A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing
Abstract
:1. Introduction
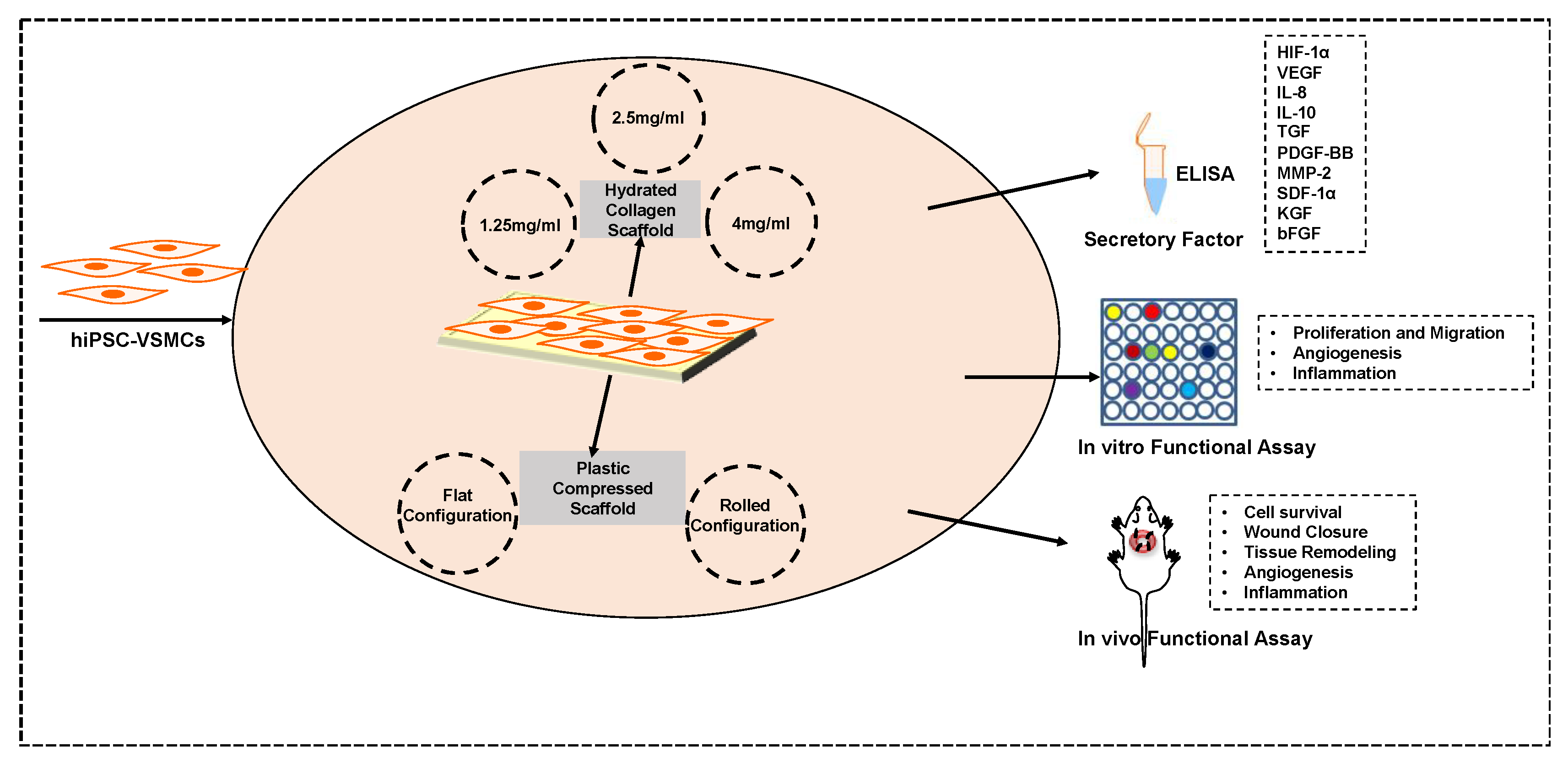
2. Methods
2.1. VSMC Differentiation and Characterization
2.2. Scaffold Characterization
2.2.1. Cellular Scaffold Preparation
2.2.2. AlamarBlue Assay
2.2.3. Cell Proliferation, Apoptosis, and Hypoxia
2.2.4. Phenotype Characterization
2.2.5. ELISA
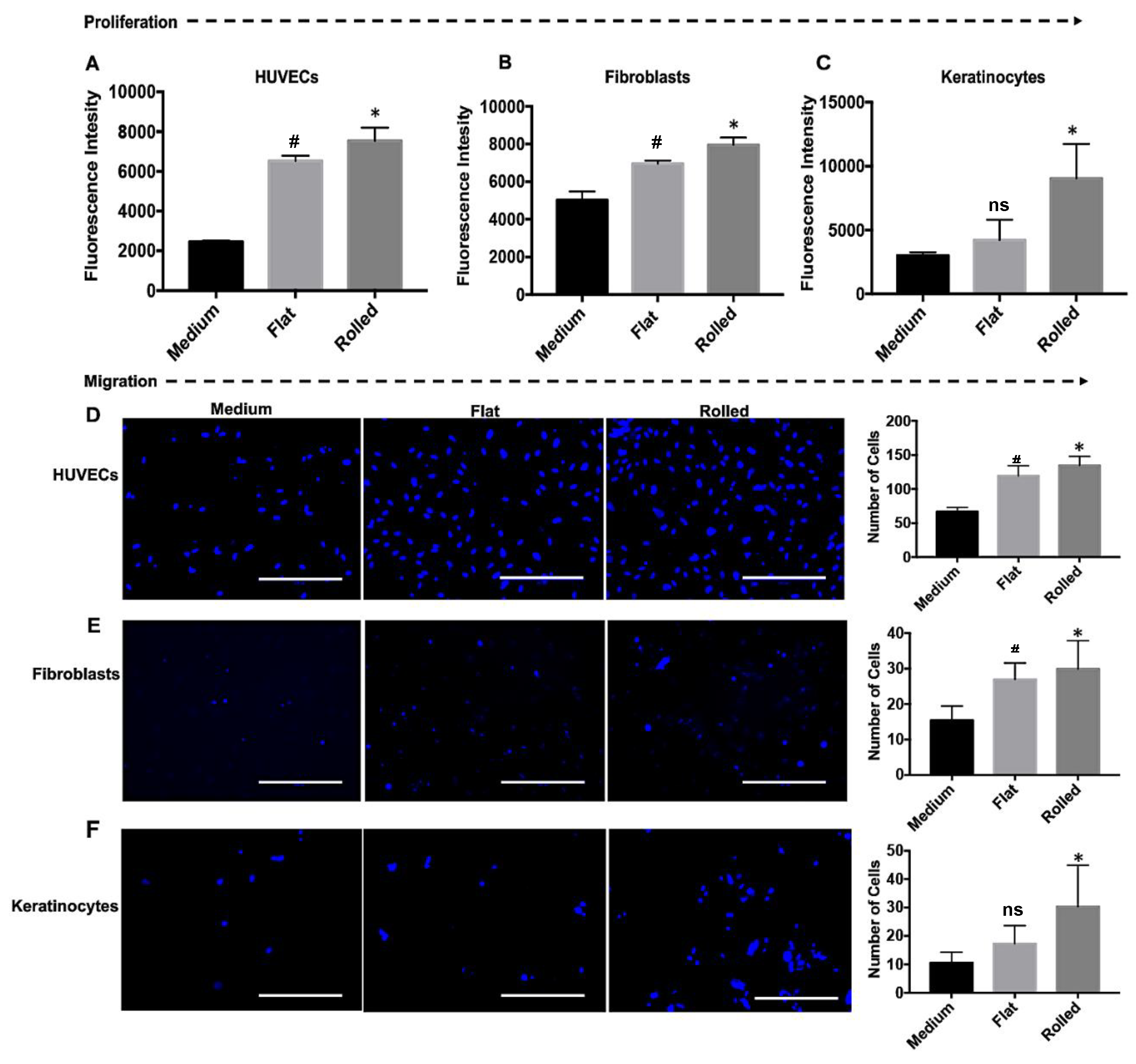
2.2.6. Cell Proliferation and Migration Assay
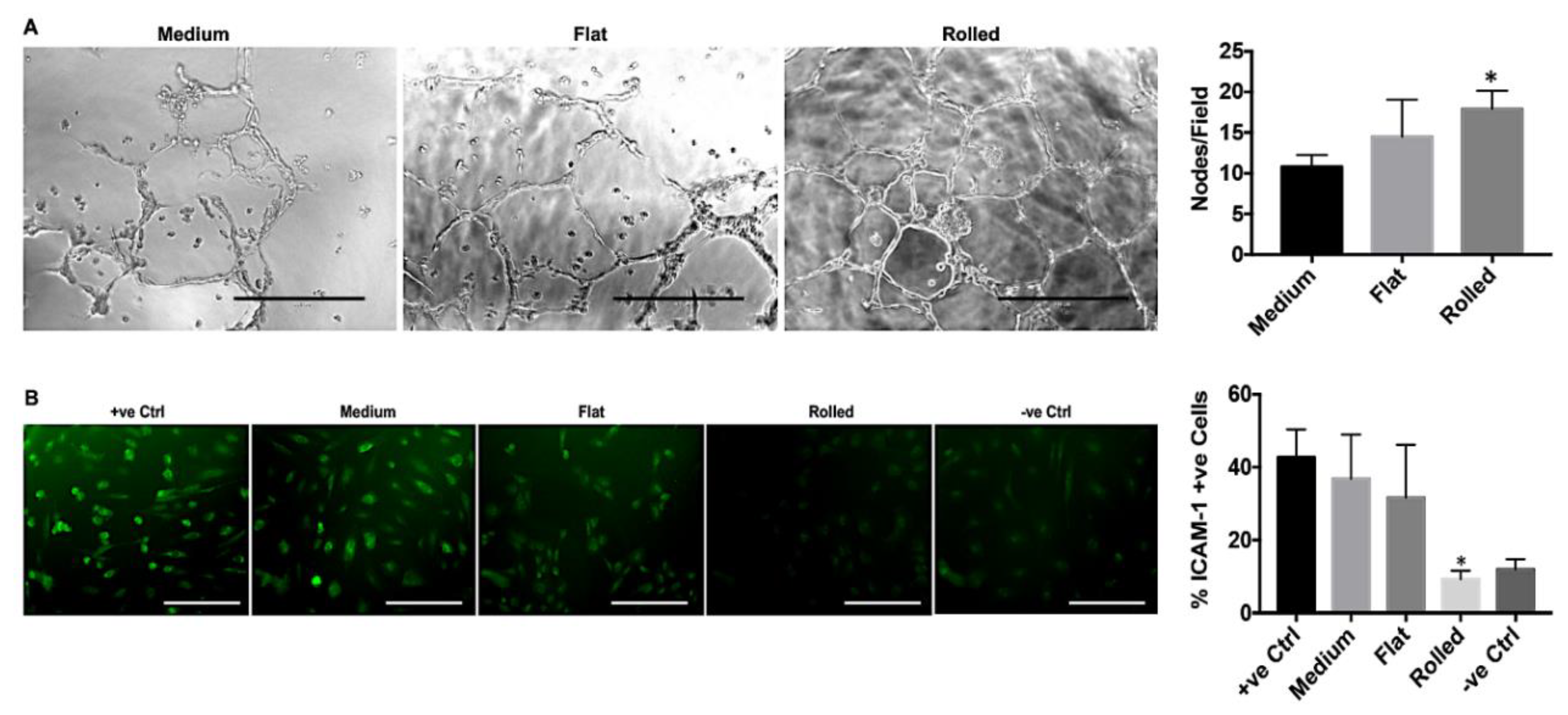
2.2.7. In Vitro Angiogenesis Assay
2.2.8. ICAM-1 Expression Analysis
2.3. Animal Studies
2.3.1. Splinted Acute Back Wound Model and Grafting
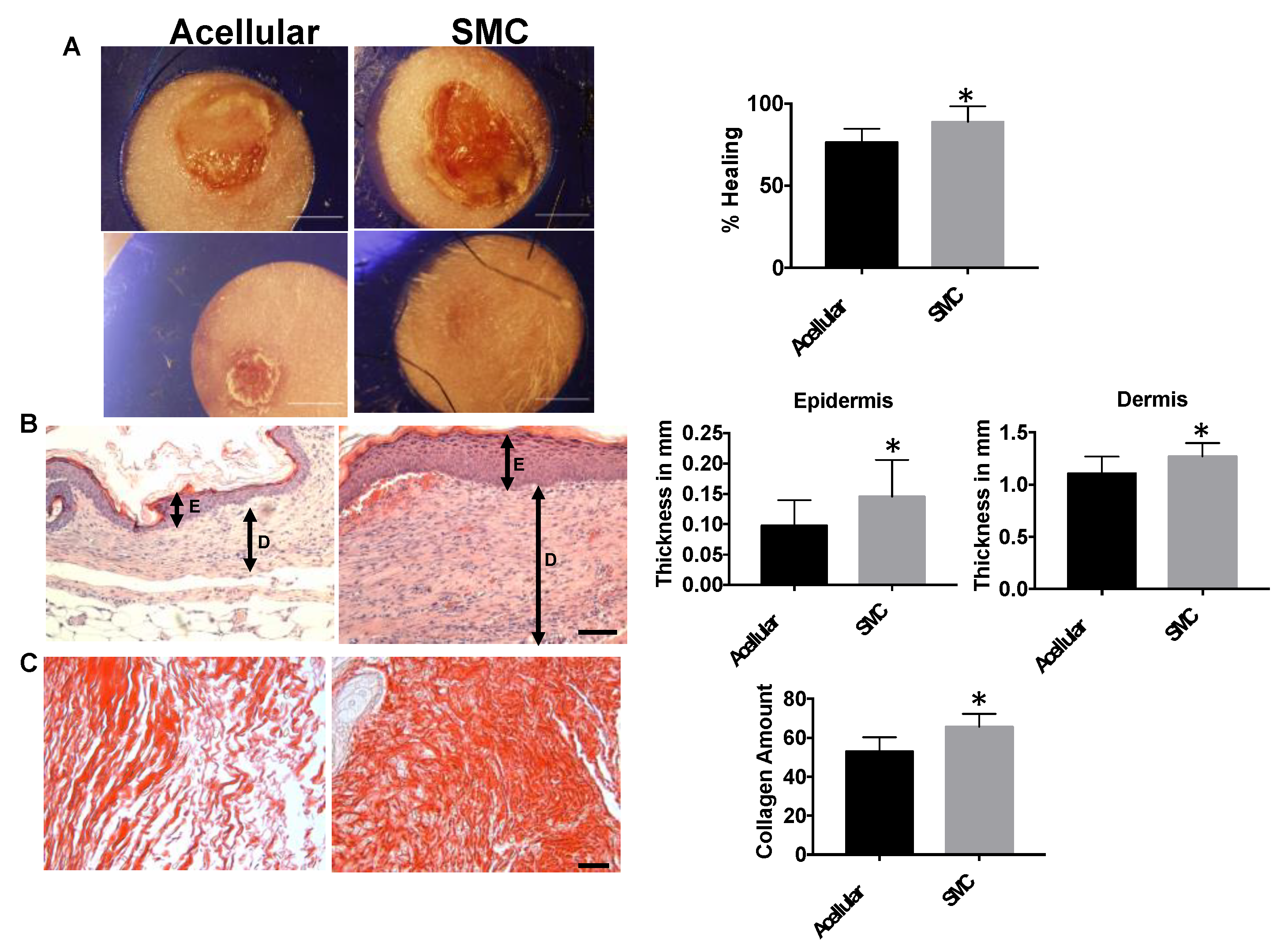
2.3.2. Histology
2.3.3. In Vvo Engraftment
2.3.4. Evaluation of Vascularization
2.3.5. Evaluation of Inflammation
3. Results
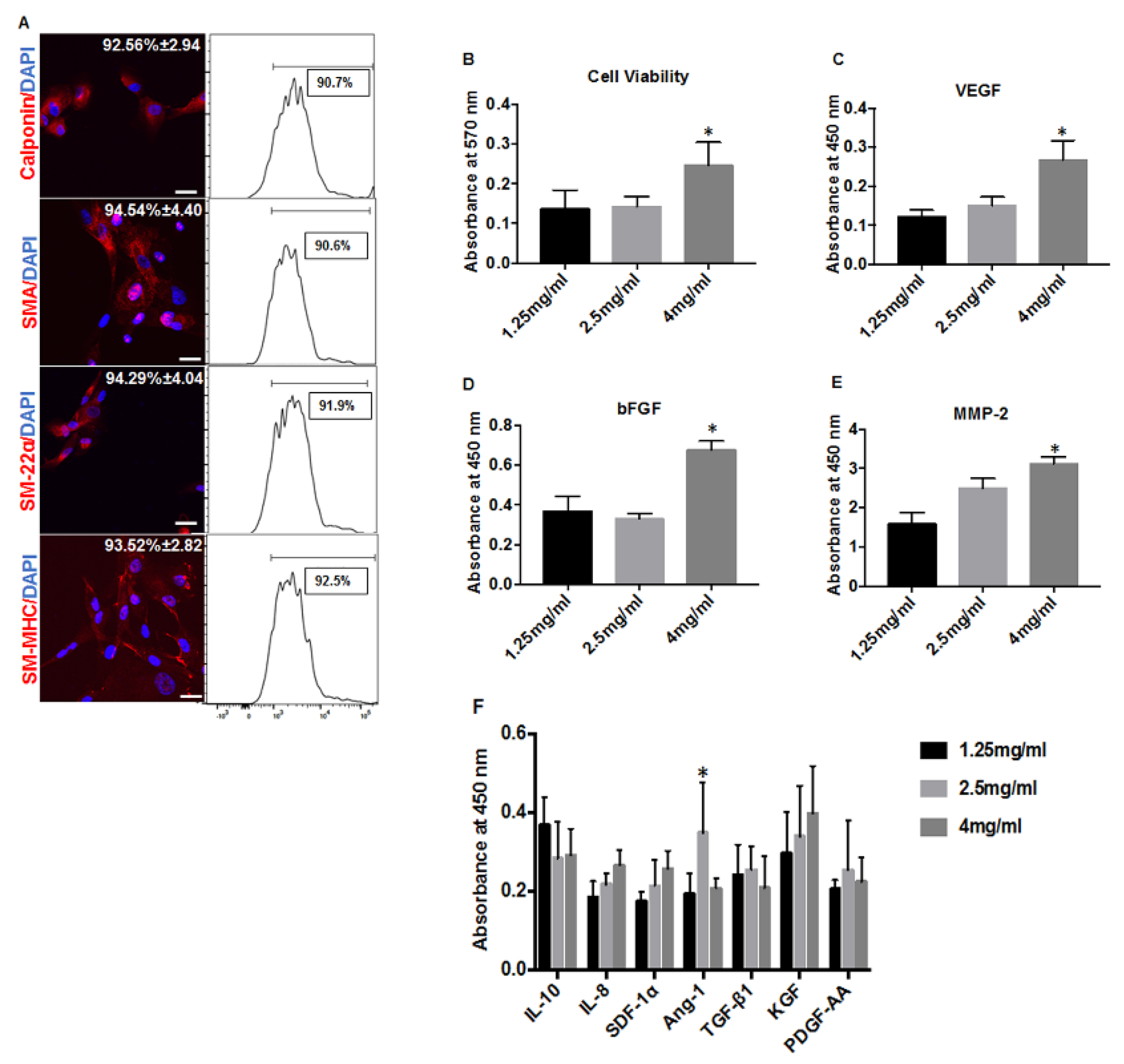
3.1. Increased Collagen Concentration Alters the Cell Proliferation and Paracrine Secretion of hiPSC-VSMCs in Hydrated Collagen Scaffolds
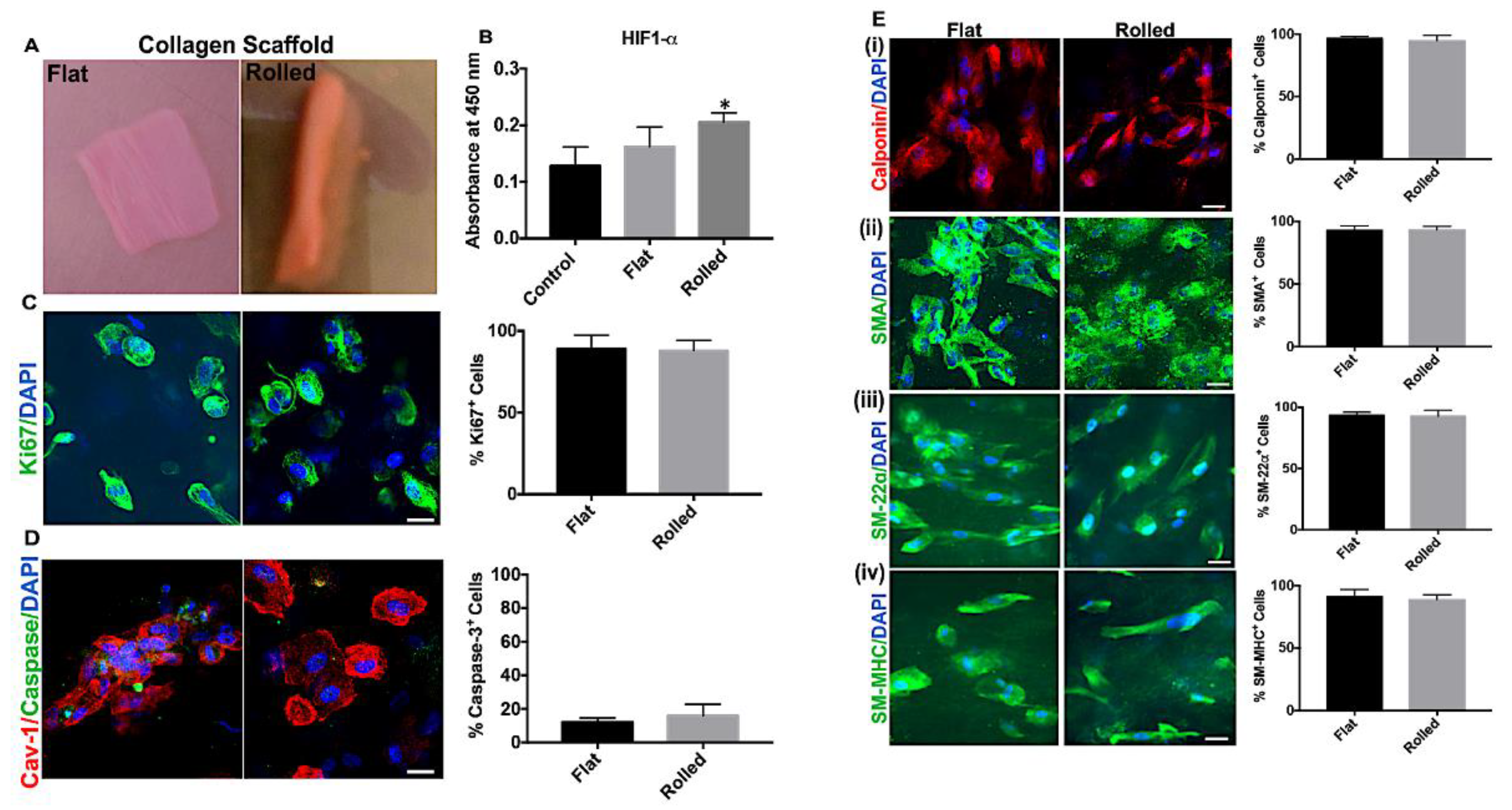
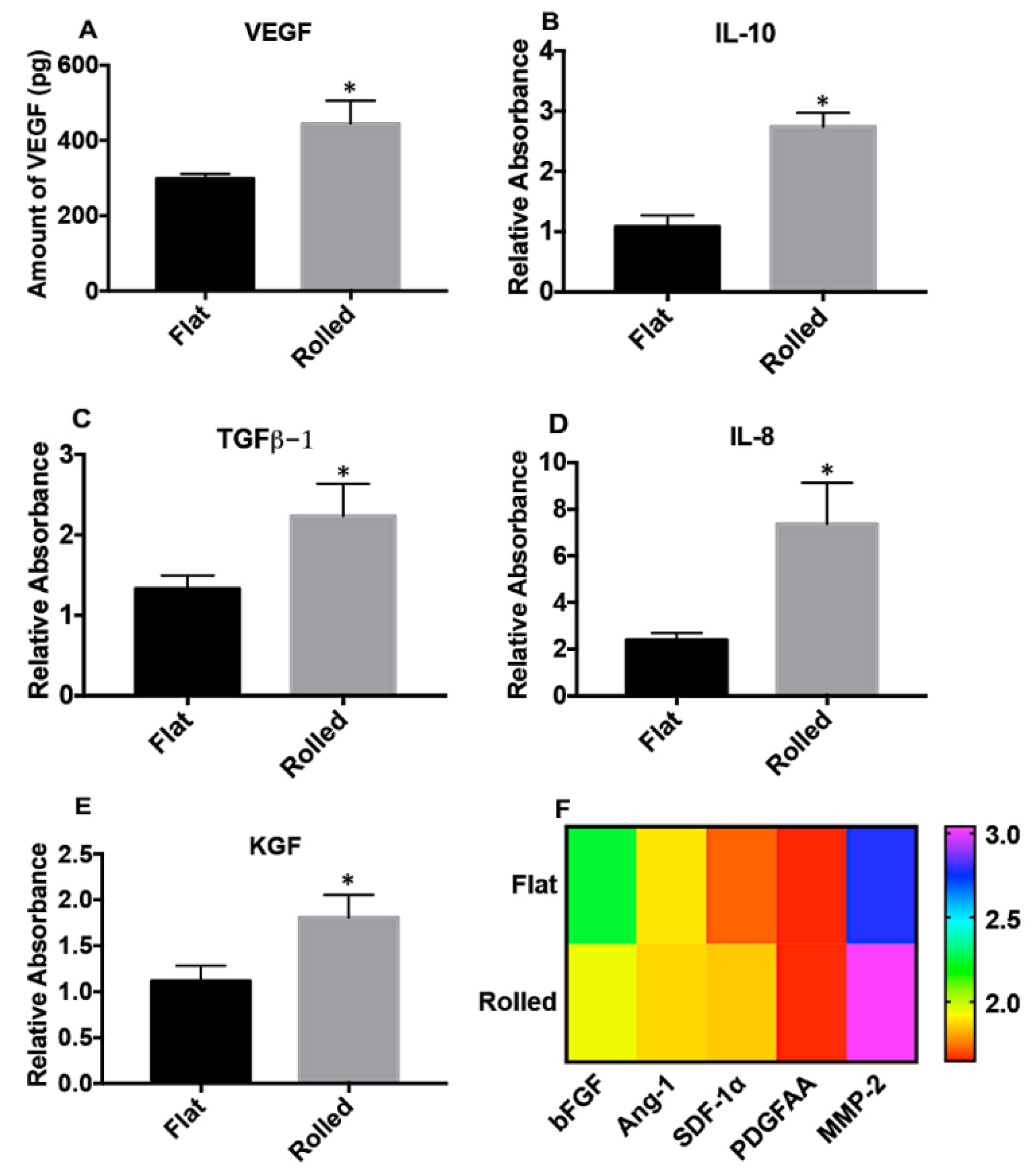
3.2. A Rolled DC Scaffold Differentially Modulates iPSC-VSMCs’ Secretory Function
3.3. The Conditioned Medium from Rolled DC Scaffold Promotes Cellular Proliferation and Migration
3.4. Conditioned Medium from Rolled DC Scaffold Has a Pro-Angiogenic and Anti-Inflammatory Effect
3.5. hIPSC-VSMCs in a Rolled DC Scaffold Enhances Wound Closure and Tissue Remodeling
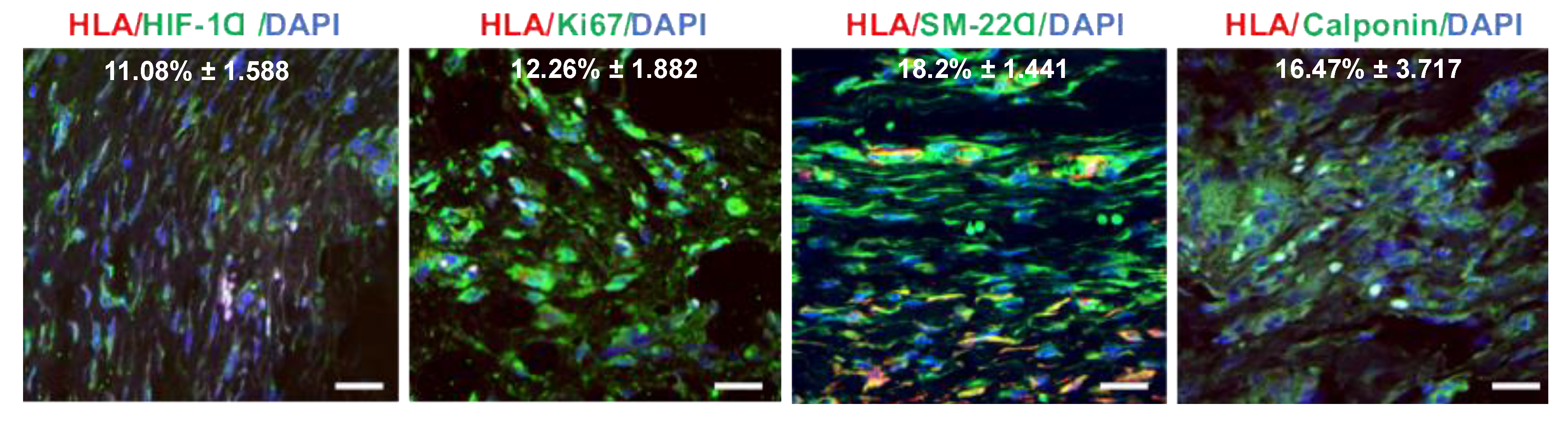
3.6. Hypoxia-Induced Activation of iPSC-VSMC Promotes Their Survival In Vivo
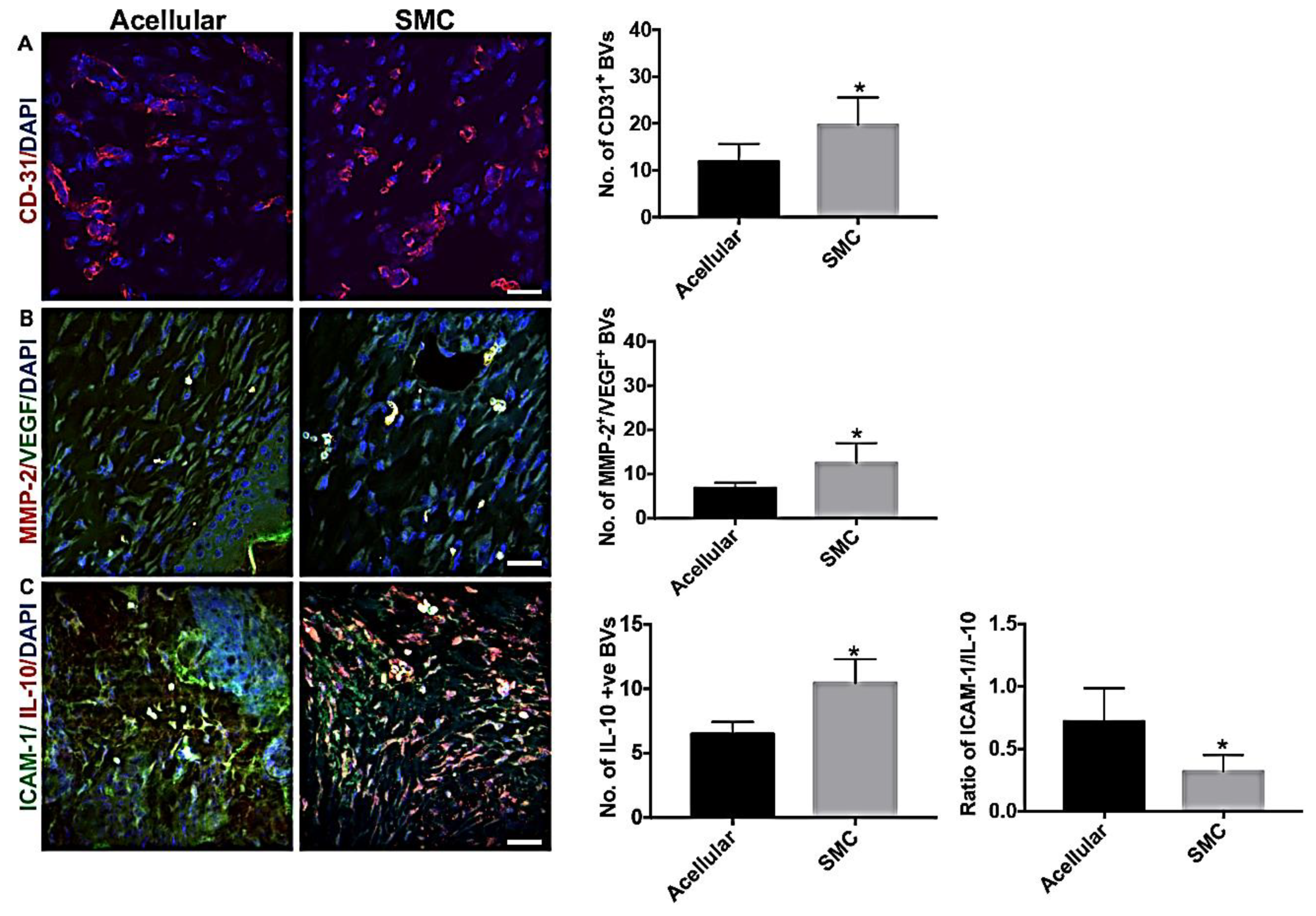
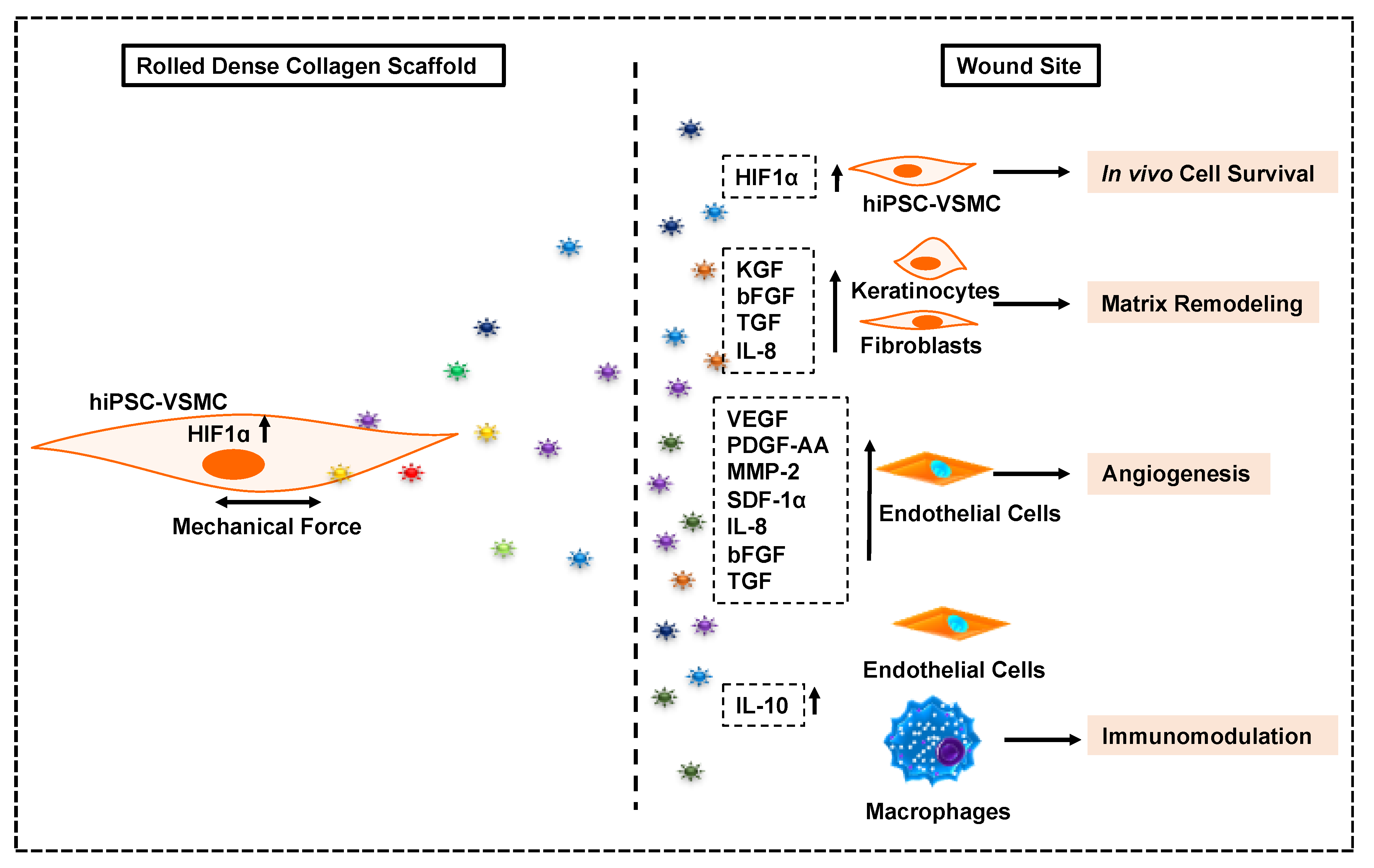
3.7. hIPSC-VSMC Secretory Factor-Mediated Vascularization and Immunomodulation in the Wound Microenvironment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maguire, E.M.; Xiao, Q.; Xu, Q. Differentiation and Application of Induced Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2017, 37, 2026–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D. iPSCs-based generation of vascular cells: Reprogramming approaches and applications. Cell Mol. Life Sci. 2018, 75, 1411–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, B.C.; Jiang, Z.; Suh, C.; Qyang, Y. Induced pluripotent stem cell-derived vascular smooth muscle cells: Methods and application. Biochem. J. 2015, 465, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoubi, S.; Sheikh, S.P.; Eskildsen, T.V. Human induced pluripotent stem cell-derived vascular smooth muscle cells: Differentiation and therapeutic potential. Cardiovasc. Res. 2017, 113, 1282–1293. [Google Scholar] [CrossRef]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef]
- Dash, B.C.; Levi, K.; Schwan, J.; Luo, J.; Bartulos, O.; Wu, H.; Qiu, C.; Yi, T.; Ren, Y.; Campbell, S.; et al. Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Rep. 2016, 7, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lian, Q.; Zhu, G.; Zhou, F.; Sui, L.; Tan, C.; Mutalif, R.A.; Navasankari, R.; Zhang, Y.; Tse, H.-F.; et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011, 8, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell Mol. Life Sci. 2014, 71, 2271–2288. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Ren, Y.; Bartulos, O.; Lee, M.Y.; Yue, Z.; Kim, K.-Y.; Li, W.; Amos, P.J.; Bozkulak, E.C.; Iyer, A.; et al. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation 2012, 126, 1695–1704. [Google Scholar] [CrossRef]
- Sundaram, S.; One, J.; Siewert, J.; Teodosescu, S.; Zhao, L.; Dimitrievska, S.; Qian, H.; Huang, A.H.; Niklason, L. Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells Transl. Med. 2014, 3, 1535–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, S.; Niklason, L.E. Smooth muscle and other cell sources for human blood vessel engineering. Cells Tissues Org. 2012, 195, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Qin, L.; Kural, M.H.; Schwan, J.; Li, X.; Bartulos, O.; Cong, X.-Q.; Ren, Y.; Gui, L.; Li, G.; et al. Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering. Biomaterials 2017, 147, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Dash, B.C.; Luo, J.; Qin, L.; Zhao, L.; Yamamoto, K.; Hashimoto, T.; Wu, H.; Dardik, A.; Tellides, G.; et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 2016, 102, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Qin, L.; Zhao, L.; Gui, L.; Ellis, M.W.; Huang, Y.; Kural, M.H.; Clark, J.A.; Ono, S.; Wang, J.; et al. Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell 2020, 26, 251–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-I.; Cho, H.; Papa, A.E.; Burke, J.A.; Chan, X.Y.; Duh, E.J.; Gerecht, S. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Kim, K.L.; Song, S.H.; Choi, K.S.; Suh, W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng. Part A 2013, 19, 2478–2485. [Google Scholar] [CrossRef]
- Collado, M.S.; Cole, B.K.; Figler, R.A.; Lawson, M.; Manka, D.; Simmers, M.B.; Hoang, S.; Serrano, F.; Blackman, B.R.; Sinha, S.; et al. Exposure of Induced Pluripotent Stem Cell-Derived Vascular Endothelial and Smooth Muscle Cells in Coculture to Hemodynamics Induces Primary Vascular Cell-Like Phenotypes. Stem Cells Transl. Med. 2017, 6, 1673–1683. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Moser, P.T.; Gilpin, S.; Okamoto, T.; Wu, T.; Tapias, L.F.; E Mercier, F.; Xiong, L.; Ghawi, R.; Scadden, D.T.; et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat. Biotechnol. 2015, 33, 1097–1102. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Coller, J.; Natu, V.; Hastie, T.J.; Huang, N.F. Combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell-derived endothelial cells in hypoxia. Acta Biomater. 2016, 44, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Fontana, G.; Thomas, D.; Collin, E.; Pandit, A. Microgel microenvironment primes adipose-derived stem cells towards an NP cells-like phenotype. Adv. Healthc. Mater. 2014, 3, 2012–2022. [Google Scholar] [CrossRef]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.U.; Berthiaume, F.; Dardik, A.; Hsia, H.C. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Fontana, G.; Chen, X.; Nogués, C.S.; Zeugolis, D.I.; Dockery, P.; O’Brien, T.; Pandit, A. A shape-controlled tuneable microgel platform to modulate angiogenic paracrine responses in stem cells. Biomaterials 2014, 35, 8757–8766. [Google Scholar] [CrossRef]
- Cheema, U.; Brown, R.A. Rapid Fabrication of Living Tissue Models by Collagen Plastic Compression: Understanding Three-Dimensional Cell Matrix Repair In Vitro. Adv. Wound Care 2013, 2, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.A.; Wiseman, M.; Chuo, C.B.; Cheema, U.; Nazhat, S.N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Muja, N.; Marelli, B.; Nazhat, S.N. Real time responses of fibroblasts to plastically compressed fibrillar collagen hydrogels. Biomaterials 2011, 32, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006, 2, 986–992. [Google Scholar] [CrossRef]
- Bitar, M.; Brown, R.A.; Salih, V.; Kidane, A.G.; Knowles, J.C.; Nazhat, S.N. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 2008, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Buxton, P.G.; Bitar, M.; Gellynck, K.; Parkar, M.; Brown, R.; Young, A.; Knowles, J.C.; Nazhat, S.N. Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone 2008, 43, 377–385. [Google Scholar] [CrossRef] [PubMed]
- DeCicco-Skinner, K.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar] [CrossRef] [PubMed]
- Modur, V.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J. Biol. Chem. 1996, 271, 13094–13102. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Longaker, M.T.; Gurtner, G.C. Murine models of human wound healing. Methods Mol. Biol. 2013, 1037, 265–274. [Google Scholar]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Bauman, T.M.; Nicholson, T.M.; Abler, L.L.; Eliceiri., K.W.; Huang, W.; Vezina, C.M.; Ricke, W.A. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS ONE 2014, 9, e109102. [Google Scholar] [CrossRef] [Green Version]
- Turin, S.Y.; Ledwon, J.K.; Bae, H.; Buganza-Tepole, A.; Topczewska, J.; Gosain, A.K. Digital analysis yields more reliable and accurate measures of dermal and epidermal thickness in histologically processed specimens compared to traditional methods. Exp. Dermatol. 2018, 27, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, S.M.; Batty, L.; Luo, J. Application of Human Induced Pluripotent Stem Cells in Generating Tissue-Engineered Blood Vessels as Vascular Grafts. Stem Cells Dev. 2019, 28, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Bargehr, J.; Low, L.; Cheung, C.; Bernard, W.; Iyer, D.; Bennett, M.R.; Gambardella, L.; Sinha, S. Embryological Origin of Human Smooth Muscle Cells Influences Their Ability to Support Endothelial Network Formation. Stem Cells Transl. Med. 2016, 5, 946–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Sarvestani, S.K.; Moeinzadeh, S.; He, X.; Jabbari, E. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: Effect of matrix modulus. Tissue Eng. Part A 2013, 19, 669–684. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Abdollahi, H.; Harris, L.J.; Zhang, P.; McIlhenny, S.; Srinivas, V.; Tulenko, T.; DiMuzio, P.J. The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 2011, 165, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Dalloul, A. Hypoxia and visualization of the stem cell niche. Methods Mol. Biol. 2013, 1035, 199–205. [Google Scholar]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, K.E.; Sharp, T.V.; McKay, T.R. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013, 8, 771–782. [Google Scholar] [CrossRef]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia inducible factor 1alpha promotes survival of mesenchymal stem cells under hypoxia. Am. J. Transl. Res. 2017, 9, 1521–1529. [Google Scholar]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring. Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Lisanti, M.P. ICAM-1: Role in inflammation and in the regulation of vascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H926–H927. [Google Scholar] [CrossRef]
- Shi, J.; Wei, P.K. Interleukin-8: A potent promoter of angiogenesis in gastric cancer. Oncol. Lett. 2016, 11, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Heidemann, J.; Ogawa, H.; Dwinell, M.B.; Rafiee, P.; Maaser, C.; Gockel, M.H.R.; Otterson, M.F.; Ota, D.M.; Lügering, N.; Domschke, W.; et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J. Biol. Chem. 2003, 278, 8508–8515. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalaf, H.H.; Al-Harbi, B.; Al-Sayed, A.; Arafah, M.; Tulbah, A.; Jarman, A.; Al-Mohanna, F.; Aboussekhra, A. Interleukin-8 Activates Breast Cancer-Associated Adipocytes and Promotes Their Angiogenesis- and Tumorigenesis-Promoting Effects. Mol. Cell Biol. 2019, 39, e00332-18. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Rennekampff, H.O.; Hansbrough, J.F.; Kiessig, V.; Dore, C.; Sticherling, M.; Schroder, J.M. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res. 2000, 93, 41–54. [Google Scholar] [CrossRef]
- Steed, D.L. The role of growth factors in wound healing. Surg Clin. N. Am. 1997, 77, 575–586. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, B.C.; Setia, O.; Gorecka, J.; Peyvandi, H.; Duan, K.; Lopes, L.; Nie, J.; Berthiaume, F.; Dardik, A.; Hsia, H.C. A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing. Cells 2020, 9, 966. https://doi.org/10.3390/cells9040966
Dash BC, Setia O, Gorecka J, Peyvandi H, Duan K, Lopes L, Nie J, Berthiaume F, Dardik A, Hsia HC. A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing. Cells. 2020; 9(4):966. https://doi.org/10.3390/cells9040966
Chicago/Turabian StyleDash, Biraja C., Ocean Setia, Jolanta Gorecka, Hassan Peyvandi, Kaiti Duan, Lara Lopes, James Nie, Francois Berthiaume, Alan Dardik, and Henry C. Hsia. 2020. "A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing" Cells 9, no. 4: 966. https://doi.org/10.3390/cells9040966
APA StyleDash, B. C., Setia, O., Gorecka, J., Peyvandi, H., Duan, K., Lopes, L., Nie, J., Berthiaume, F., Dardik, A., & Hsia, H. C. (2020). A Dense Fibrillar Collagen Scaffold Differentially Modulates Secretory Function of iPSC-Derived Vascular Smooth Muscle Cells to Promote Wound Healing. Cells, 9(4), 966. https://doi.org/10.3390/cells9040966





