The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Cell Culture
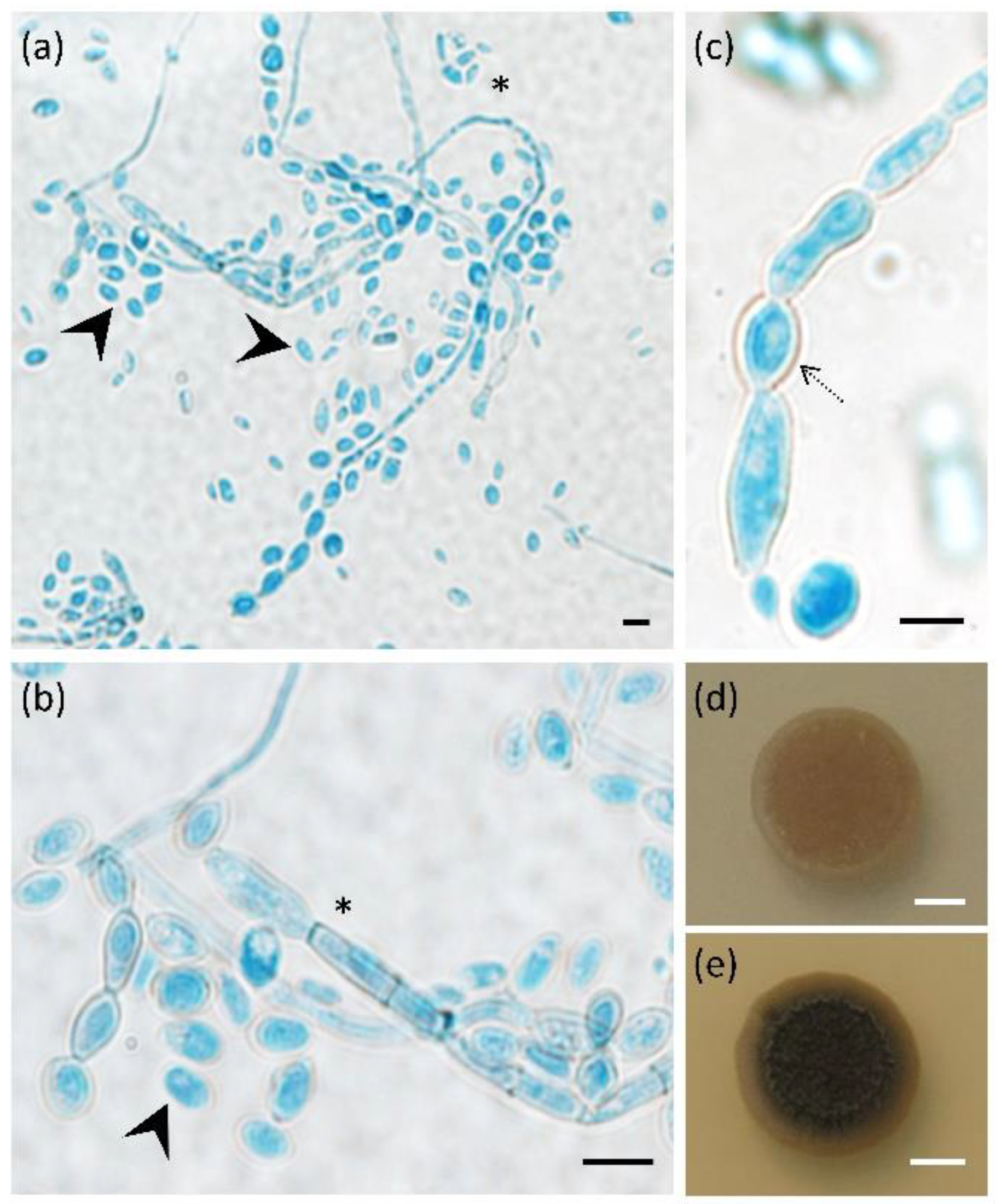
2.3. Morphological Observations
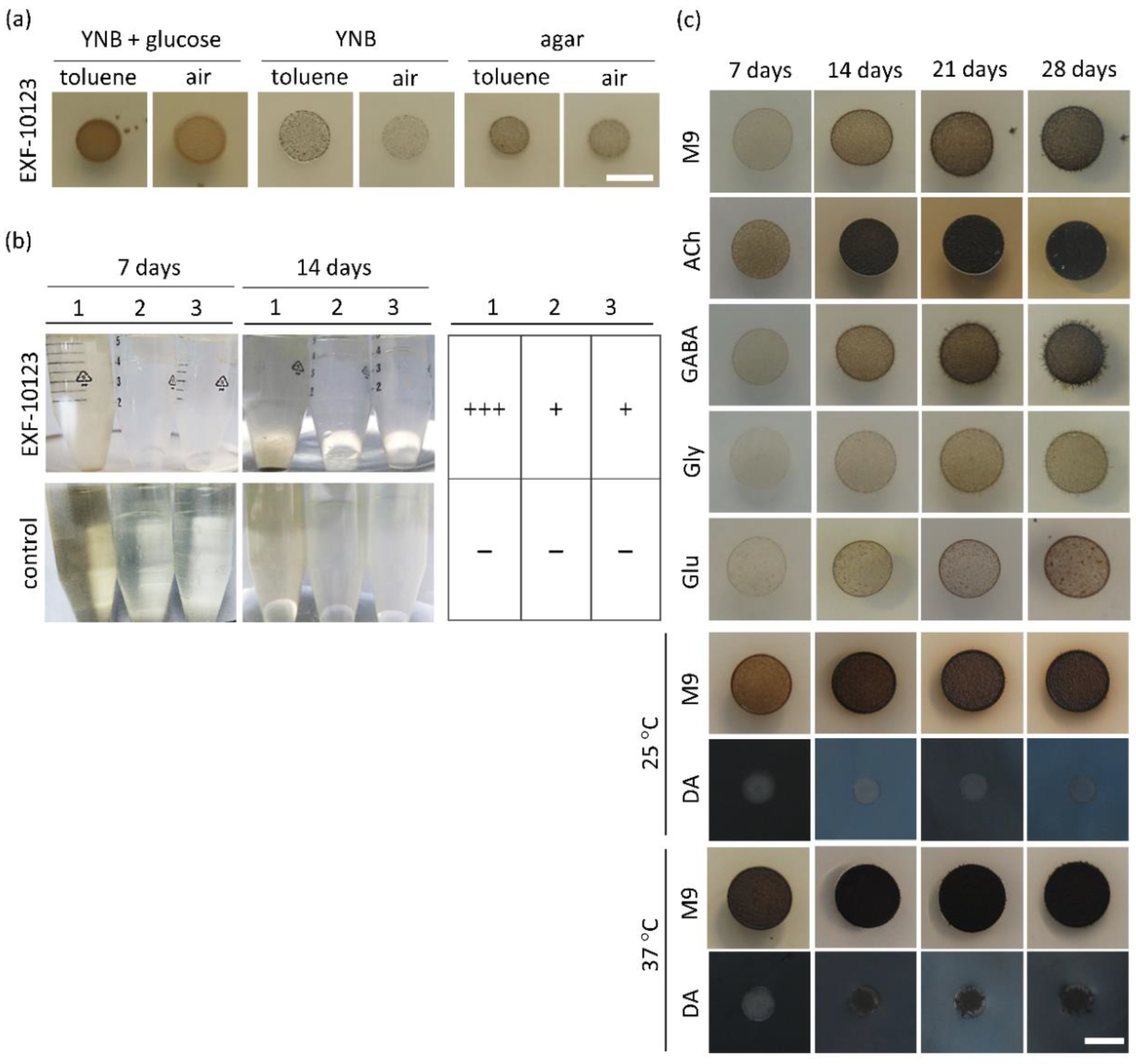
2.4. Assimilation of Hydrocarbons
2.5. Assimilation of Neurotransmitters
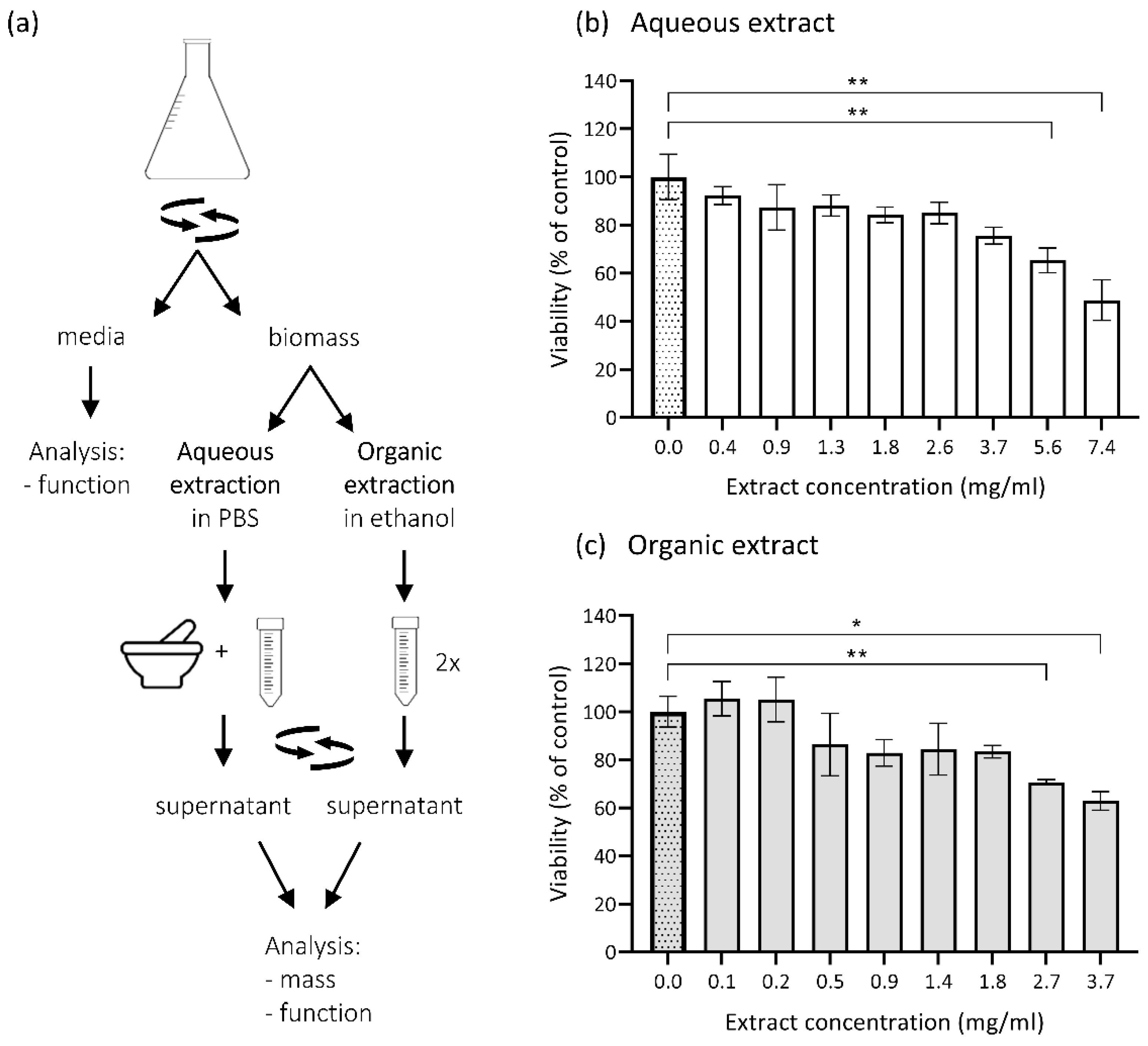
2.6. Biomass Extraction
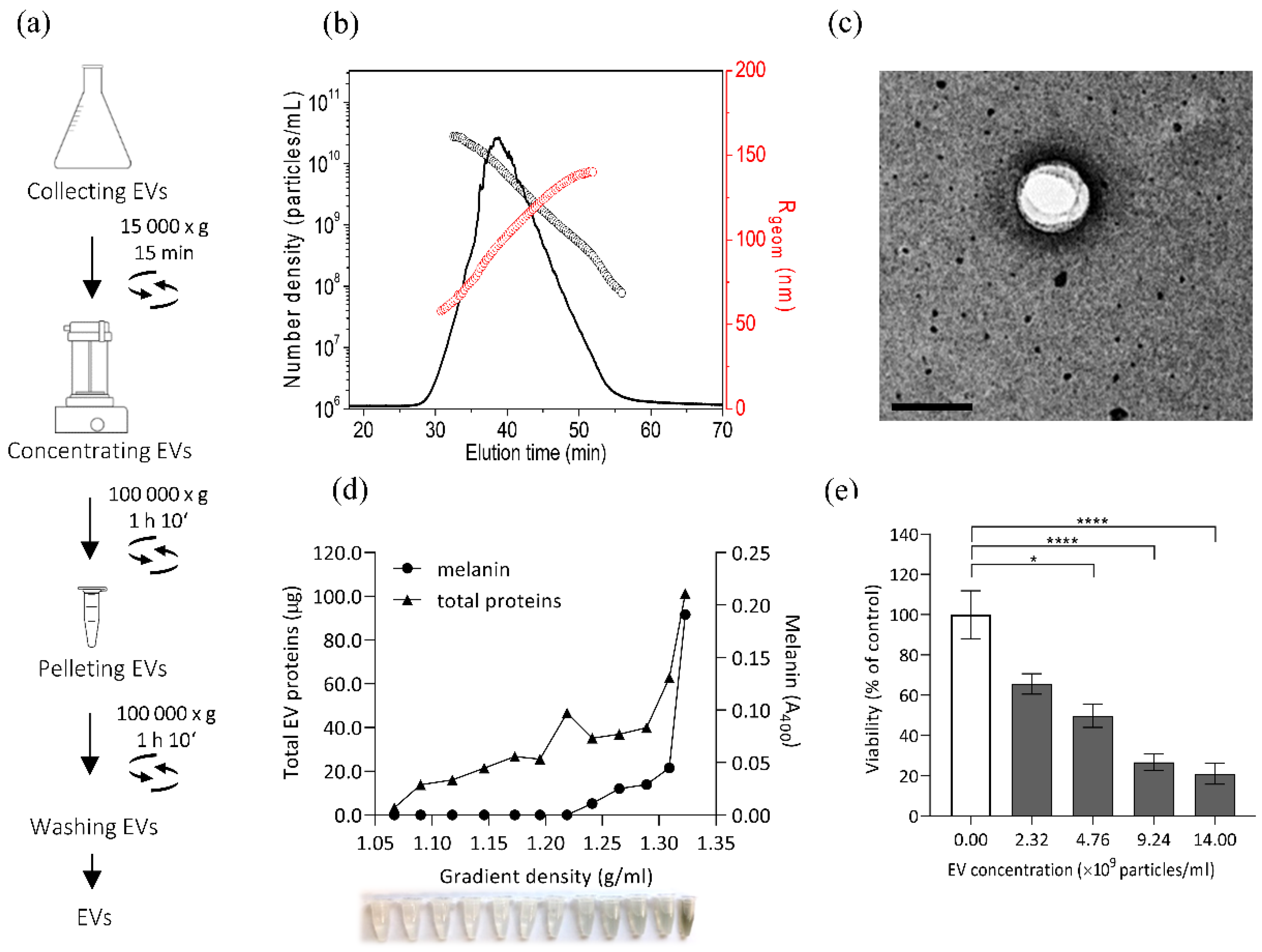
2.7. Isolation of Fungal Extracellular Vesicles (EVs)
2.8. Characterisation of Extracellular Vesicles
2.9. Effect of Volatile Organic Compounds (VOCs) of E. dermatitidis on SH-SY5Y Cells
2.10. Neutral Red Uptake Assay
2.11. Scanning Electron Microscopy (SEM)
2.12. Immunofluorescence
3. Results
3.1. Exophiala dermatitidis Increases Melanisation and Yeast-like Growth at Human Body Temperature
3.2. Exophiala dermatitidis Assimilates Selected Hydrocarbons and Neurotransmitters as Sole Carbon Sources
3.3. Cytotoxicity of Metabolites Present in E. dermatitidis Extracts on SH-SY5Y Cells
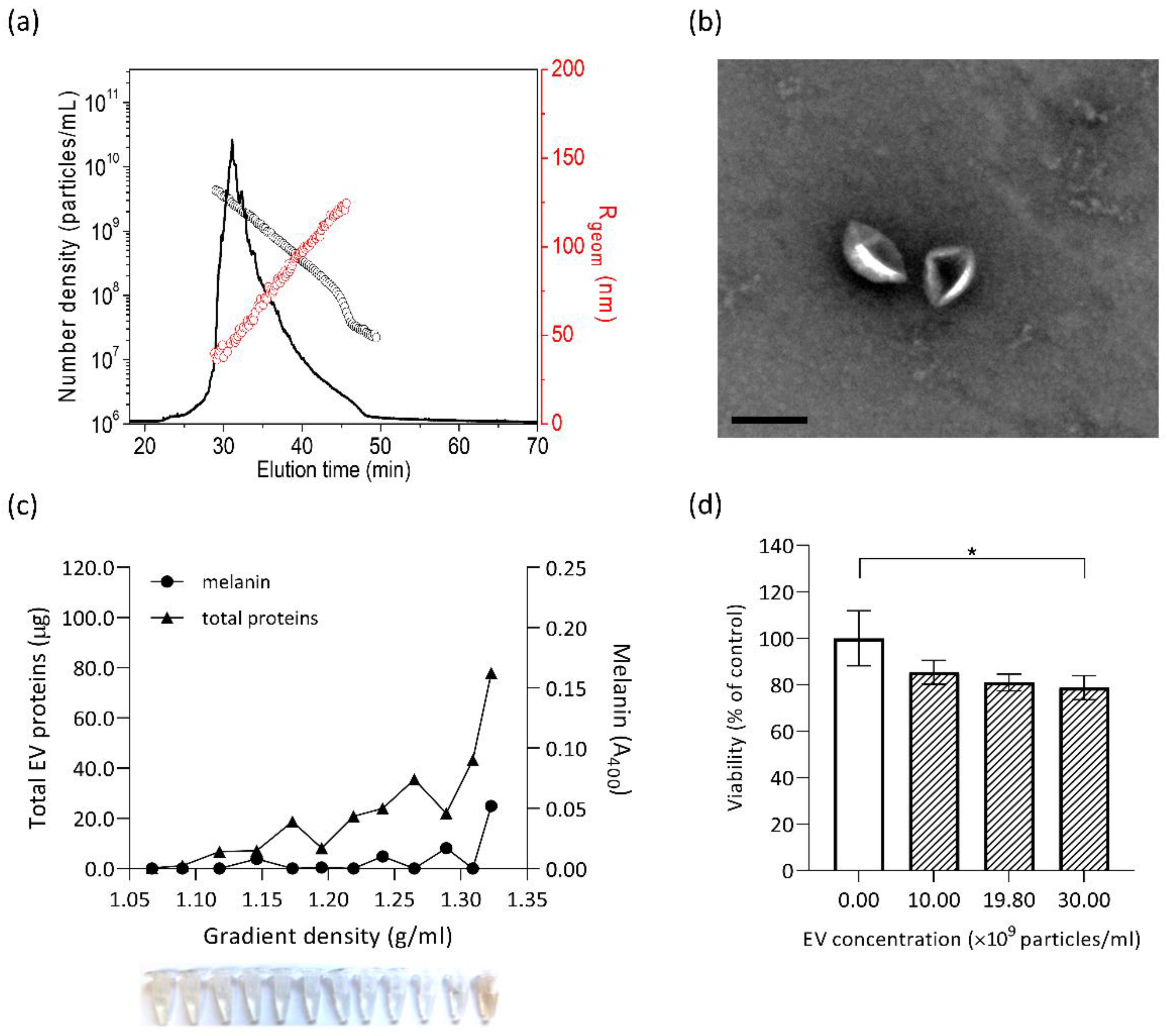
3.4. Isolation and Characterisation of E. dermatitidis EVs and Their Cytotoxic Effect on SH-SY5Y Cells
3.5. Cytotoxic Effect on SH-SY5Y Cells is Significantly Lower with Non-Melanised EVs
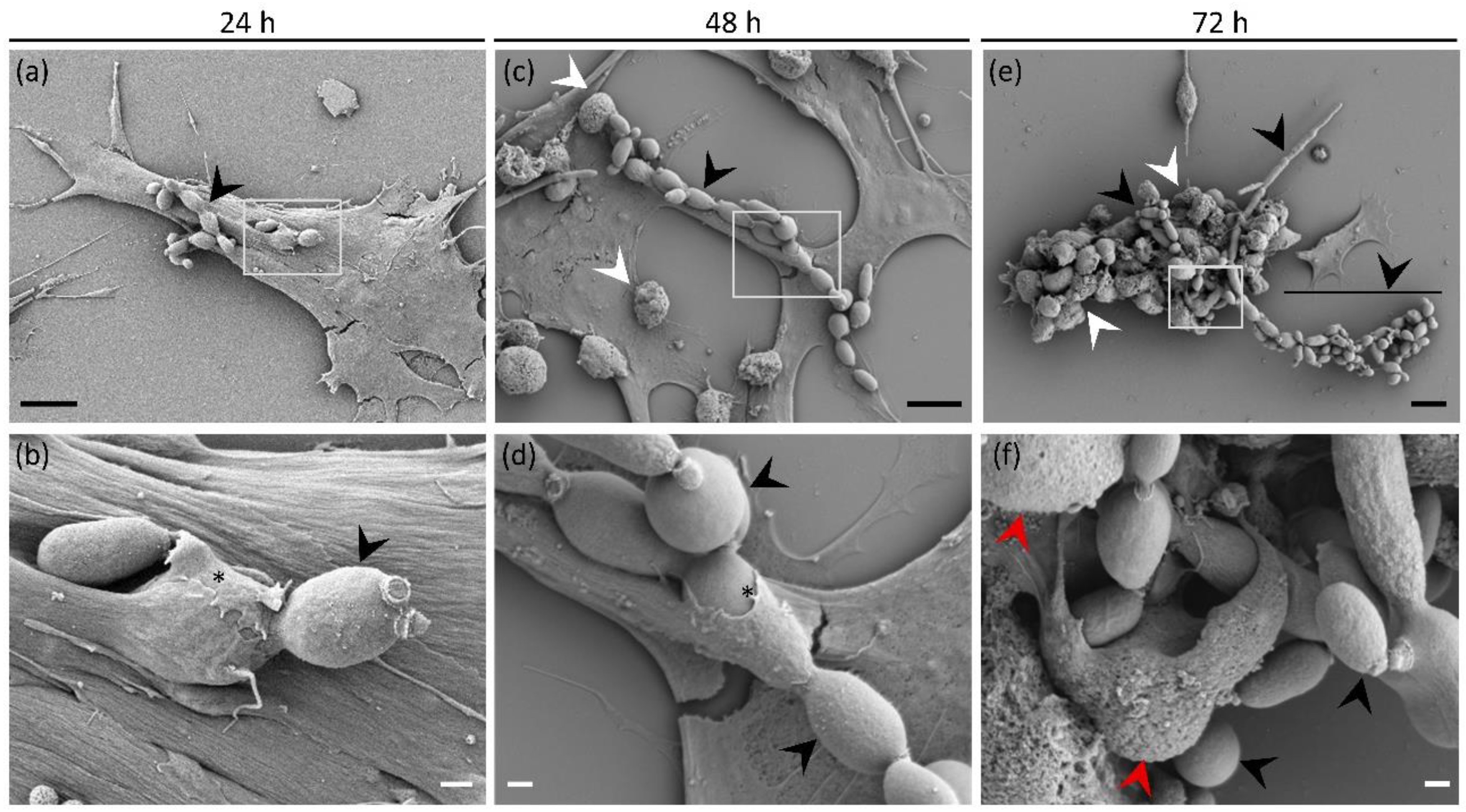
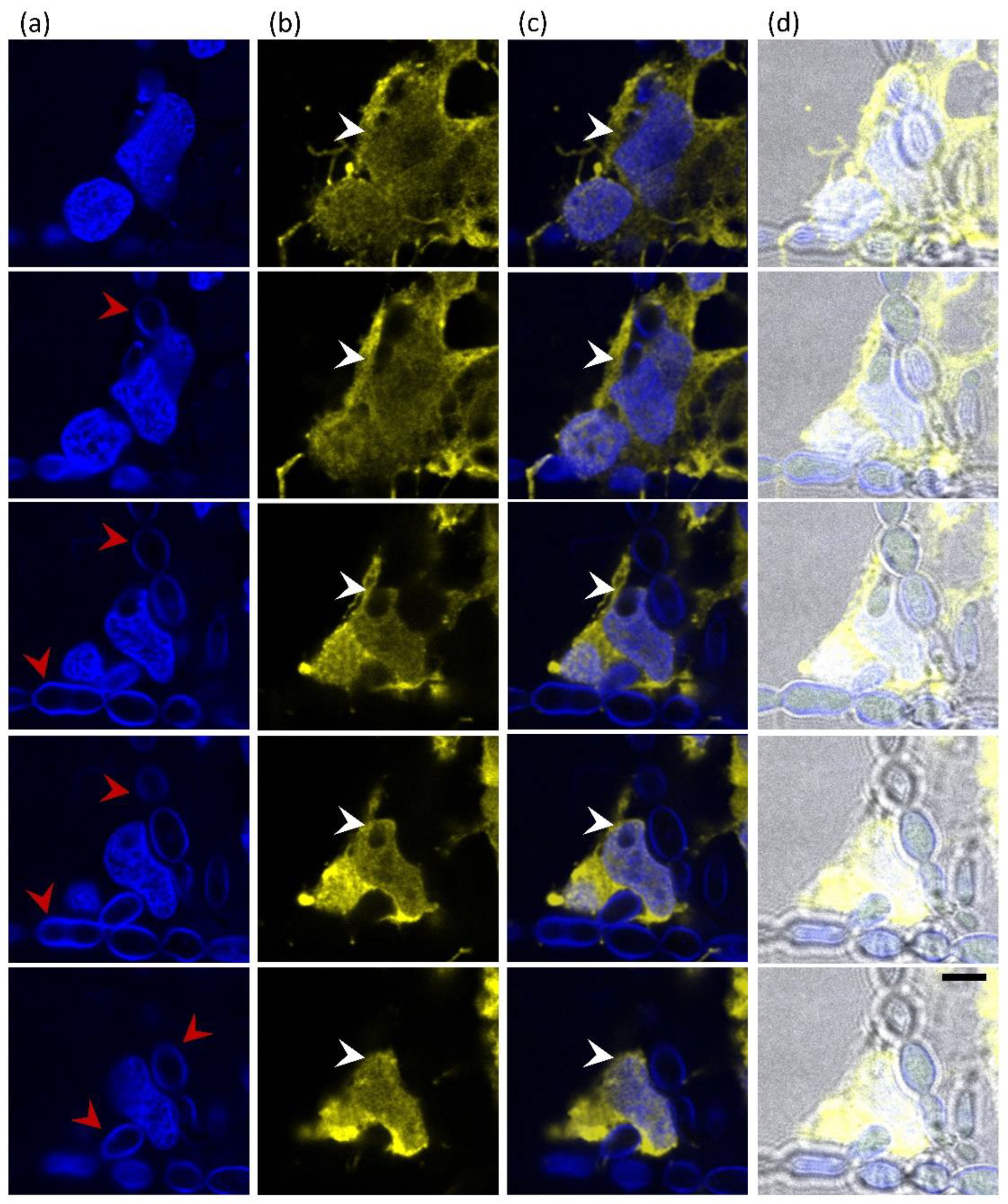
3.6. Direct Interaction of E. dermatitidis with SH-SY5Y Cells Shows Fungal Internalisation Followed by Neuroblastoma Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sterflinger, K.; De Hoog, G.S.; Haase, G. Phylogeny and ecology of meristematic ascomycetes. Stud. Mycol. 1999, 43, 5–22. [Google Scholar]
- Zalar, P.; De Hoog, G.S.; Gunde-Cimerman, N. Ecology of halotolerant dothideaceous black yeasts. Stud. Mycol. 1999, 43, 38–48. [Google Scholar]
- De Hoog, G.S.; Queiroz-Telles, F.; Haase, G.; Fernandez-Zeppenfeldt, G.; Angelis, D.A.; Ende, A.H.G.G.; Van den Matos, T.; Peltroche-Llacsahuanga, H.; Pizzirani-Kleiner, A.; Rainer, J.; et al. Black fungi: Clinical and pathogenic approaches. Med. Mycol. 2000, 38, 243–250. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gene, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; De Hoog, G.S., Ed.; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2000; p. 1160. ISBN 9789070351434. [Google Scholar]
- Prenafeta-Boldú, F.X.; Kuhn, A.; Luykx, D.M.A.M.; Anke, H.; Van Groenestijn, J.W.; De Bont, J.A.M. Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycol. Res. 2001, 105, 477–484. [Google Scholar]
- Prenafeta-Boldú, F.X.; Summerbell, R.; Sybren de Hoog, G. Fungi growing on aromatic hydrocarbons: Biotechnology’s unexpected encounter with biohazard? FEMS Microbiol. Rev. 2006, 30, 109–130. [Google Scholar] [CrossRef]
- Zeng, J.S.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Harrak, M.J.; De Hoog, G.S. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 2007, 45, 3713–3720. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; De Hoog, G.S.; Summerbell, R.C. Fungal Communities in Hydrocarbon Degradation. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; McGenity, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. ISBN 978-3-319-60063-5. [Google Scholar]
- Kirchhoff, L.; Olsowski, M.; Rath, P.-M.; Steinmann, J. Exophiala dermatitidis: Key issues of an opportunistic fungal pathogen. Virulence 2019, 10, 984–998. [Google Scholar] [CrossRef]
- Matsumoto, T.; Padhye, A.A.; Ajello, L. Medical significance of the so-called black yeasts. Eur. J. Epidemiol. 1987, 3, 87–95. [Google Scholar] [CrossRef]
- Chang, X.; Li, R.; Yu, J.; Bao, X.; Qin, J. Phaeohyphomycosis of the Central Nervous System Caused by Exophiala dermatitidis in a 3-Year-Old Immunocompetent Host. J. Child Neurol. 2009, 24, 342–345. [Google Scholar] [CrossRef]
- Kondori, N.; Gilljam, M.; Lindblad, A.; Jonsson, B.; Moore, E.R.B.; Wenneras, C. High Rate of Exophiala dermatitidis Recovery in the Airways of Patients with Cystic Fibrosis Is Associated with Pancreatic Insufficiency. J. Clin. Microbiol. 2011, 49, 1004–1009. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, A.; Fujieda, A.; Nakase, K.; Katayama, N. Pulmonary infection caused by Exophiala dermatitidis in a patient with multiple myeloma: A case report and a review of the literature. Med. Mycol. Case Rep. 2012, 1, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kusenbach, G.; Skopnik, H.; Haase, G.; Friedrichs, F.; Döhmen, H. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur. J. Pediatr. 1992, 151, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, M.; Kawada, A.; Ohata, H.; Ohnishi, Y.; Takahashi, H.; Yamazaki, M.; Ishibashi, A.; Hatsuse, K.; Kakihara, M.; Yoshida, M. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 1993, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Horré, R.; De Hoog, G.S. Primary cerebral infections by melanized fungi: A review. Stud. Mycol. 1999, 1999, 176–193. [Google Scholar]
- Kerkmann, M.-L.; Piontek, K.; Mitze, H.; Haase, G. Isolation of Exophiala (Wangiella) dermatitidis in a Case of Otitis Externa. Clin. Infect. Dis. 1999, 29, 939–940. [Google Scholar] [CrossRef][Green Version]
- Chang, C.L.; Kim, D.-S.; Park, D.J.; Kim, H.J.; Lee, C.H.; Shin, J.H. Acute Cerebral Phaeohyphomycosis due to Wangiella dermatitidis Accompanied by Cerebrospinal Fluid Eosinophilia. J. Clin. Microbiol. 2000, 38, 1965–1966. [Google Scholar] [CrossRef]
- Al-Obaid, I.; Ahmad, S.; Khan, Z.U.; Dinesh, B.; Hejab, H.M. Catheter-associated fungemia due to Exophiala oligosperma in a leukemic child and review of fungemia cases caused by Exophiala species. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 729–732. [Google Scholar] [CrossRef]
- Mukaino, T.; Koga, T.; Oshita, Y.; Narita, Y.; Obata, S.; Aizawa, H. Exophiala dermatitidis infection in non-cystic fibrosis bronchiectasis. Respir. Med. 2006, 100, 2069–2071. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J.; El Shafie, S.; Alsoub, H.; Eldeeb, Y.; De Hoog, G.S. Isolation of Exophiala dermatitidis from endotracheal aspirate of a cancer patient. Mycoses 2006, 49, 504–509. [Google Scholar] [CrossRef]
- Grenouillet, F.; Cimon, B.; Pana-Katatali, H.; Person, C.; Gainet-Brun, M.; Malinge, M.-C.; Le Govic, Y.; Richaud-Thiriez, B.; Bouchara, J.-P. Exophiala dermatitidis Revealing Cystic Fibrosis in Adult Patients with Chronic Pulmonary Disease. Mycopathologia 2018, 183, 71–79. [Google Scholar] [CrossRef]
- Lang, R.; Minion, J.; Skinner, S.; Wong, A. Disseminated Exophiala dermatitidis causing septic arthritis and osteomyelitis. BMC Infect. Dis. 2018, 18, 255. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Zavasky, D.; Chow, N.A.; Gade, L.; Zlatanic, E.; Elkind, S.; Litvintseva, A.P.; Pappas, P.G.; Perfect, J.R.; Revankar, S.; et al. Management of an Outbreak of Exophiala dermatitidis Bloodstream Infections at an Outpatient Oncology Clinic. Clin. Infect. Dis. 2018, 66, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Klasinc, R.; Riesenhuber, M.; Bacher, A.; Willinger, B. Invasive Fungal Infection Caused by Exophiala dermatitidis in a Patient after Lung Transplantation: Case Report and Literature Review. Mycopathologia 2019, 184, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.L.; Cognialli, R.C.R.; Barros, R.C.; Pinto, T.D.A.; Cunha, M.F.M.; Tahan, T.T.; Voidaleski, M.F.; Gomes, R.R.; Becker, G.N.; Andrade, L.V.; et al. Peritonitis by Exophiala dermatitidis in a pediatric patient. Med. Mycol. Case Rep. 2019, 24, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, H.; Jiang, X.; Zeng, J.; Liu, Z.; Chen, J.; Wu, Y. Cerebral Phaeohyphomycosis Caused by Exophiala dermatitidis in a Chinese CARD9-Deficient Patient: A Case Report and Literature Review. Front. Neurol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Noverr, M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef]
- Rizzetto, L.; De Filippo, C.; Cavalieri, D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Eur. J. Immunol. 2014, 44, 3166–3181. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Laureijssen-van de Sande, W.W.J.; Moreno, L.F.; Van den Ende, B.G.; Li, R.; De Hoog, G.S. Comparative Ecology of Capsular Exophiala Species Causing Disseminated Infection in Humans. Front. Microbiol. 2017, 8, 1–25. [Google Scholar] [CrossRef]
- Schnitzler, N.; Peltroche-Llacsahuanga, H.; Bestier, N.; Zündorf, J.; Lütticken, R.; Haase, G. Effect of Melanin and Carotenoids ofExophiala (Wangiella) dermatitidis on Phagocytosis, Oxidative Burst, and Killing by Human Neutrophils. Infect. Immun. 1999, 67, 94–101. [Google Scholar] [CrossRef]
- Taylor, B.E.; Wheeler, M.H.; Szaniszlo, P.J. Evidence for Pentaketide Melanin Biosynthesis in Dematiaceous Human Pathogenic Fungi. Mycologia 1987, 79, 320–322. [Google Scholar] [CrossRef]
- Kogej, T.; Stein, M.; Volkmann, M.; Gorbushina, A.A.; Galinski, E.A.; Gunde-Cimerman, N. Osmotic adaptation of the halophilic fungus Hortaea werneckii: Role of osmolytes and melanization. Microbiology 2007, 153, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Kejžar, A.; Gobec, S.; PlemenitaŠ, A.; Lenassi, M. Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol. 2013, 117, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Miyaji, M.; Taguchi, H.; Tanaka, R. Fungi in bathwater and sludge of bathroom drainpipes. 1. Frequent isolation of Exophiala species. Mycopathologia 1987, 97, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.; De Hoog, G.S.; De Boer, A.G.; De Crom, I.; Haase, G. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 2002, 45, 373–377. [Google Scholar] [CrossRef]
- Zalar, P.; Novak, M.; De Hoog, G.S.; Gunde-Cimerman, N. Dishwashers — A man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 2011, 115, 997–1007. [Google Scholar] [CrossRef]
- Gümral, R.; Özhak-Baysan, B.; Tümgör, A.; Saraçlı, M.A.; Yıldıran, Ş.T.; Ilkit, M.; Zupančič, J.; Novak-Babič, M.; Gunde-Cimerman, N.; Zalar, P.; et al. Dishwashers provide a selective extreme environment for human-opportunistic yeast-like fungi. Fungal Divers. 2016, 76, 1–9. [Google Scholar] [CrossRef]
- Zupančič, J.; Novak Babič, M.; Zalar, P.; Gunde-Cimerman, N. The Black Yeast Exophiala dermatitidis and Other Selected Opportunistic Human Fungal Pathogens Spread from Dishwashers to Kitchens. PLoS ONE 2016, 11, e0148166. [Google Scholar] [CrossRef]
- Garber, G. An Overview of Fungal Infections. Drugs 2001, 61, 1–12. [Google Scholar] [CrossRef]
- Sharma, R.R. Fungal infections of the nervous system: Current perspective and controversies in management. Int. J. Surg. 2010, 8, 591–601. [Google Scholar] [CrossRef]
- Mursch, K.; Trnovec, S.; Ratz, H.; Hammer, D.; Horré, R.; Klinghammer, A.; De Hoog, S.; Behnke-Mursch, J. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Child’s Nerv. Syst. 2006, 22, 189–192. [Google Scholar] [CrossRef]
- Chen, T.-C.; Ho, M.-W.; Chien, W.-C.; Lin, H.-H. Disseminated Scedosporium apiospermum infection in a near-drowning patient. J. Formos. Med. Assoc. 2016, 115, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Yu, S.-L.; Chen, S.; Zhang, W.-H. CNS infection caused by Pseudallescheria boydii in a near-drowning traveller from a traffic accident. J. Travel Med. 2016, 23, tav018. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, S.; Nakashima, K.; Suzuki, F.; Otsuki, A.; Watanabe, J.; Takai, M.; Katsurada, M.; Katsurada, N.; Ohkuni, Y.; Misawa, M.; et al. Rice-Field Drowning-Associated Pneumonia in which Pseudomonas spp., Aspergillus fumigatus, and Cunninghamella sp. Are Isolated. Intern. Med. 2016, 55, 825–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vicente, V.A.; Attili-Angelis, D.; Pie, M.R.; Queiroz-Telles, F.; Cruz, L.M.; Najafzadeh, M.J.; De Hoog, G.S.; Zhao, J.; Pizzirani-Kleiner, A. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 2008, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zeng, J.; De Hoog, G.S.; Attili-Angelis, D.; Prenafeta-Boldú, F.X. Isolation and Identification of Black Yeasts by Enrichment on Atmospheres of Monoaromatic Hydrocarbons. Microb. Ecol. 2010, 60, 149–156. [Google Scholar] [CrossRef]
- Voglmayr, H.; Mayer, V.; Maschwitz, U.; Moog, J.; Djieto-Lordon, C.; Blatrix, R. The diversity of ant-associated black yeasts: Insights into a newly discovered world of symbiotic interactions. Fungal Biol. 2011, 115, 1077–1091. [Google Scholar] [CrossRef]
- Duarte, A.P.M.; Attili-Angelis, D.; Baron, N.C.; Forti, L.C.; Pagnocca, F.C. Leaf-cutting ants: An unexpected microenvironment holding human opportunistic black fungi. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2014, 106, 465–473. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Costi, P.; Tampellini, D.; Gatti, A.; Gerlach, M.; Riederer, P.; Fariello, R.G.; Ito, S.; Gallorini, M.; et al. The neuromelanin of human substantia nigra: Structure, synthesis and molecular behaviour. J. Neural Transm. Suppl. 2003, 65, 145–155. [Google Scholar]
- Lian, X.; De Hoog, G.S. Indoor wet cells harbour melanized agents of cutaneous infection. Med. Mycol. 2010, 48, 622–628. [Google Scholar] [CrossRef]
- Isola, D.; Selbmann, L.; De Hoog, G.S.; Fenice, M.; Onofri, S.; Prenafeta-Boldú, F.X.; Zucconi, L. Isolation and Screening of Black Fungi as Degraders of Volatile Aromatic Hydrocarbons. Mycopathologia 2013, 175, 369–379. [Google Scholar] [CrossRef]
- Francis, P.T. The Interplay of Neurotransmitters in Alzheimer’s Disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Rábano, A.; Carrasco, L. Alzheimer’s disease and disseminated mycoses. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Marina, A.I.; Morato, E.; Rábano, A.; Rodal, I.; Carrasco, L. Evidence for Fungal Infection in Cerebrospinal Fluid and Brain Tissue from Patients with Amyotrophic Lateral Sclerosis. Int. J. Biol. Sci. 2015, 11, 546–558. [Google Scholar] [CrossRef]
- Watabe-Rudolph, M.; Song, Z.; Lausser, L.; Schnack, C.; Begus-Nahrmann, Y.; Scheithauer, M.O.; Rettinger, G.; Otto, M.; Tumani, H.; Thal, D.R.; et al. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology 2012, 78, 569–577. [Google Scholar] [CrossRef]
- Pisa, D.; Alonso, R.; Juarranz, A.; Rábano, A.; Carrasco, L. Direct visualization of fungal infection in brains from patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.V.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef] [PubMed]
- Pisa, D.; Alonso, R.; Rábano, A.; Rodal, I.; Carrasco, L. Different Brain Regions are Infected with Fungi in Alzheimer’s Disease. Sci. Rep. 2015, 5, 15015–15028. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Klimko, N.; Busca, A.; Di Blasi, R.; Shadrivova, O.; Cesaro, S.; Zannier, M.E.; Verga, L.; Forghieri, F.; Calore, E.; et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019, 62, 252–260. [Google Scholar] [CrossRef]
- Gavito-Higuera, J.; Mullins, C.B.; Ramos-Duran, L.; Olivas Chacon, C.I.; Hakim, N.; Palacios, E. Fungal Infections of the Central Nervous System: A Pictorial Review. J. Clin. Imaging Sci. 2016, 6, 24–30. [Google Scholar] [CrossRef]
- Bloch, K.C.; Bailin, S.S. Update on fungal infections of the central nervous system. Curr. Opin. Infect. Dis. 2019, 32, 277–284. [Google Scholar] [CrossRef]
- Swinburne, N.C.; Bansal, A.G.; Aggarwal, A.; Doshi, A.H. Neuroimaging in Central Nervous System Infections. Curr. Neurol. Neurosci. Rep. 2017, 17, 49–63. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066–27126. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular Polysaccharide Export in Cryptococcus neoformans Is a Eukaryotic Solution to the Problem of Fungal Trans-Cell Wall Transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef]
- Bielska, E.; May, R.C. Extracellular vesicles of human pathogenic fungi. Curr. Opin. Microbiol. 2019, 52, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.P.; Nosanchuk, J.D.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of Yeast Extracellular Vesicles: Evidence for the Participation of Different Pathways of Cellular Traffic in Vesicle Biogenesis. PLoS ONE 2010, 5, e11113. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Wu, C.-H.; Chang, Y.C.; Kwon-Chung, K.J.; Brown, R.J.; Jong, A. Cryptococcus neoformans-Derived Microvesicles Enhance the Pathogenesis of Fungal Brain Infection. PLoS ONE 2012, 7, e48570. [Google Scholar] [CrossRef]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans Extracellular Vesicles with the Cell Wall. Eukaryot. Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Godinho, R.M.C.; Zamith-Miranda, D.; Nimrichter, L. Traveling into Outer Space: Unanswered Questions about Fungal Extracellular Vesicles. PLOS Pathog. 2015, 11, e1005240. [Google Scholar] [CrossRef]
- Štalekar, M.; Yin, X.; Rebolj, K.; Darovic, S.; Troakes, C.; Mayr, M.; Shaw, C.E.; Rogelj, B. Proteomic analyses reveal that loss of TDP-43 affects RNA processing and intracellular transport. Neuroscience 2015, 293, 157–170. [Google Scholar] [CrossRef]
- Satow, M.M.; Attili-Angelis, D.; De Hoog, G.S.; Angelis, D.F.; Vicente, V.A. Selective factors involved in oil flotation isolation of black yeasts from the environment. Stud. Mycol. 2008, 61, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Gostinčar, C.; Černoša, A.; Gunde-Cimerman, N. Stress-Tolerant Yeasts: Opportunistic Pathogenicity Versus Biocontrol Potential. Genes 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R.; Cutting, S.M. Appendix 1—Media. Molecular Biological Methods for Bacillus; Wiley: Chichester, UK; New York, NY, USA, 1990; ISBN 9780471923930. [Google Scholar]
- Pužar Dominkuš, P.; Ferdin, J.; Plemenitaš, A.; Peterlin, B.M.; Lenassi, M. Nef is secreted in exosomes from Nef.GFP-expressing and HIV-1-infected human astrocytes. J. Neurovirol. 2017, 23, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Forest, S.E.; Simon, J.D. Wavelength-dependent photoacoustic calorimetry study of melanin. Photochem. Photobiol. 1998, 68, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Shaw, M.; Hole, P.; Smith, J.; Tannetta, D.; Redman, C.W.; Sargent, I.L. Measurement of refractive index by nanoparticle tracking analysis reveals heterogeneity in extracellular vesicles. J. Extracell. Vesicles 2014, 3, 25361. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Joffe, L.S.; Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Potential Roles of Fungal Extracellular Vesicles during Infection. mSphere 2016, 1, e00099-16. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotič, T.; Cheng, Y.; Krogan, N.J.; Plemenitaš, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.P.; Nosanchuk, J.D.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Biogenesis of extracellular vesicles in yeast. Commun. Integr. Biol. 2010, 3, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. In Neuronal Cell Culture: Methods and Protocols; Amini, S., White, M.K., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1078, pp. 9–21. ISBN 978-1-62703-639-9. [Google Scholar]
- Novak Babič, M.; Zalar, P.; Ženko, B.; Schroers, H.J.; Džeroski, S.; Gunde-Cimerman, N. Candida and Fusarium species known as opportunistic human pathogens from customer-accessible parts of residential washingmachines. Fungal Biol. 2015, 119, 95–113. [Google Scholar] [CrossRef]
- Hamada, N.; Abe, N. Physiological characteristics of 13 common fungal species in bathrooms. Mycoscience 2009, 50, 421–429. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Deatheragea, B.L.; Cooksona, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef]
- Vargas, G.; Rocha, J.D.B.; Oliveira, D.L.; Albuquerque, P.C.; Frases, S.; Santos, S.S.; Nosanchuk, J.D.; Gomes, A.M.O.; Medeiros, L.C.A.S.; Miranda, K.; et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell. Microbiol. 2015, 17, 389–407. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Casadevall, A. A two-way road: Novel roles for fungal extracellular vesicles. Mol. Microbiol. 2018, 110, 11–15. [Google Scholar] [CrossRef]
- Casadevall, A. Melanin triggers antifungal defences. Nature 2018, 555, 319–320. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Frases, S.; Nicola, A.M.; Rodrigues, M.L.; Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 2009, 155, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Upadhyay, S.; Xu, X.; Lowry, D.; Jackson, J.C.; Roberson, R.W.; Lin, X. Subcellular Compartmentalization and Trafficking of the Biosynthetic Machinery for Fungal Melanin. Cell Rep. 2016, 14, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Franquet, T.; Giménez, A.; Hidalgo, A. Imaging of opportunistic fungal infections in immunocompromised patient. Eur. J. Radiol. 2004, 51, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gaviani, P.; Schwartz, R.B.; Hedley-Whyte, E.T.; Ligon, K.L.; Robicsek, A.; Schaefer, P.; Henson, J.W. Diffusion-weighted imaging of fungal cerebral infection. Am. J. Neuroradiol. 2005, 26, 1115–1121. [Google Scholar] [PubMed]
- Jain, K.K.; Mittal, S.K.; Kumar, S.; Gupta, R.K. Imaging features of central nervous system fungal infections Krishan. Neurol. India 2007, 55, 241–250. [Google Scholar]
- Brouwer, M.C.; Coutinho, J.M.; Van de Beek, D. Clinical characteristics and outcome of brain abscess: Systematic review and meta-analysis. Neurology 2014, 82, 806–813. [Google Scholar] [CrossRef]
- Shi, M.; Calaruso, P.; Mody, C.H. Real-time in vivo imaging of fungal migration to the central nervous system. Cell. Microbiol. 2012, 14, 1819–1827. [Google Scholar] [CrossRef]
- Jong, A.Y.; Stins, M.F.; Huang, S.-H.; Chen, S.H.M.; Kim, K.S. Traversal of Candida albicans across Human Blood-Brain Barrier. Am. Soc. Microbiol. 2001, 69, 4536–4544. [Google Scholar] [CrossRef]
- Tirado, F.H.S.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. MBio 2017, 8, e02183-16. [Google Scholar]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vignais, M.-L.; Caicedo, A.; Brondello, J.-M.; Jorgensen, C. Cell Connections by Tunneling Nanotubes: Effects of Mitochondrial Trafficking on Target Cell Metabolism, Homeostasis, and Response to Therapy. Stem Cells Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Pasonen-Seppänen, S.; Deen, A.J.; Koistinen, V.V.T.; Wojciechowski, S.; Oikari, S.; Kärnä, R.; Bart, G.; Törrönen, K.; Tammi, R.H.; et al. Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp. Cell Res. 2013, 319, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrin, T.; Konte, T.; Kostanjšek, R.; Sitar, S.; Sepčič, K.; Prpar Mihevc, S.; Žagar, E.; Župunski, V.; Lenassi, M.; Rogelj, B.; et al. The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death. Cells 2020, 9, 963. https://doi.org/10.3390/cells9040963
Lavrin T, Konte T, Kostanjšek R, Sitar S, Sepčič K, Prpar Mihevc S, Žagar E, Župunski V, Lenassi M, Rogelj B, et al. The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death. Cells. 2020; 9(4):963. https://doi.org/10.3390/cells9040963
Chicago/Turabian StyleLavrin, Teja, Tilen Konte, Rok Kostanjšek, Simona Sitar, Kristina Sepčič, Sonja Prpar Mihevc, Ema Žagar, Vera Župunski, Metka Lenassi, Boris Rogelj, and et al. 2020. "The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death" Cells 9, no. 4: 963. https://doi.org/10.3390/cells9040963
APA StyleLavrin, T., Konte, T., Kostanjšek, R., Sitar, S., Sepčič, K., Prpar Mihevc, S., Žagar, E., Župunski, V., Lenassi, M., Rogelj, B., & Gunde Cimerman, N. (2020). The Neurotropic Black Yeast Exophiala dermatitidis Induces Neurocytotoxicity in Neuroblastoma Cells and Progressive Cell Death. Cells, 9(4), 963. https://doi.org/10.3390/cells9040963






