Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Cell Cultures
2.2. Western Blot Analysis
2.3. Animal Experiments
2.4. siRNA Transfection
2.5. Flow Cytometry Analysis
2.6. Immunofluorescence Analysis
2.7. Adenovirus and Stable Cell Lines
2.8. RT-PCR Analysis
2.9. Zymography
2.10. Cell Binding Assay
2.11. In Vitro Transmigration Assay
2.12. Statistical Analysis
3. Results
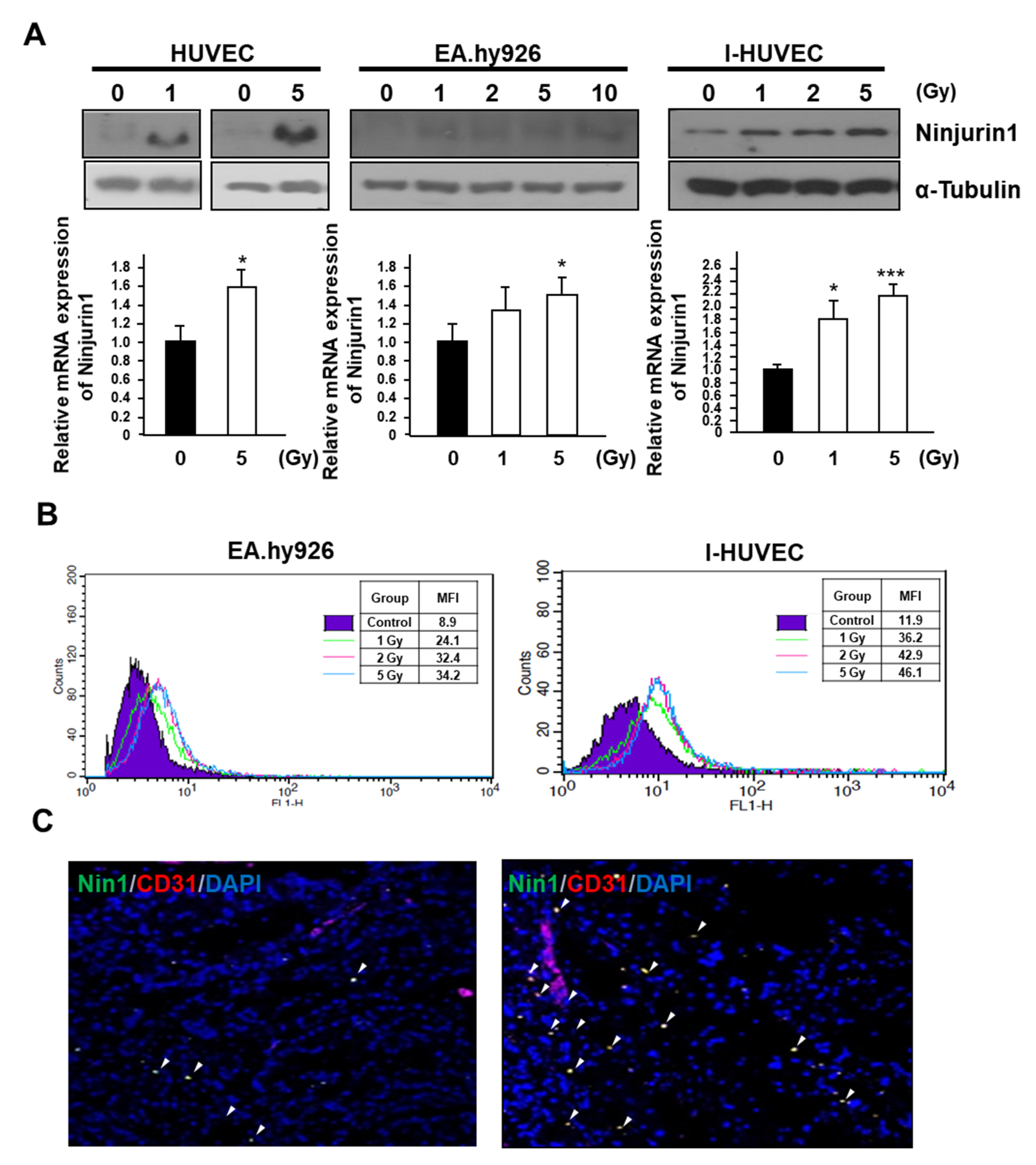
3.1. Surface Expression of Ninj1 Following Treatment with Radiation in Endothelial Cell Lines
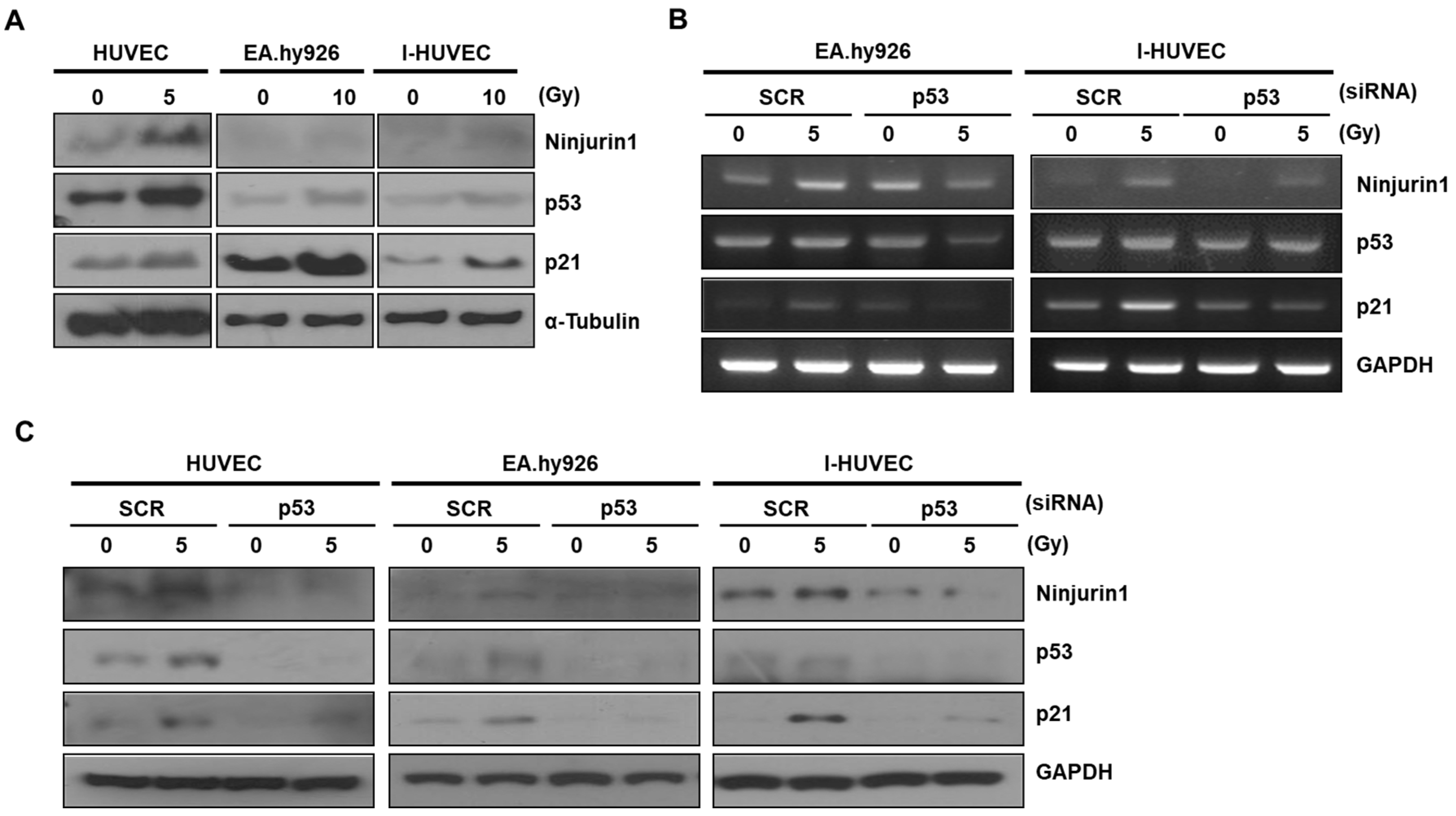
3.2. p53 Enhanced Ninj1 Expression in Endothelial Cell Lines
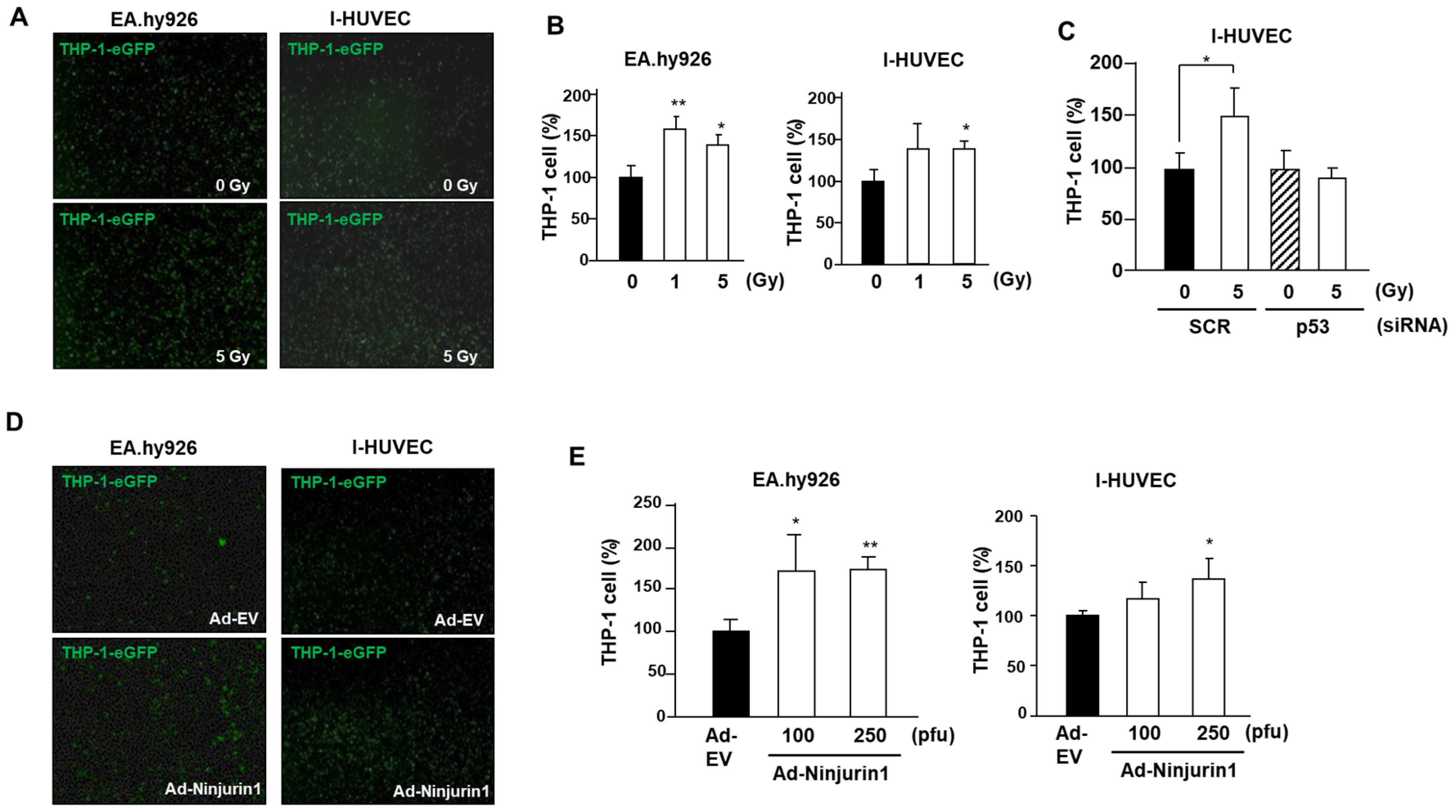
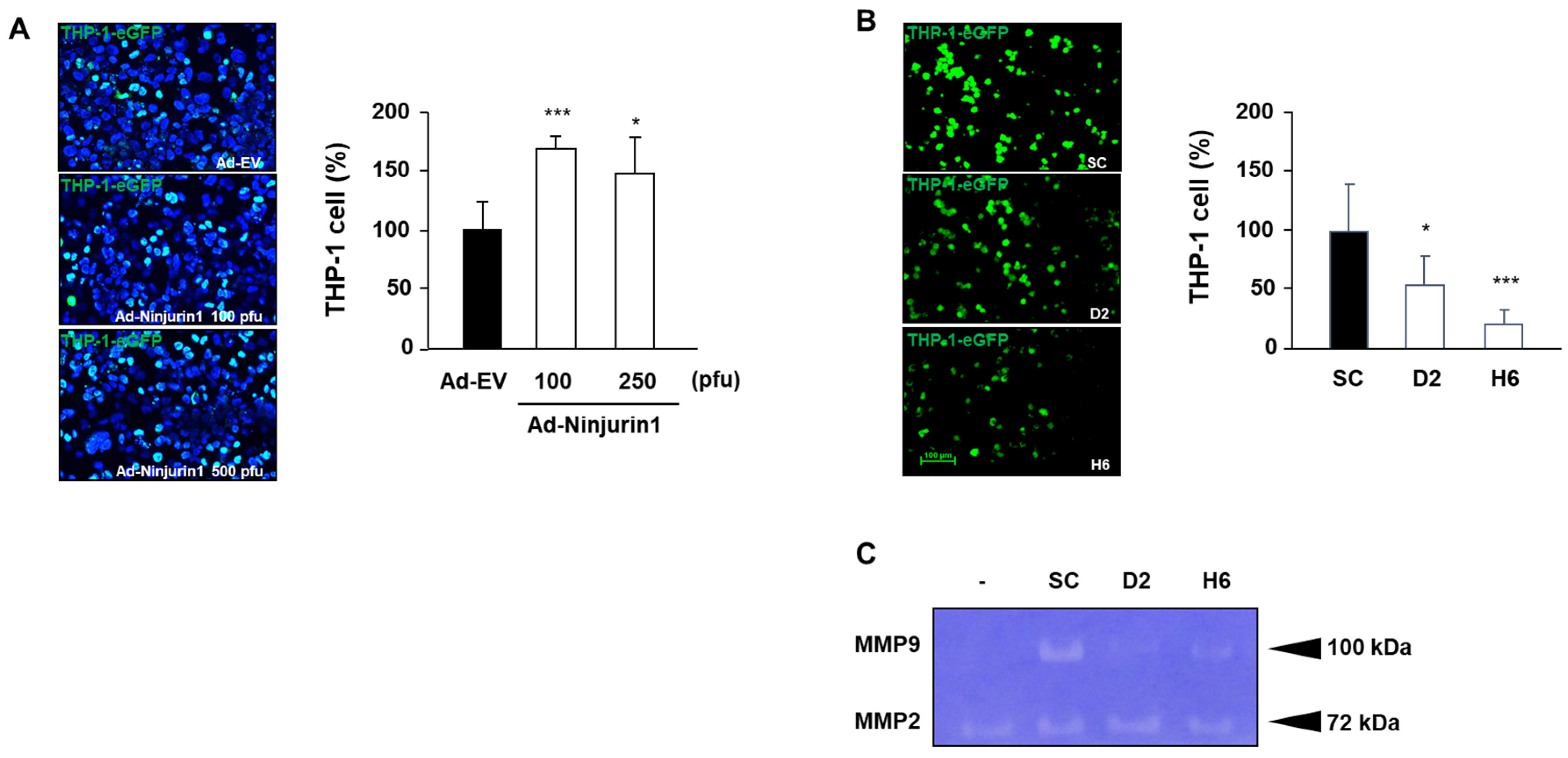
3.3. Over-Expressed Ninj1 in Endothelial Cells Enhanced Monocyte Attachment
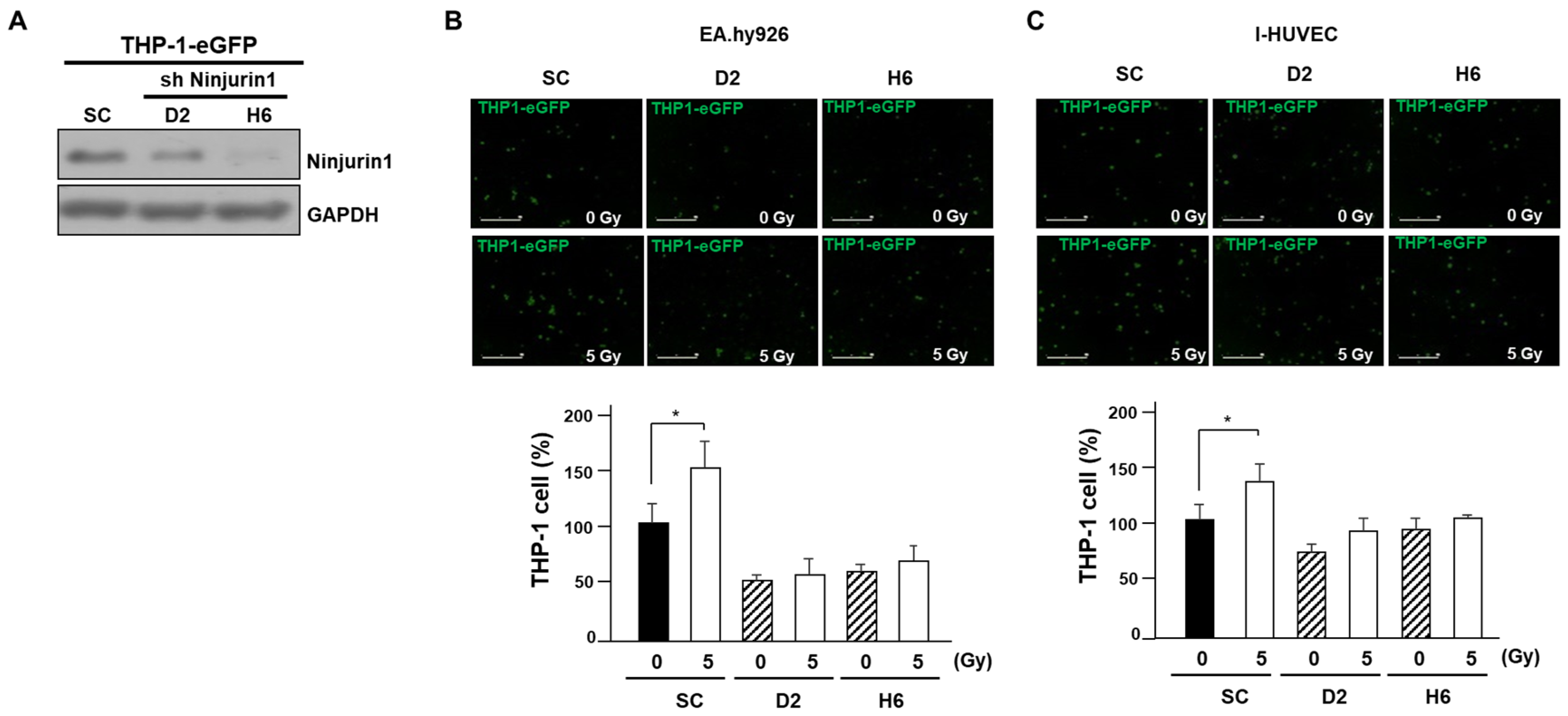
3.4. Over-Expressed Ninj1 in Endothelial Cells Increases Monocyte Transmigration and Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoshimura, M.; Itasaka, S.; Harada, H.; Hiraoka, M. Microenvironment and radiation therapy. BioMed Res. Int. 2013, 2013, 685308. [Google Scholar] [CrossRef] [PubMed]
- Grabham, P.; Sharma, P. The effects of radiation on angiogenesis. Vasc. Cell 2013, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patricia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Kang, J.H.; Shin, D.; Park, S.H.; Kang, K.; Nho, C.W.; Seong, J.K.; Lee, S.J.; Oh, S.H. Daurinol Enhances the Efficacy of Radiotherapy in Lung Cancer via Suppression of Aurora Kinase A/B Expression. Mol. Cancer Ther. 2015, 14, 1693–1704. [Google Scholar] [CrossRef]
- Oh, E.T.; Park, M.T.; Song, M.J.; Lee, H.; Cho, Y.U.; Kim, S.J.; Chu, Y.C.; Choi, E.K.; Park, H.J. Radiation-induced angiogenic signaling pathway in endothelial cells obtained from normal and cancer tissue of human breast. Oncogene 2014, 33, 1229–1238. [Google Scholar] [CrossRef]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef]
- Fang, H.; Declerck, Y.A. Targeting the tumor microenvironment: From understanding pathways to effective clinical trials. Cancer Res. 2013, 73, 4965–4977. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–207. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Quaranta, V.; Schmid, M.C. Macrophage-Mediated Subversion of Anti-Tumour Immunity. Cells 2019, 8, 747. [Google Scholar] [CrossRef]
- Russell, J.S.; Brown, J.M. The irradiated tumor microenvironment: Role of tumor-associated macrophages in vascular recovery. Front. Physiol. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Rahat, M.A.; Hemmerlein, B.; Iragavarapu-Charyulu, V. The regulation of angiogenesis by tissue cell-macrophage interactions. Front. Physiol. 2014, 5, 262. [Google Scholar] [CrossRef]
- Sofia Vala, I.; Martins, L.R.; Imaizumi, N.; Nunes, R.J.; Rino, J.; Kuonen, F.; Carvalho, L.M.; Ruegg, C.; Grillo, I.M.; Barata, J.T.; et al. Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PLoS ONE 2010, 5, e11222. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Zimonjic, D.B.; Popescu, N.C.; Milbrandt, J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J. Biol. Chem. 1997, 272, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 1996, 17, 353–361. [Google Scholar] [CrossRef]
- Choi, S.; Woo, J.K.; Jang, Y.S.; Kang, J.H.; Hwang, J.I.; Seong, J.K.; Yoon, Y.S.; Oh, S.H. Ninjurin1 Plays a Crucial Role in Pulmonary Fibrosis by Promoting Interaction between Macrophages and Alveolar Epithelial Cells. Sci. Rep. 2018, 8, 17542. [Google Scholar] [CrossRef]
- Ifergan, I.; Kebir, H.; Terouz, S.; Alvarez, J.I.; Lecuyer, M.A.; Gendron, S.; Bourbonniere, L.; Dunay, I.R.; Bouthillier, A.; Moumdjian, R.; et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann. Neurol. 2011, 70, 751–763. [Google Scholar] [CrossRef]
- Ahn, B.J.; Le, H.; Shin, M.W.; Bae, S.J.; Lee, E.J.; Wee, H.J.; Cha, J.H.; Lee, H.J.; Lee, H.S.; Kim, J.H.; et al. Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice. J. Biol. Chem. 2014, 289, 3328–3338. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Ninomiya, Y.; Koike, A. Characterization of Ninjurin and TSC22 induction after X-irradiation of normal human skin cells. J. Dermatol. 2008, 35, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Rossi, A.; Jung, Y.S.; Yan, W.; Liu, G.; Zhang, J.; Zhang, M.; Chen, X. Ninjurin1, a target of p53, regulates p53 expression and p53-dependent cell survival, senescence, and radiation-induced mortality. Proc. Natl. Acad. Sci. USA 2013, 110, 9362–9367. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kang, J.H.; Woo, J.K.; Kim, H.M.; Hwang, J.I.; Lee, S.J.; Lee, H.Y.; Oh, S.H. Ninjurin1 suppresses metastatic property of lung cancer cells through inhibition of interleukin 6 signaling pathway. Int. J. Cancer 2016, 139, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Jang, Y.-S.; Lee, H.J.; Lee, C.-Y.; Shin, D.Y.; Oh, S.H. Inhibition of STAT3 signaling induces apoptosis and suppresses growth of lung cancer: Good and bad. Lab. Anim. Res. 2019, 35, 30. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, A.R.; Nam, J.K.; Kim, J.M.; Kim, J.Y.; Seo, H.R.; Lee, H.J.; Cho, J.; Lee, Y.J. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat. Commun. 2018, 9, 5108. [Google Scholar] [CrossRef]
- Abdulkarim, B.; Deutsch, E. Endothelial-cell apoptosis and tumour response to radiotherapy. Lancet Oncol. 2004, 5, 9. [Google Scholar] [CrossRef]
- Cao, C.; Shinohara, E.T.; Subhawong, T.K.; Geng, L.; Kim, K.W.; Albert, J.M.; Hallahan, D.E.; Lu, B. Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol. Cancer Ther. 2006, 5, 411–417. [Google Scholar] [CrossRef]
- Burdelya, L.G.; Komarova, E.A.; Hill, J.E.; Browder, T.; Tararova, N.D.; Mavrakis, L.; DiCorleto, P.E.; Folkman, J.; Gudkov, A.V. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006, 66, 9356–9361. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, J.; Yan, W.; Cho, S.J.; Lucchesi, C.; Chen, M.; Huang, E.C.; Scoumanne, A.; Zhang, W.; Chen, X. Ninjurin 1 has two opposing functions in tumorigenesis in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, 11500–11505. [Google Scholar] [CrossRef]
- Ahn, G.O.; Brown, J.M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008, 13, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ahn, B.J.; Shin, M.W.; Jeong, J.W.; Kim, J.H.; Kim, K.W. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009, 16, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Jang, Y.S.; Kang, J.H.; Hwang, J.I.; Seong, J.K.; Lee, S.J.; Jeon, S.; Oh, G.T.; Lee, H.Y.; Oh, S.H. Ninjurin1 inhibits colitis-mediated colon cancer development and growth by suppression of macrophage infiltration through repression of FAK signaling. Oncotarget 2016, 7, 29592–29604. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.W.; Bae, S.J.; Wee, H.J.; Lee, H.J.; Ahn, B.J.; Le, H.; Lee, E.J.; Kim, R.H.; Lee, H.S.; Seo, J.H.; et al. Ninjurin1 regulates lipopolysaccharide-induced inflammation through direct binding. Int. J. Oncol. 2016, 48, 821–828. [Google Scholar] [CrossRef]
- Matsuki, M.; Kabara, M.; Saito, Y.; Shimamura, K.; Minoshima, A.; Nishimura, M.; Aonuma, T.; Takehara, N.; Hasebe, N.; Kawabe, J. Ninjurin1 is a novel factor to regulate angiogenesis through the function of pericytes. Circ. J. 2015, 79, 1363–1371. [Google Scholar] [CrossRef]
- Galliera, E.; Randelli, P.; Dogliotti, G.; Dozio, E.; Colombini, A.; Lombardi, G.; Cabitza, P.; Corsi, M.M. Matrix metalloproteases MMP-2 and MMP-9: Are they early biomarkers of bone remodelling and healing after arthroscopic acromioplasty? Injury 2010, 41, 1204–1207. [Google Scholar] [CrossRef]
- Masson, V. Roles of serine proteases and matrix metalloproteinases in tumor invasion and angiogenesis. Bull. Mem. Acad. R. Med. Belg. 2006, 161, 320–326. [Google Scholar]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Li, Z.; Takino, T.; Endo, Y.; Sato, H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017, 108, 347–353. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-H.; Woo, J.K.; Jang, Y.-S.; Oh, S.H. Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells. Cells 2020, 9, 1086. https://doi.org/10.3390/cells9051086
Kang J-H, Woo JK, Jang Y-S, Oh SH. Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells. Cells. 2020; 9(5):1086. https://doi.org/10.3390/cells9051086
Chicago/Turabian StyleKang, Ju-Hee, Jong Kyu Woo, Yeong-Su Jang, and Seung Hyun Oh. 2020. "Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells" Cells 9, no. 5: 1086. https://doi.org/10.3390/cells9051086
APA StyleKang, J.-H., Woo, J. K., Jang, Y.-S., & Oh, S. H. (2020). Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells. Cells, 9(5), 1086. https://doi.org/10.3390/cells9051086




