Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Trials and Patients
2.2. FFPE Block Collection
2.3. Pathological Revision
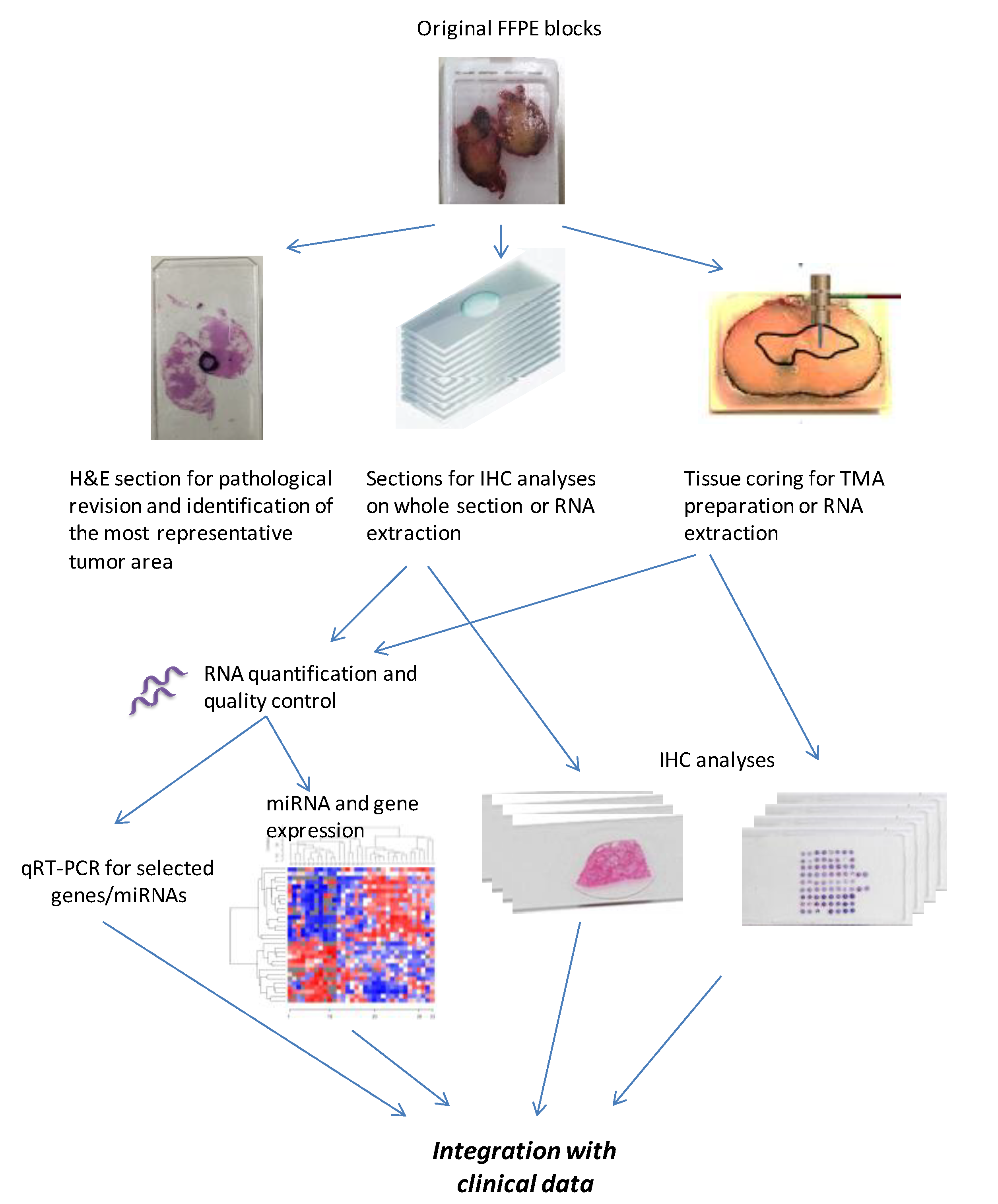
2.4. FFPE Blocks Processing
2.5. TMA Building for IHC Analysis
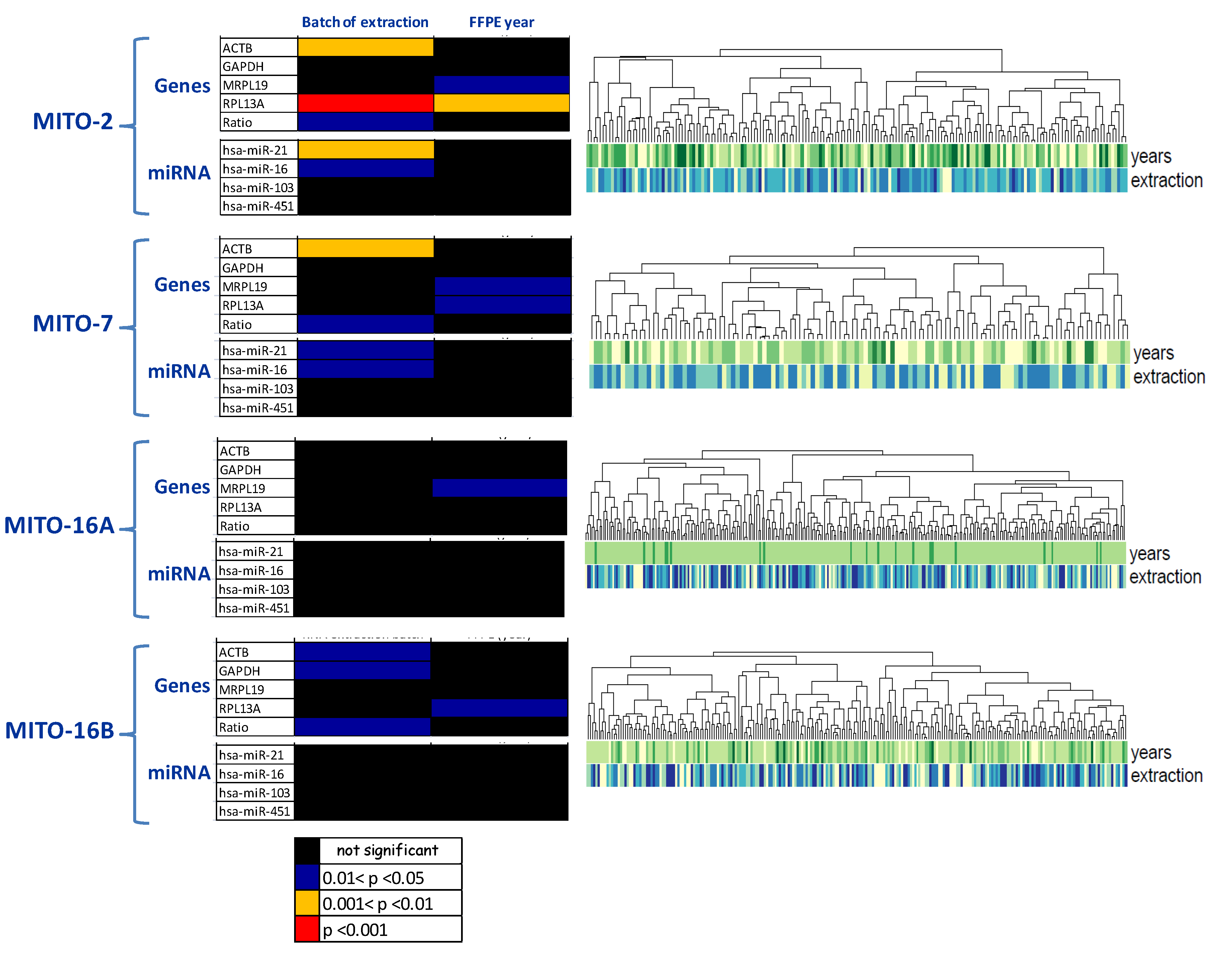
2.6. RNA Extraction and Quality Controls
3. Results
3.1. FFPE Block Collection
3.2. Performance of FFPE Block Collection over 10 Years of Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Dancey, J.E.; Dobbin, K.K.; Groshen, S.; Jessup, J.M.; Hruszkewycz, A.H.; Koehler, M.; Parchment, R.; Ratain, M.J.; Shankar, L.K.; Stadler, W.M.; et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010, 16, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Scambia, G.; Ferrandina, G.; Savarese, A.; Sorio, R.; Breda, E.; Gebbia, V.; Musso, P.; Frigerio, L.; Del Medico, P.; et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: The MITO-2 randomized phase III trial. J. Clin. Oncol. 2011, 29, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Calzolari, A.; Napolitano, M.; Bravo, E. Review of the Italian current legislation on research biobanking activities on the eve of the participation of national biobanks’ network in the legal consortium BBMRI-ERIC. Biopreserv. Biobank 2013, 11, 124–128. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Bagnoli, M.; Shi, T.Y.; Gourley, C.; Speiser, P.; Reuss, A.; Nijman, H.W.; Creutzberg, C.L.; Scholl, S.; Negrouk, A.; Brady, M.F.; et al. Gynecological Cancers Translational, Research Implementation, and Harmonization: Gynecologic Cancer InterGroup Consensus and Still Open Questions. Cells 2019, 8, 200. [Google Scholar] [CrossRef]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Tamayo, P.; Yang, J.Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H.; et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2013, 123, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Wang, C.; Hamidi, H.; Winterhoff, B.; Kalli, K.R.; Dering, J.; Ginther, C.; Chen, H.W.; Dowdy, S.; Cliby, W.; et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.M.; Kannan, L.; Geistlinger, L.; Kofia, V.; Safikhani, Z.; Gendoo, D.M.A.; Parmigiani, G.; Birrer, M.; Haibe-Kains, B.; Waldron, L. Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2018, 24, 5037–5047. [Google Scholar] [CrossRef] [PubMed]
- Bais, C.; Mueller, B.; Brady, M.F.; Mannel, R.S.; Burger, R.A.; Wei, W.; Marien, K.M.; Kockx, M.M.; Husain, A.; Birrer, M.J.; et al. Tumor Microvessel Density as a Potential Predictive Marker for Bevacizumab Benefit: GOG-0218 Biomarker Analyses. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Kommoss, S.; Winterhoff, B.; Oberg, A.L.; Konecny, G.E.; Wang, C.; Riska, S.M.; Fan, J.B.; Maurer, M.J.; April, C.; Shridhar, V.; et al. Bevacizumab May Differentially Improve Ovarian Cancer Outcome in Patients with Proliferative and Mesenchymal Molecular Subtypes. Clin. Cancer Res. 2017, 23, 3794–3801. [Google Scholar] [CrossRef]
- Alvarez Secord, A.; Bell Burdett, K.; Owzar, K.; Tritchler, D.; Sibley, A.B.; Liu, Y.; Starr, M.D.; Brady, J.C.; Lankes, H.A.; Hurwitz, H.I.; et al. Predictive Blood-Based Biomarkers in Patients with Epithelial Ovarian Cancer Treated with Carboplatin and Paclitaxel with or without Bevacizumab: Results from GOG-0218. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Gourley, C.; Mc Cavigan, A.; Perren, T.; Paul, J.; Michie, C.O.; ChurchmanAlistair, M.W.; Glenn McCluggage, W.; Parmar, M.; Kaplan, R.S.; Hill, L.A.; et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J. Clin. Oncol. 2014, 32, 5502. [Google Scholar] [CrossRef]
- Gori, S.; Barberis, M.; Bella, M.A.; Buttitta, F.; Capoluongo, E.; Carrera, P.; Colombo, N.; Cortesi, L.; Genuardi, M.; Gion, M.; et al. Recommendations for the implementation of BRCA testing in ovarian cancer patients and their relatives. Crit. Rev. Oncol. Hematol. 2019, 140, 67–72. [Google Scholar] [CrossRef]
- Longo, D.L. Personalized Medicine for Primary Treatment of Serous Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2471–2474. [Google Scholar] [CrossRef]
- Winterhoff, B.; Kommoss, S.; Heitz, F.; Konecny, G.E.; Dowdy, S.C.; Mullany, S.A.; Park-Simon, T.W.; Baumann, K.; Hilpert, F.; Brucker, S.; et al. Developing a Clinico-Molecular Test for Individualized Treatment of Ovarian Cancer: The interplay of Precision Medicine Informatics with Clinical and Health Economics Dimensions. AMIA Annu. Symp. Proc. 2018, 2018, 1093–1102. [Google Scholar]
- Hutter, C.; Zenklusen, J.C. The Cancer Genome Atlas: Creating Lasting Value beyond Its Data. Cell 2018, 173, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, C.; Speed, T.P.; Wang, W.; Symmans, F.S. Accurate RNA Sequencing From Formalin-Fixed Cancer Tissue To Represent High-Quality Transcriptome From Frozen Tissue. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Zhao, Y.; Mehta, M.; Walton, A.; Talsania, K.; Levin, Y.; Shetty, J.; Gillanders, E.M.; Tran, B.; Carrick, D.M. Robustness of RNA sequencing on older formalin-fixed paraffin-embedded tissue from high-grade ovarian serous adenocarcinomas. PLoS ONE 2019, 14, e0216050. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, M.; Canevari, S.; Califano, D.; Losito, S.; Maio, M.D.; Raspagliesi, F.; Carcangiu, M.L.; Toffoli, G.; Cecchin, E.; Sorio, R.; et al. Development and validation of a microRNA-based signature (MiROvaR) to predict early relapse or progression of epithelial ovarian cancer: A cohort study. Lancet Oncol. 2016, 17, 1137–1146. [Google Scholar] [CrossRef]
- Perrone, F.; Baldassarre, G.; Indraccolo, S.; Signoriello, S.; Chiappetta, G.; Esposito, F.; Ferrandina, G.; Franco, R.; Mezzanzanica, D.; Sonego, M.; et al. Biomarker analysis of the MITO2 phase III trial of first-line treatment in ovarian cancer: Predictive value of DNA-PK and phosphorylated ACC. Oncotarget 2016, 7, 72654–72661. [Google Scholar] [CrossRef][Green Version]
- Schwarz, R.F.; Ng, C.K.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef]

| MITO2 | MITO7 | MITO16A | MITO16B | |
|---|---|---|---|---|
| Type of trial | First line Randomized phase III | First line Randomized phase III | Observational phase IV | Second line Randomized phase III |
| Explorable biomarkers | Prognostic and predictive | Prognostic and predictive | Prognostic only | Prognostic and predictive |
| Samples collection and translational end-point | Retrospective Secondary | Retrospective Secondary | Prospective Primary | Prospective Secondary |
| N. centers and type of participation to TR analyses | 17/43 Voluntary | 12/67Voluntary | 47/47Mandatory | 78/82Mandatory |
| N Pts. Enrolled in Clinical Trial | N Pts. with Approval for TR Analyses * | Patients with FFPE Blocks Centralized | |||
|---|---|---|---|---|---|
| Number | % Centralized/Enrolled | % Centralized/TR Approved * | |||
| MITO2 | 820 | 549 | 269 | 33% | 49% |
| MITO7 | 810 | 457 | 176 | 22% | 38% |
| MITO16A | 400 | 400 | 385 | 96% | 96% |
| MITO16B | 406 | 406 | 366 | 90% | 90% |
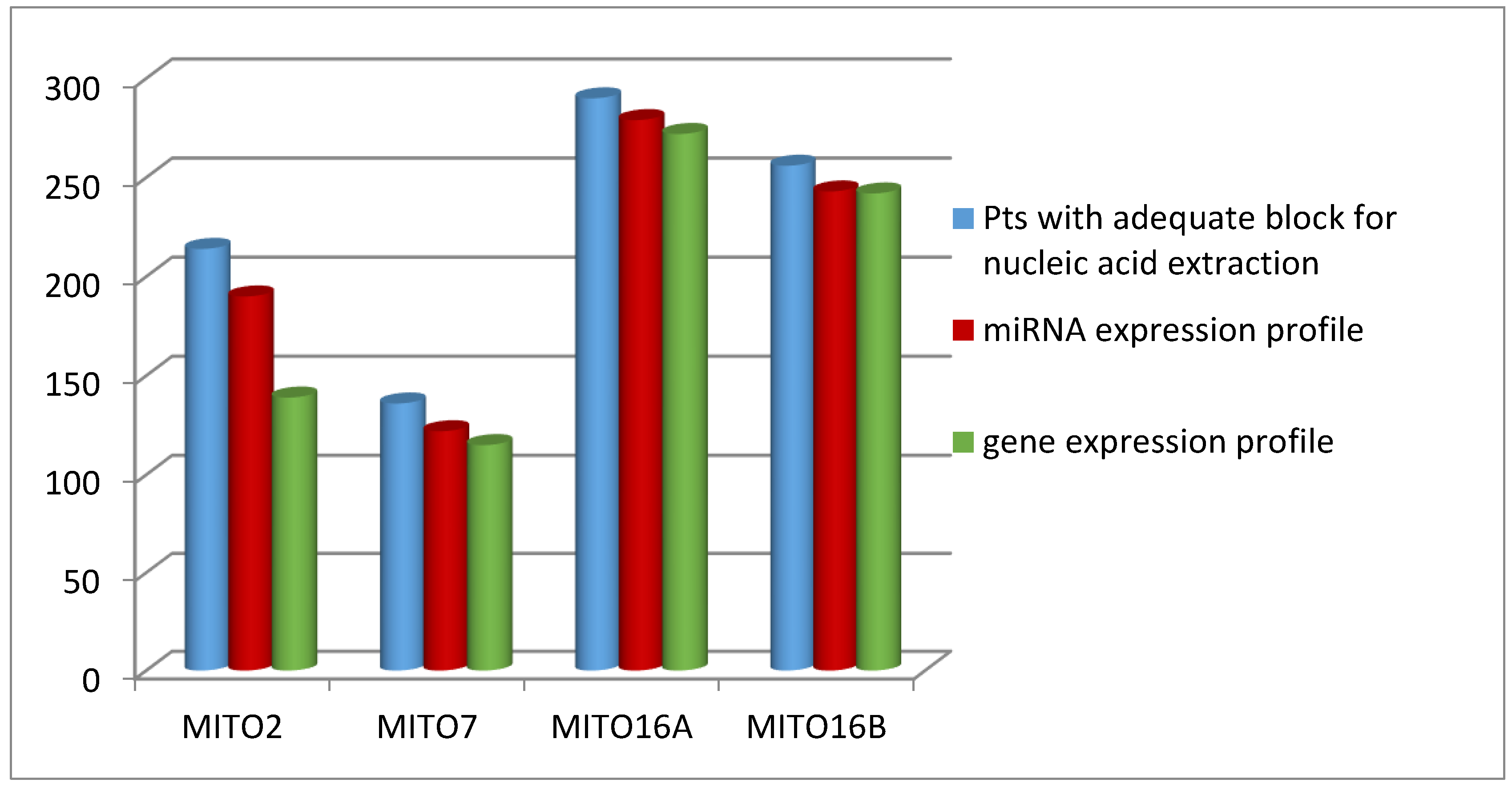
| Trial | Type of TR Analysis | Pts with Adequate Block | Pts with Primary Tumor Samples | Pts. with Synchronous Primary and Secondary Lesions | Pts. with Secondary Lesion Only |
|---|---|---|---|---|---|
| MITO2 | IHC | 239 | 230 | 52 | 8 |
| Molecular * | 214 | 180 | 42 | 34 | |
| MITO7 | IHC | 158 | 127 | 49 | 31 |
| Molecular | 136 | 108 | 41 | 28 | |
| MITO16A | IHC | 358 | 252 | 58 | 106 |
| Molecular | 290 | 223 | 41 | 67 | |
| MITO16B | IHC | 313 | 180 | 31 | 133 |
| Molecular | 256 | 165 | 26 | 91 |
| Parameters | Limits | Solutions | Future Improvements |
|---|---|---|---|
| Histological blocks’ retrival |
|
|
|
|
|
| |
|
| ||
| Histological blocks’ characteristics and processing |
|
|
|
|
| ||
|
| ||
| IHC on whole sections |
|
|
|
| TMA construction |
|
| |
| RNA extraction and quality |
|
| |
|
| ||
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Califano, D.; Russo, D.; Scognamiglio, G.; Losito, N.S.; Spina, A.; Bello, A.M.; Capiluongo, A.; Galdiero, F.; De Cecio, R.; Bevilacqua, S.; et al. Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience. Cells 2020, 9, 903. https://doi.org/10.3390/cells9040903
Califano D, Russo D, Scognamiglio G, Losito NS, Spina A, Bello AM, Capiluongo A, Galdiero F, De Cecio R, Bevilacqua S, et al. Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience. Cells. 2020; 9(4):903. https://doi.org/10.3390/cells9040903
Chicago/Turabian StyleCalifano, Daniela, Daniela Russo, Giosuè Scognamiglio, Nunzia Simona Losito, Anna Spina, Anna Maria Bello, Anna Capiluongo, Francesca Galdiero, Rossella De Cecio, Simona Bevilacqua, and et al. 2020. "Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience" Cells 9, no. 4: 903. https://doi.org/10.3390/cells9040903
APA StyleCalifano, D., Russo, D., Scognamiglio, G., Losito, N. S., Spina, A., Bello, A. M., Capiluongo, A., Galdiero, F., De Cecio, R., Bevilacqua, S., Gargiulo, P., Marchesi, E., Canevari, S., Perrone, F., Daniele, G., De Cecco, L., Mezzanzanica, D., & Pignata, S. (2020). Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience. Cells, 9(4), 903. https://doi.org/10.3390/cells9040903






