Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Human Corneal Epithelial Cells
2.1.2. Human Corneal Fibroblasts
2.1.3. Two-Dimensional (2D) Conventional Cultures
2.1.4. Three-Dimensional (3-D) Stromal Cultures
2.2. EV Isolation
2.3. EV-Labelling
2.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Western Blot
2.6. Stimulated Emission Depletion (STED) Nanoscopy
2.7. Immunofluorescence Microscopy
2.8. EV Uptake
2.9. Analysis of F-Actin Organization
2.10. Collagen Contraction Assay
2.11. Proliferation Assay
2.12. Migration Assay
2.13. Proteomics
2.14. Statistical Analysis
3. Results
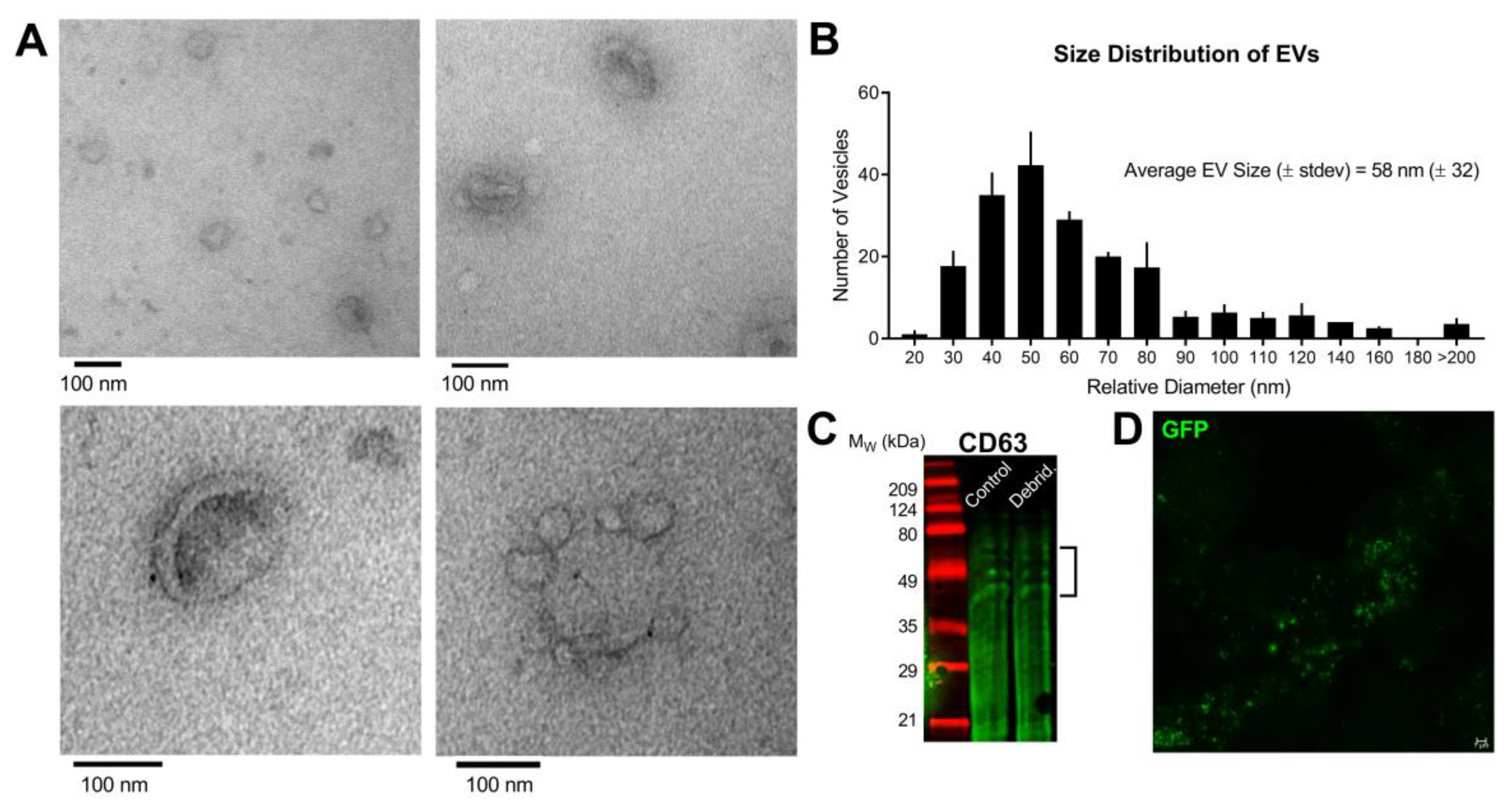
3.1. Characterization of Epithelial-Derived EVs
3.2. EV Uptake by hCFs
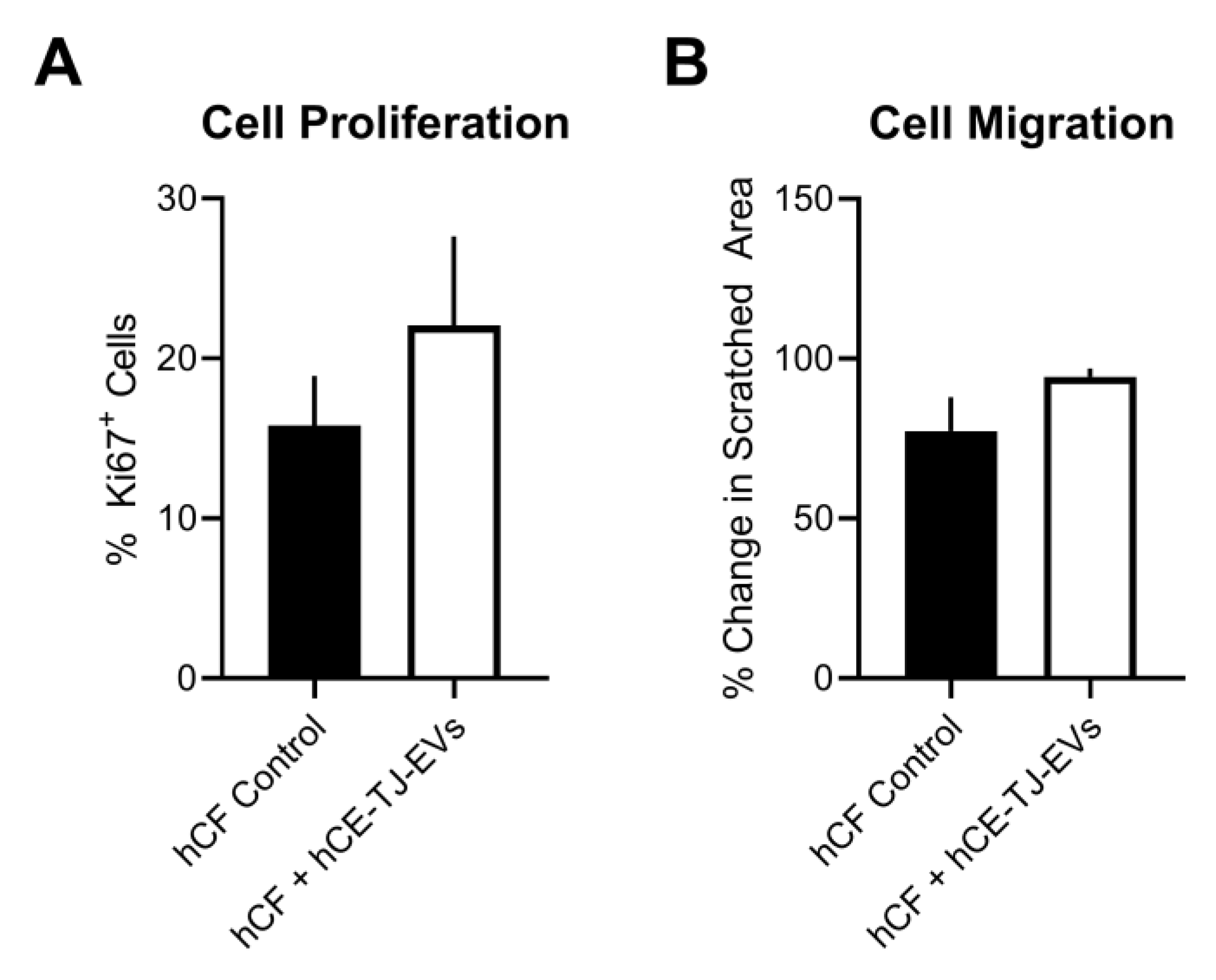
3.3. Functional Effects of hCE-TJ-Derived EVs on hCFs
3.4. EV Protein Cargo
3.5. hCE-TJ-Derived EV Protein Localization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2011, 96, 614–618. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Heal. Organ. 2003, 79, 214–221. [Google Scholar]
- Borderie, V.M.; Boëlle, P.Y.; Touzeau, O.; Allouch, C.; Boutboul, S.; Laroche, L. Predicted long-term outcome of corneal transplantation. Ophthalmology 2009, 116, 2354–2360. [Google Scholar] [CrossRef]
- Payant, J.A.; Gordon, L.W.; VanderZwaag, R.; Wood, T.O. Cataract Formation Following Corneal Transplantation in Eyes with Fuchs’ Endothelial Dystrophy. Cornea 1990, 9, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, H.L.; Gedde, S.J. Glaucoma management after corneal transplantation surgeries. Curr. Opin. Ophthalmol. 2016, 27, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 1. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal stromal wound healing in refractive surgery: The role of myofibroblasts. Prog. Retin. Eye Res. 1999, 18, 311–356. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Boil. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Breakefield, X.O.; Wood, M. Extracellular vesicles: Emerging targets for cancer therapy. Trends Mol. Med. 2014, 20, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell-Cell Communication in the Cornea. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2019. [Google Scholar] [CrossRef]
- McKay, T.B.; Karamichos, D.; Hutcheon, A.; Guo, X.; Zieske, J. Corneal Epithelial-Stromal Fibroblast Constructs to Study Cell-Cell Communication in Vitro. Bioengineering 2019, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Higashijima, S.C.; Spurr-Michaud, S.J.; Gipson, I.K. Biosynthetic responses of the rabbit cornea to a keratectomy wound. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1668–1677. [Google Scholar]
- Han, K.-Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Opthalmology Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, N.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. STEM CELLS Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Marino, G.K.; Santhiago, M.R.; Santhanam, A.; Torricelli, A.A.M.; Wilson, S.E. Regeneration of Defective Epithelial Basement Membrane and Restoration of Corneal Transparency After Photorefractive Keratectomy. J. Refract. Surg. 2017, 33, 337–346. [Google Scholar] [CrossRef]
- Marino, G.K.; Santhiago, M.R.; Torricelli, A.A.M.; Santhanam, A.; Wilson, S.E. Corneal Molecular and Cellular Biology for the Refractive Surgeon: The Critical Role of the Epithelial Basement Membrane. J. Refract. Surg. 2016, 32, 118–125. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D. Biology of corneal fibrosis: Soluble mediators, integrins, and extracellular vesicles. Eye 2019, 34, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Guo, X.; Hutcheon, A.E.K.; Tran, J.A.; Zieske, J.D. TGF-β-target genes are differentially regulated in corneal epithelial cells and fibroblasts. New Front. Ophthalmol. 2017, 3, 3. [Google Scholar] [CrossRef]
- Ren, R.; Hutcheon, A.; Guo, X.; Saeidi, N.; Melotti, S.; Ruberti, J.; Zieske, J.; Trinkaus-Randall, V. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev. Dyn. 2008, 237, 2705–2715. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Melotti, S.A.; Zieske, J.; Trinkaus-Randall, V.; Ruberti, J. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investig. Opthalmology Vis. Sci. 2007, 48, 4050–4060. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Boil. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Taylor, U.D.; Zacharias, W.; Gercel-Taylor, C. Exosome Isolation for Proteomic Analyses and RNA Profiling. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; Volume 728, pp. 235–246. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Boudaoud, A.; Burian, A.; Borowska-Wykręt, R.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Budnik, B.; Levy, E.; Harmange, G.; Slavov, N. Scope-ms: Mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018, 19, 161. [Google Scholar] [CrossRef]
- Wallace, E.W.J.; Kear-Scott, J.L.; Pilipenko, E.V.; Schwartz, M.H.; Laskowski, P.R.; Rojek, A.E.; Katanski, C.; Riback, J.A.; Dion, M.; Franks, A.; et al. Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 2015, 162, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Slavov, N.; Semrau, S.; Airoldi, E.; Budnik, B.; Van Oudenaarden, A. Differential Stoichiometry among Core Ribosomal Proteins. Cell Rep. 2015, 13, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. Uniprot: A worldwide hub of protein knowledge. Nucleic Acids Research 2018, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, J.; Fink, J.L.; Karunaratne, S.; Hanson, K.; Hamilton, N.; Teasdale, R.D. LOCATE: A mammalian protein subcellular localization database. Nucleic Acids Res. 2007, 36, D230–D233. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.D.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabro’, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes – vesicular carriers for intercellular communication. Curr. Opin. Cell Boil. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Metzelaar, M.J.; Wijngaard, P.L.; Peters, P.J.; Sixma, J.J.; Nieuwenhuis, H.K.; Clevers, H.C. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J. Boil. Chem. 1991, 266, 3239–3245. [Google Scholar]
- Gruenberg, J. The endocytic pathway: A mosaic of domains. Nat. Rev. Mol. Cell Boil. 2001, 2, 721–730. [Google Scholar] [CrossRef]
- Pols, M.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Kennel, S.; Lankford, P.; Foote, L.; Davis, I. Monoclonal Antibody to Rat CD63 Detects Different Molecular Forms in Rat Tissue. Hybridoma 1998, 17, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The ki-67 protein: From the known and the unknown. J.Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Gupta, A.; Monroy, D.; Ji, Z.; Yoshino, K.; Huang, A.; Pflugfelder, S.C. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr. Eye Res. 1996, 15, 605–614. [Google Scholar] [CrossRef]
- Tuominen, I.S.; Tervo, T.M.; Teppo, A.-M.; Valle, T.U.; Grönhagen-Riska, C.; Vesaluoma, M.H. Human Tear Fluid PDGF-BB, TNF-α and TGF-β1 vs Corneal Haze and Regeneration of Corneal Epithelium and Subbasal Nerve Plexus after PRK. Exp. Eye Res. 2001, 72, 631–641. [Google Scholar] [CrossRef]
- Vesaluoma, M.; Teppo, A.-M.; Grönhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-H.; Ebner, R.; Derynck, R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-beta activities. Science 1993, 260, 1335–1338. [Google Scholar] [CrossRef]
- Kim, W.J.; Mohan, R.R.; Wilson, S.E. Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: Expression of the platelet-derived growth factor system in the cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1364–1372. [Google Scholar]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 Is a Major Activator of TGF-β1 In Vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Breuza, L.; Poux, S.; Estreicher, A.; Famiglietti, M.L.; Magrane, M.; Tognolli, M.; Bridge, A.; Baratin, D.; Redaschi, N. UniProt Consortium The UniProtKB guide to the human proteome. Database 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Nishida, T.; Ohashi, Y.; Nakagawa, S.; Manabe, R. Fibronectin appears at the site of corneal stromal wound in rabbits. Curr. Eye Res. 1981, 1, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lee, P.; Yu, F.-S.X. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci. Rep. 2016, 6, 36414. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Razavi, Y.; Chinnery, H.R.; McMenamin, P.G. A Novel Association Between Resident Tissue Macrophages and Nerves in the Peripheral Stroma of the Murine Cornea. Investig. Opthalmology Vis. Sci. 2014, 55, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Lim, J.W.E.; Tauro, B.J.; Ji, H.; Moritz, R.L.; Simpson, R. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteom. 2009, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2008, 23, 1541–1557. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Simpson, R.; Jensen, S.; Lim, J.W.E. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Couzin, J. CELL BIOLOGY: The Ins and Outs of Exosomes. Science 2005, 308, 1862–1863. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adhes. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef] [PubMed]






| Pathway Name | Entitites | Reactions | ||||
|---|---|---|---|---|---|---|
| Found | Ratio | p-Value | FDR * | Found | Ratio | |
| Formation of the ternary complex, and subsequently, the 43S complex | 28/52 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 3/3 | 2.44 × 10−4 |
| Regulation of activated PAK-2p34 by proteasome mediated degradation | 26/50 | 0.004 | 1.11 × 10−16 | 3.11 × 10−15 | 2/2 | 1.62 × 10−4 |
| AUF1 (hnRNP D0) binds and destabilizes mRNA | 29/56 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 4/4 | 3.25 × 10−4 |
| Translation initiation complex formation | 30/59 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 2/2 | 1.62 × 10−4 |
| Ribosomal scanning and start codon recognition | 30/59 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 2/2 | 1.62 × 10−4 |
| Eukaryotic Translation Elongation | 42/95 | 0.008 | 1.11 × 10−16 | 3.11 × 10−15 | 9/9 | 7.31 × 10−4 |
| Peptide chain elongation | 39/90 | 0.008 | 1.11 × 10−16 | 3.11 × 10−15 | 5/5 | 4.06 × 10−4 |
| Formation of a pool of free 40S subunits | 43/102 | 0.009 | 1.11 × 10−16 | 3.11 × 10−15 | 2/2 | 1.62 × 10−4 |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 47/113 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 3/3 | 2.44 × 10−4 |
| Nonsense-Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | 38/96 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 1/1 | 8.12 × 10−5 |
| Viral mRNA Translation | 37/101 | 0.009 | 1.11 × 10−16 | 3.11 × 10−15 | 2/2 | 1.62 × 10−4 |
| Nonsense-Mediated Decay (NMD) | 40/117 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 6/6 | 4.87 × 10−4 |
| Nonsense-Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) | 40/117 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 5/5 | 4.06 × 10−4 |
| SRP-dependent cotranslational protein targeting to membrane | 37/113 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 5/5 | 4.06 × 10−4 |
| Cap-dependent Translation Initiation | 47/120 | 0.011 | 1.11 × 10−16 | 3.11 × 10−15 | 17/18 | 0.001 |
| Eukaryotic Translation Initiation | 47/120 | 0.011 | 1.11 × 10−16 | 3.11 × 10−15 | 19/21 | 0.002 |
| Activation of the mRNA upon binding of the cap-binding complex and eIFs, and subsequent binding to 43S | 30/60 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 5/6 | 4.87 × 10−4 |
| Regulation of Apoptosis | 26/53 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 4/5 | 4.06 × 10−4 |
| Eukaryotic Translation Termination | 38/94 | 0.008 | 1.11 × 10−16 | 3.11 × 10−15 | 4/5 | 4.06 × 10−4 |
| Hh mutants that do not undergo autocatalytic processing are degraded by ERAD | 26/56 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 4/6 | 4.87 × 10−4 |
| L13a-mediated translational silencing of Ceruloplasmin expression | 47/112 | 0.01 | 1.11 × 10−16 | 3.11 × 10−15 | 2/3 | 2.44 × 10−4 |
| Host Interactions of HIV factors | 41/144 | 0.013 | 1.11 × 10−16 | 3.11 × 10−15 | 34/54 | 0.004 |
| Translation | 67/294 | 0.026 | 1.11 × 10−16 | 3.11 × 10−15 | 57/99 | 0.008 |
| Hh mutants abrogate ligand secretion | 26/59 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 4/7 | 5.69 × 10−4 |
| Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A | 25/52 | 0.005 | 1.11 × 10−16 | 3.11 × 10−15 | 2/4 | 3.25 × 10−4 |
| Protein Name | UniProt Id | Relative Protein Abundance [Average (± Stdev)] |
|---|---|---|
| Alanine-tRNA ligase | P49588 | 1.0 × 105 (± 2.0 × 104) |
| Aminoacyl tRNA synthase complex | Q12904 | 2.2 × 104 (± 3.4 × 103) |
| Asparagine-tRNA ligase | O43776 | 1.6 × 104 (± 4.1 × 103) |
| Aspartate-tRNA ligase | P14868 | 5.9 × 103 (± 8.8 × 102) |
| Elongation factor 1-alpha 1 | P68104 | 5.2 × 105 (± 3.6 × 104) |
| Elongation factor 1-gamma | P26641 | 2.9 × 105 (± 2.3 × 104) |
| Elongation factor 2 | P13639 | 3.6 × 105 (± 4.3 × 104) |
| Eukaryotic translation initiation factor 3 subunit F | O00303 | 2.1 × 104 (± 4.2 × 103) |
| Glycine-tRNA ligase | P41250 | 1.0 × 105 (± 9.4 × 103) |
| Serine-tRNA ligase | P49591 | 1.5 × 104 (± 8.7 × 102) |
| Threonine-tRNA ligase | P26639 | 2.6 × 104 (± 3.8 × 103) |
| Tryptophan-tRNA ligase | P23381 | 6.4 × 103 (± 8.3 × 102) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020, 9, 1080. https://doi.org/10.3390/cells9051080
McKay TB, Hutcheon AEK, Zieske JD, Ciolino JB. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells. 2020; 9(5):1080. https://doi.org/10.3390/cells9051080
Chicago/Turabian StyleMcKay, Tina B., Audrey E. K. Hutcheon, James D. Zieske, and Joseph B. Ciolino. 2020. "Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation" Cells 9, no. 5: 1080. https://doi.org/10.3390/cells9051080
APA StyleMcKay, T. B., Hutcheon, A. E. K., Zieske, J. D., & Ciolino, J. B. (2020). Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells, 9(5), 1080. https://doi.org/10.3390/cells9051080




