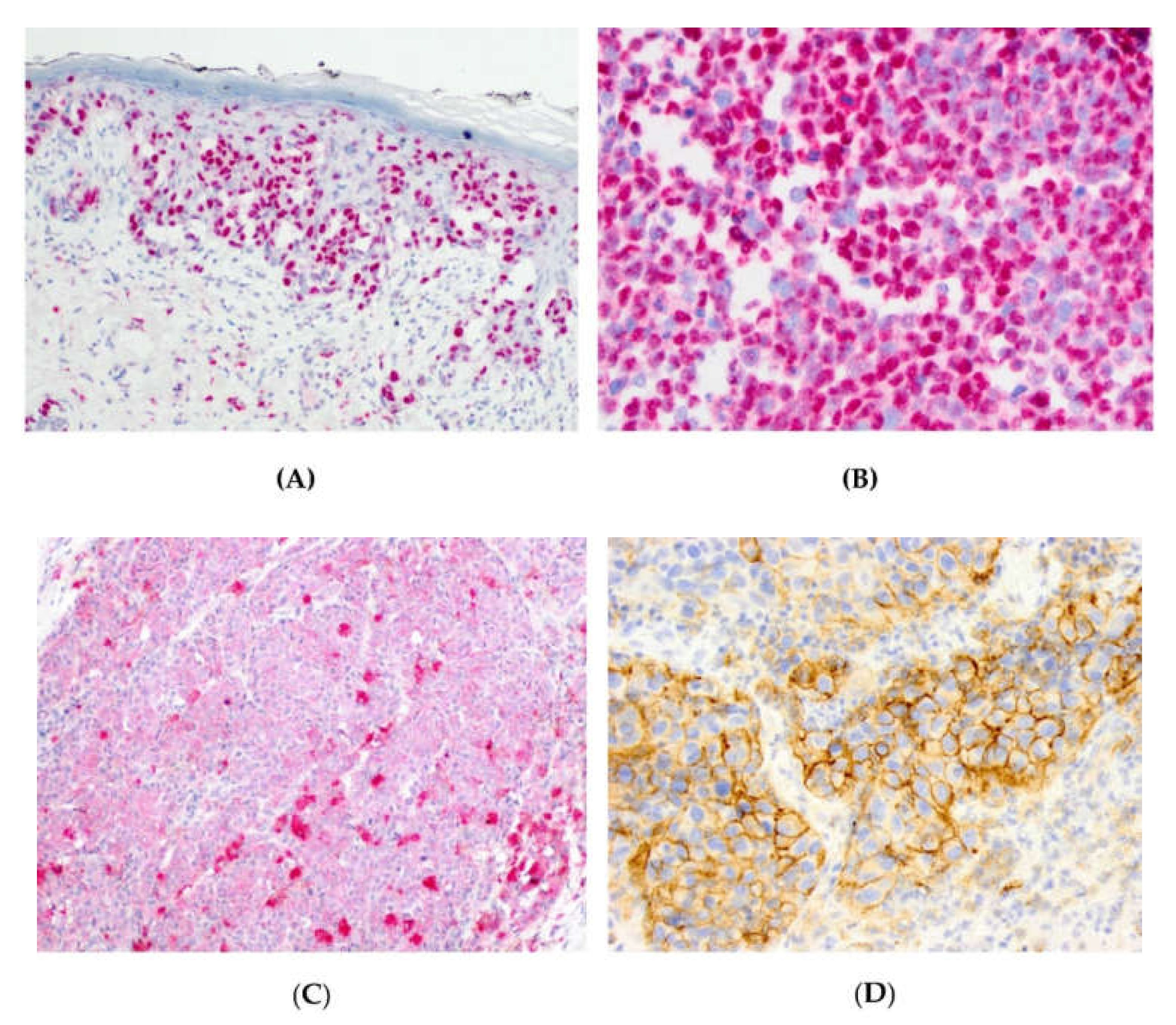
3.1. Expression of PARP1, IDO1, and PD-L1 in Mucosal Melanoma Cells.
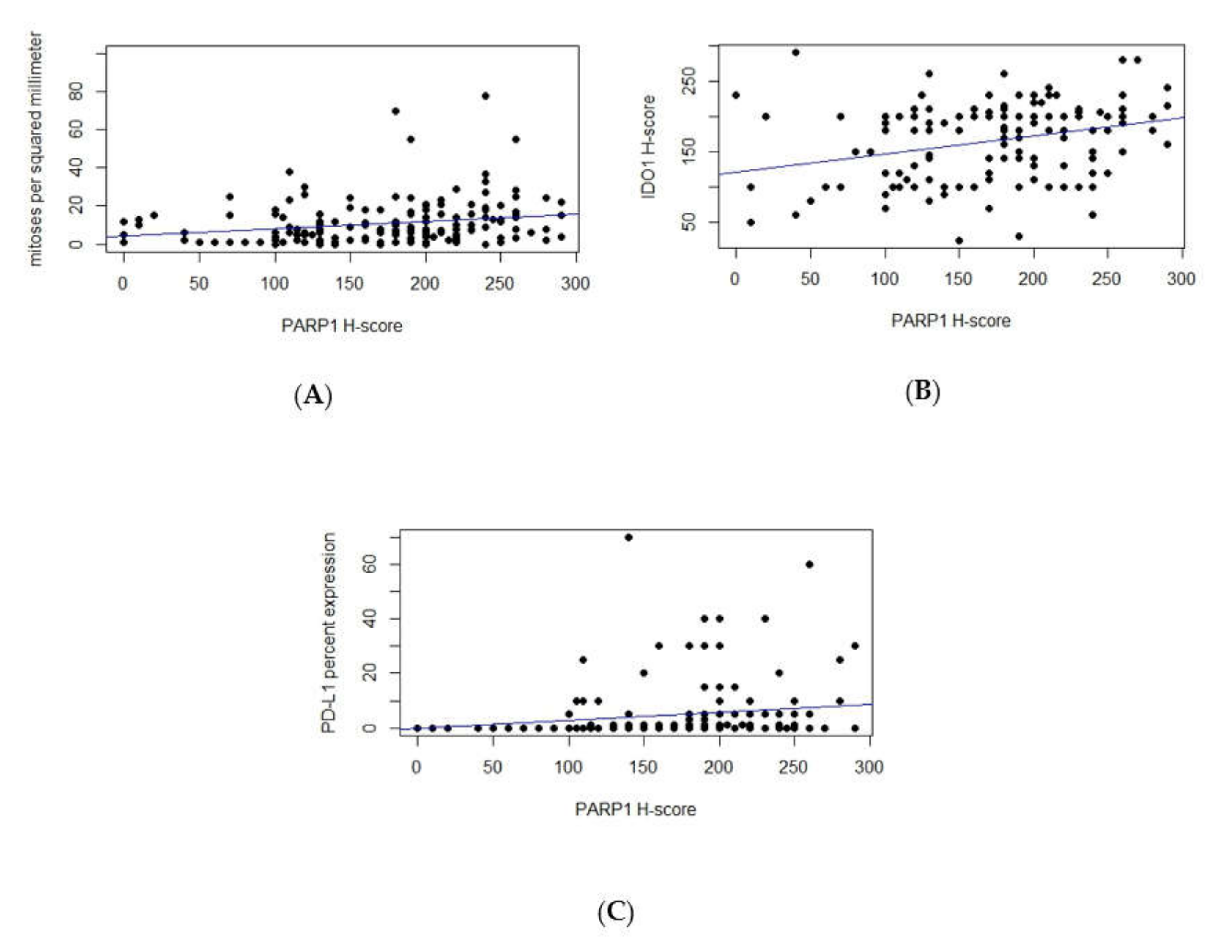
3.2. Correlations between PARP1, IDO1, and PD-L1 Expression and Clinicopathologic Variables
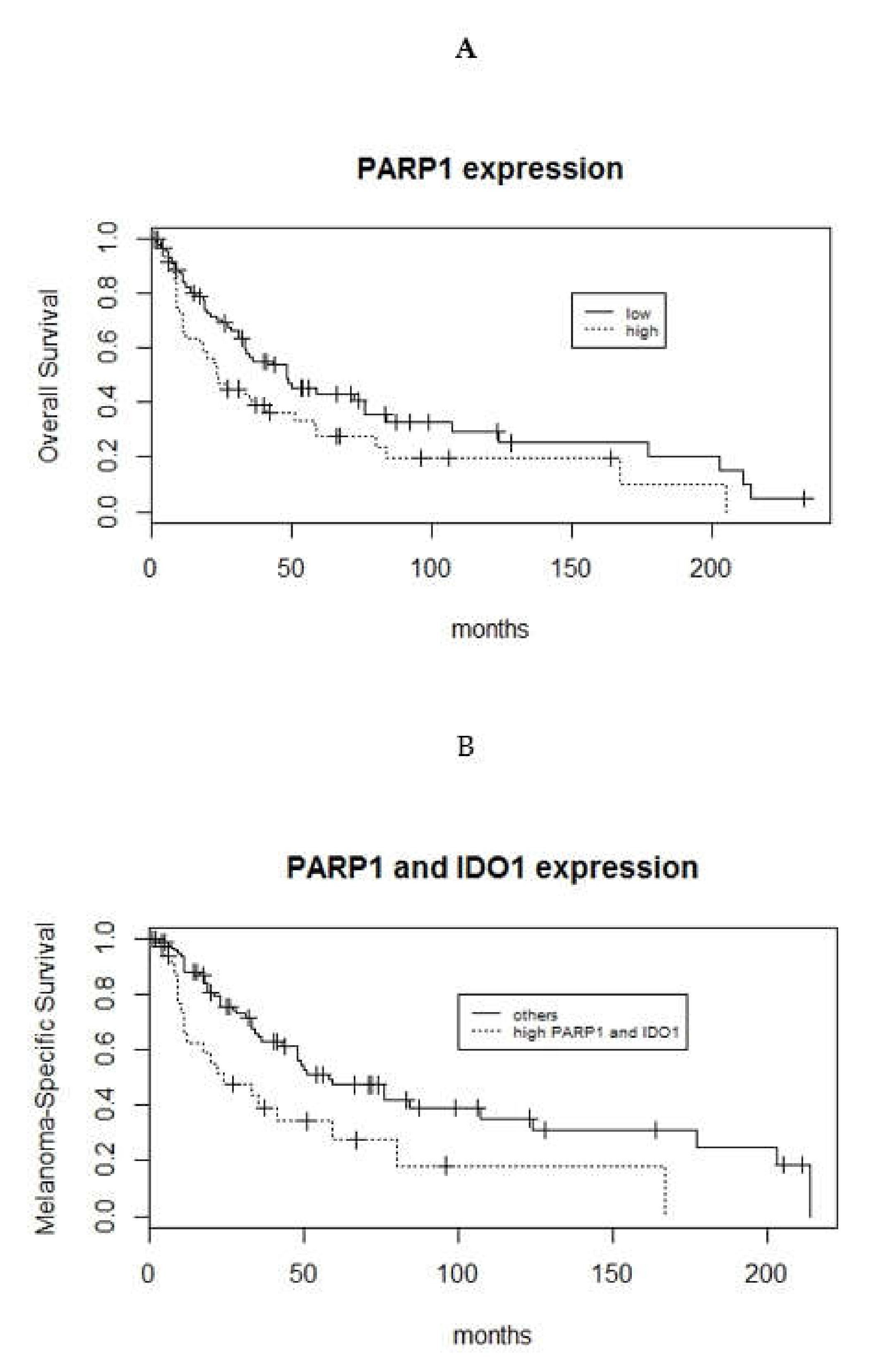
3.3. Survival Analyses of PARP1, IDO1, and PD-L1 Expression in Mucosal Melanoma Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, A.E.; Kamell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Hahn, H.M.; Lee, K.G.; Choi, W.; Cheong, S.H.; Myung, K.B.; Hahn, H.J. An updated review of mucosal melanoma: Survival meta-analysis. Mol. Clin. Oncol. 2019, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.D.; Olszewski, A.J. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int. J. Cancer 2014, 134, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.J.; Zhang, F.; Zhang, G.S.; Deng, X.W.; Zhang, W.J.; Lawrence, W.R.; Zou, L.; Zhang, X.S.; Lu, L.X. Efficacy and safety of primary surgery with postoperative radiotherapy in head and neck mucosal melanoma: A single-arm Phase II study. Cancer Manag. Res. 2018, 10, 6985–6996. [Google Scholar] [CrossRef] [PubMed]
- Mignard, C.; Deschamps Huvier, A.; Gillibert, A.; Duval Modeste, A.B.; Dutriaux, C.; Khammari, A.; Avril, M.F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J. Oncol. 2018, 2018, 1908065. [Google Scholar] [CrossRef] [PubMed]
- Langelier, M.F.; Eisemann, T.; Riccio, A.A.; Pascal, J.M. PARP family enzymes: Regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 2018, 53, 187–198. [Google Scholar] [CrossRef]
- Slade, D. Mitotic functions of poly(ADP-ribose) polymerases. Biochem. Pharmacol. 2019, 167, 33–43. [Google Scholar] [CrossRef]
- Iglesias, P.; Costoya, J.A. The antimitotic potential of PARP inhibitors, an unexplored therapeutic alternative. Curr. Top Med. Chem. 2014, 14, 2346–2365. [Google Scholar] [CrossRef]
- Lodhi, N.; Kossenkov, A.V.; Tulin, A.V. Bookmarking promoters in mitotic chromatin: Poly(ADP-ribose)polymerase-1 as an epigenetic mark. Nucleic Acids Res. 2014, 42, 7028–7038. [Google Scholar] [CrossRef]
- Perdoni, F.; Bottone, M.G.; Soldani, C.; Veneroni, P.; Alpini, C.; Pellicciari, C.; Scovassi, A.I. Distribution of centromeric proteins and PARP-1 during mitosis and apoptosis. Ann. N. Y. Acad. Sci. 2009, 1171, 32–37. [Google Scholar] [CrossRef]
- Madison, D.L.; Stauffer, D.; Lundblad, J.R. The PARP inhibitor PJ34 causes a PARP1-independent, p21 dependent mitotic arrest. DNA Repair (Amst.) 2011, 10, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Colicchia, V.; Petroni, M.; Guarguaglini, G.; Sardina, F.; Sahún-Roncero, M.; Carbonari, M.; Ricci, B.; Heil, C.; Capalbo, C.; Belardinilli, F.; et al. PARP inhibitors enhance replication stress and cause mitotic catastrophe in MYCN-dependent neuroblastoma. Oncogene 2017, 36, 4682–4691. [Google Scholar] [CrossRef] [PubMed]
- Staibano, S.; Pepe, S.; Lo Muzio, L.; Somma, P.; Mascolo, M.; Argenziano, G.; Scalvenzi, M.; Salvatore, G.; Fabbrocini, G.; Molea, G.; et al. Poly(adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Hum. Pathol. 2005, 36, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bennici, E.; Novelli, F.; Pioli, C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology 2013, 139, 428–437. [Google Scholar] [CrossRef]
- Munn, D.H. Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr. Med. Chem. 2011, 18, 2240–2246. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Theate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Brochez, L.; Chevolet, I.; Kruse, V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer 2017, 76, 167–182. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—Challenges and opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Khan, J.A.; Forouhar, F.; Tao, X.; Tong, L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin. Ther. Targets 2007, 11, 695–705. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 4 March 2020).
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kähler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer 2017, 81, 36–44. [Google Scholar] [CrossRef]
- Lodhi, N.; Ji, Y.; Tulin, A. Mitotic bookmarking: Maintaining post-mitotic reprogramming of transcription reactivation. Curr. Mol. Biol. Rep. 2016, 2, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tie, X.; Han, S.; Meng, L.; Wang, Y.; Wu, A. NFAT1 is highly expressed in, and regulates the invasion of, glioblastoma multiforme cells. PLoS ONE 2013, 8, e66008. [Google Scholar] [CrossRef] [PubMed]
- Kashima, L.; Idogawa, M.; Mita, H.; Shitashige, M.; Yamada, T.; Ogi, K.; Suzuki, H.; Toyota, M.; Ariga, H.; Sasaki, Y.; et al. CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation. J. Biol. Chem. 2012, 287, 12975–12984. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Perez, R.; Muñoz-Gámez, J.A.; Ruiz-Extremera, A.; O’Valle, F.; Sanjuán-Nuñez, L.; Martín-Alvarez, A.B.; Martín-Oliva, D.; Caballero, T.; Muñoz de Rueda, P.; León, J.; et al. Inhibition of poly adenosine diphosphate-ribose polymerase decreases hepatocellular carcinoma growth by modulation of tumor-related gene expression. Hepatology 2010, 51, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Jin, J.; Wang, J.; Huang, J.; Ma, Z.; Huang, X.; He, X.; Zhou, Y.; Xu, Y.; et al. High PARP-1 expression predicts poor survival in acute myeloid leukemia and PARP-1 inhibitor and SAHA-bendamustine hybrid inhibitor combination treatment synergistically enhances anti-tumor effects. EBioMedicine 2018, 38, 47–56. [Google Scholar] [CrossRef]
- Jacot, W.; Thezenas, S.; Senal, R.; Viglianti, C.; Laberenne, A.C.; Lopez-Crapez, E.; Bibeau, F.; Bleuse, J.P.; Romieu, G.; Lamy, P.J. BRCA1 promoter hypermethylation, 53BP1 protein expression and PARP-1 activity as biomarkers of DNA repair deficit in breast cancer. BMC Cancer 2013, 13, 523. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Monneur, A.; Perrot, D.; Chevreau, C.; Le Cesne, A.; Blay, J.Y.; Mir, O.; Birnbaum, D. PARP1 expression in soft tissue sarcomas is a poor-prognosis factor and a new potential therapeutic target. Mol. Oncol. 2019, 13, 1577–1588. [Google Scholar] [CrossRef]
- Yélamos, J.; Moreno-Lama, L.; Jimeno, J.; Ali, S.O. Immunomodulatory Roles of PARP-1 and PARP-2: Impact on PARP-Centered Cancer Therapies. Cancers (Basel) 2020, 12, E392. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, E41. [Google Scholar] [CrossRef]
- Luo, X.; Nie, J.; Wang, S.; Chen, Z.; Chen, W.; Li, D.; Hu, H.; Li, B. Poly(ADP-ribosyl)ation of FOXP3 Protein Mediated by PARP-1 Protein regulates the function of regulatory t cells. J. Biol. Chem. 2015, 290, 28675–28682. [Google Scholar] [CrossRef]
- Heyman, B.; Jamieson, C. To PARP or not to PARP? Toward sensitizing acute myeloid leukemia stem cells to immunotherapy. EMBO J. 2019, 38, e103479. [Google Scholar] [CrossRef] [PubMed]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Tandler, C.; Mbarga, M.; Schaefer, T.; Falcone, M.; et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019, 572, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Godin-Ethier, J.; Hanafi, L.A.; Piccirillo, C.A.; Lapointe, R. Indoleamine 2,3-dioxygenase expression in human cancers: Clinical and immunologic perspectives. Clin. Cancer Res. 2011, 17, 6985–6991. [Google Scholar] [CrossRef]
- Zheng, X.; Koropatnick, J.; Li, M.; Zhang, X.; Ling, F.; Ren, X.; Hao, X.; Sun, H.; Vladau, C.; Franek, J.A.; et al. Reinstalling antitumor immunity by inhibiting tumor derived immunosuppressive molecule IDO through RNA interference. J. Immunol. 2006, 177, 5639–5646. [Google Scholar] [CrossRef]
- Munn, D.H.; Shafizadeh, E.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef]
- Maleki Vareki, S.; Rytelewski, M.; Figueredo, R.; Chen, D.; Ferguson, P.J.; Vincent, M.; Min, W.; Zheng, X.; Koropatnick, J. Indoleamine 2,3-dioxygenase mediates immune-independent human tumor cell resistance to olaparib, gamma radiation, and cisplatin. Oncotarget 2014, 5, 2778–2791. [Google Scholar] [CrossRef]
- Li, A.; Yi, M.; Qin, S.; Chu, Q.; Luo, S.; Wu, K. Prospects for combining immune checkpoint blockade with PARP inhibition. J. Hematol. Oncol. 2019, 12, 98. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Xu, X.; Qian, Y.; Liang, G.; Yao, F.; Yao, Z.; Wu, H.; Zhang, J.; He, Q.; et al. PARP1 Suppresses the Transcription of PD-L1 by Poly(ADP-Ribosyl)ating STAT3. Cancer Immunol. Res. 2019, 7, 136–149. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, K.; Xiao, Y.; Feng, B.; Mikule, K.; Ma, X.; Feng, N.; Vellano, C.P.; Federico, L.; Marszalek, J.R.; et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci. Rep. 2019, 9, 1853. [Google Scholar] [CrossRef] [PubMed]

| PARP1 N = 167 | PD-L1 N = 174 | IDO1 N = 159 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| High | Low | p-Value | High | Low | p-Value | High | Low | p-Value | |
| Age | |||||||||
| >65 years | 41 | 46 | 0.44 | 20 | 62 | 0.4 | 39 | 45 | 0.43 |
| < = 65 years | 42 | 38 | 28 | 64 | 40 | 35 | |||
| Ulceration | |||||||||
| Present | 66 | 62 | 0.47 | 42 | 88 | 0.019* | 70 | 53 | 0.0011* |
| Absent | 17 | 22 | 6 | 38 | 9 | 27 | |||
| Mitoses | |||||||||
| >4 / mm2 | 49 | 34 | 0.02* | 24 | 60 | 0.87 | 69 | 60 | 0.067 |
| <= 4 / mm2 | 34 | 50 | 24 | 66 | 10 | 20 | |||
| Lymphovascular Invasion | |||||||||
| Present | 18 | 13 | 0.33 | 9 | 21 | 0.82 | 22 | 11 | 0.032 * |
| Absent | 65 | 71 | 38 | 105 | 57 | 69 | |||
| Perineural invasion | |||||||||
| Present | 11 | 14 | 0.67 | 5 | 16 | 0.80 | 9 | 16 | 0.19 |
| Absent | 72 | 70 | 42 | 110 | 70 | 64 | |||
| Overall Survival | Melanoma Specific Survival | |||
|---|---|---|---|---|
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | |
| Age | 1.14 | 0.49 | 1.10 | 0.65 |
| Stage (1–2 versus 3–4) | 1.6 | 0.095 | 2.19 | 0.0062 * |
| Ulceration | 1.69 | 0.029 * | 1.78 | 0.035 * |
| Mitoses | 1.47 | 0.12 | 1.54 | 0.13 |
| Lymphovascular invasion | 1.03 | 0.91 | 1.2 | 0.52 |
| Perineural invasion | 1.05 | 0.86 | 1.2 | 0.64 |
| PARP1 expression | 1.59 | 0.027 * | 1.61 | 0.049 * |
| IDO1 expression | 1.3 | 0.22 | 1.31 | 0.28 |
| PD-L1 expression | 0.75 | 0.23 | 0.71 | 0.21 |
| PARP1 and IDO1 expression | 1.77 | 0.017 * | 2.14 | 0.0043 * |
| PARP1 and PD-L1 expression | 1.35 | 0.28 | 1.37 | 0.33 |
| IDO1 and PD-L1 expression | 1.24 | 0.46 | 1.33 | 0.39 |
| Overall Survival | Melanoma Specific Survival | |||
|---|---|---|---|---|
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | |
| PARP1 expression | 1.53 | 0.047 * | 1.68 | 0.04 * |
| Ulceration | 1.31 | 0.30 | 1.48 | 0.2 |
| Stage (1-2 versus 3-4) | - | - | 2.43 | 0.0051 * |
| PARP1 and IDO1 expression | 1.75 | 0.025 * | 2.14 | 0.0069 * |
| Ulceration | 1.13 | 0.66 | 1.25 | 0.5 |
| Stage (1-2 versus 3-4) | - | - | 1.96 | 0.039 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donizy, P.; Wu, C.-L.; Mull, J.; Fujimoto, M.; Chłopik, A.; Peng, Y.; Shalin, S.C.; Selim, M.A.; Puig, S.; Fernandez-Figueras, M.-T.; et al. Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas. Cells 2020, 9, 1135. https://doi.org/10.3390/cells9051135
Donizy P, Wu C-L, Mull J, Fujimoto M, Chłopik A, Peng Y, Shalin SC, Selim MA, Puig S, Fernandez-Figueras M-T, et al. Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas. Cells. 2020; 9(5):1135. https://doi.org/10.3390/cells9051135
Chicago/Turabian StyleDonizy, Piotr, Cheng-Lin Wu, Jason Mull, Masakazu Fujimoto, Agata Chłopik, Yan Peng, Sara C. Shalin, M. Angelica Selim, Susana Puig, Maria-Teresa Fernandez-Figueras, and et al. 2020. "Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas" Cells 9, no. 5: 1135. https://doi.org/10.3390/cells9051135
APA StyleDonizy, P., Wu, C.-L., Mull, J., Fujimoto, M., Chłopik, A., Peng, Y., Shalin, S. C., Selim, M. A., Puig, S., Fernandez-Figueras, M.-T., Shea, C. R., Biernat, W., Ryś, J., Marszalek, A., & Hoang, M. P. (2020). Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas. Cells, 9(5), 1135. https://doi.org/10.3390/cells9051135







