Revisiting the Role of Neurotrophic Factors in Inflammation
Abstract
1. Introduction
2. Phenotype Associated with GFLs and Their Receptor Defects
3. Neurotrophic Factors in Inflammation
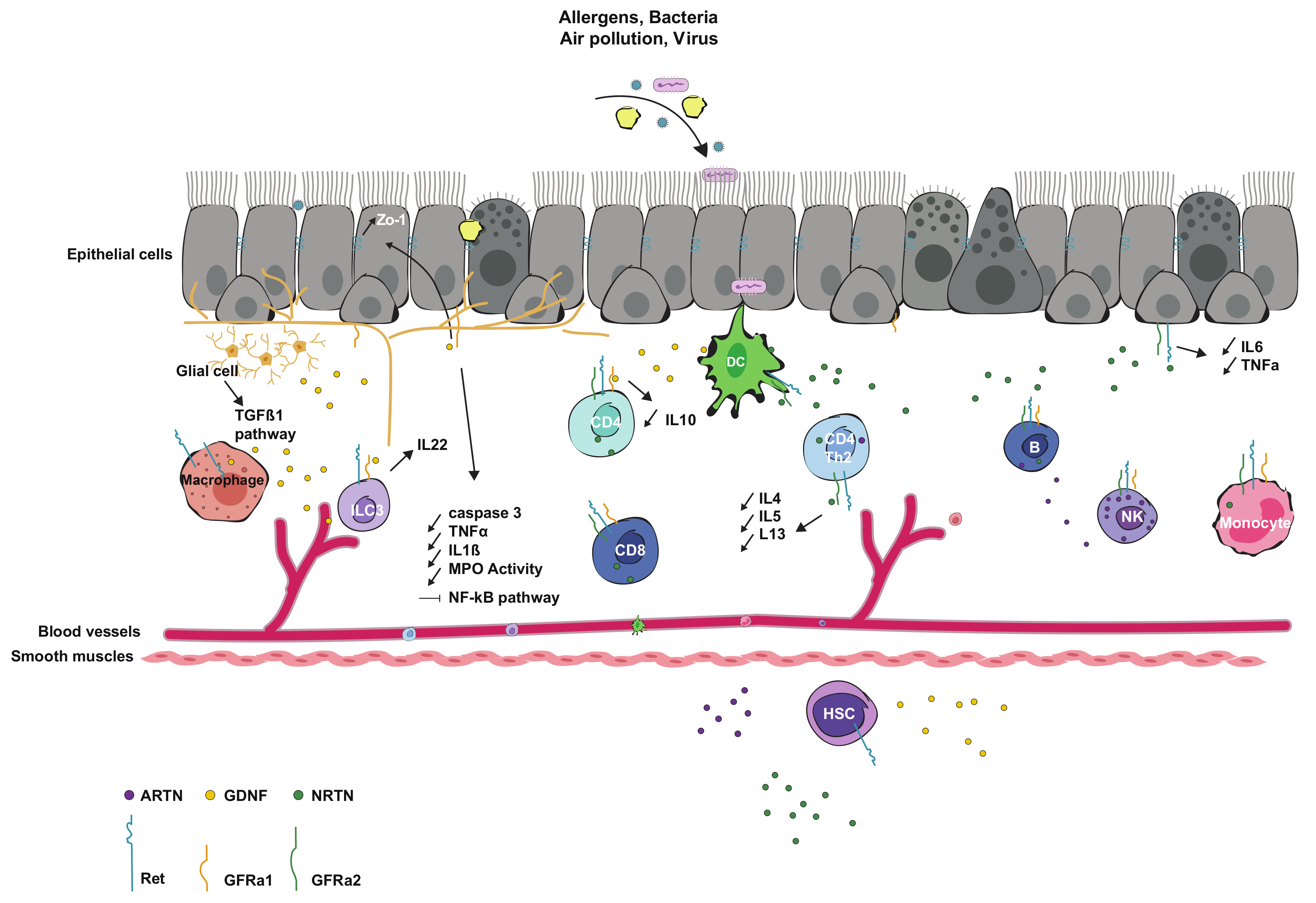
3.1. Interaction and Expression in Immune Cells
3.2. Interaction and Expression in Epithelial Cells
3.3. Interaction with Microbiota
4. Roles of GFLs in Pathologies
4.1. In Gut Diseases
4.2. In Kidney Diseases
4.3. In Diabetes
4.4. In Asthma and Allergic Rhinitis
4.5. In Skin Diseases
4.6. In Neuropsychiatric Disorders
4.7. In Cancers
4.8. In Pain Sensitivity
5. Therapeutic Potential and Pharmaceutic Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trupp, M.; Raynoschek, C.; Belluardo, N.; Ibanez, C.F. Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol. Cell Neurosci. 1998, 11, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ledda, F.; Ibanez, C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 2003, 113, 867–879. [Google Scholar] [CrossRef]
- Sandmark, J.; Dahl, G.; Oster, L.; Xu, B.; Johansson, P.; Akerud, T.; Aagaard, A.; Davidsson, P.; Bigalke, J.M.; Winzell, M.S.; et al. Structure and biophysical characterization of the human full-length neurturin-GFRa2 complex: A role for heparan sulfate in signaling. J. Biol. Chem 2018, 293, 5492–5508. [Google Scholar] [CrossRef] [PubMed]
- Horger, B.A.; Nishimura, M.C.; Armanini, M.P.; Wang, L.C.; Poulsen, K.T.; Rosenblad, C.; Kirik, D.; Moffat, B.; Simmons, L.; Johnson, E., Jr.; et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J. Neurosci. 1998, 18, 4929–4937. [Google Scholar] [CrossRef]
- Fjord-Larsen, L.; Johansen, J.L.; Kusk, P.; Tornoe, J.; Gronborg, M.; Rosenblad, C.; Wahlberg, L.U. Efficient in vivo protection of nigral dopaminergic neurons by lentiviral gene transfer of a modified Neurturin construct. Exp. Neurol. 2005, 195, 49–60. [Google Scholar] [CrossRef]
- Biju, K.C.; Santacruz, R.A.; Chen, C.; Zhou, Q.; Yao, J.; Rohrabaugh, S.L.; Clark, R.A.; Roberts, J.L.; Phillips, K.A.; Imam, S.Z.; et al. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci. Lett. 2013, 535, 24–29. [Google Scholar] [CrossRef]
- Bartus, R.T.; Kordower, J.H.; Johnson, E.M., Jr.; Brown, L.; Kruegel, B.R.; Chu, Y.; Baumann, T.L.; Lang, A.E.; Olanow, C.W.; Herzog, C.D. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with alpha-synucleinopathies. Neurobiol. Dis. 2015, 78, 162–171. [Google Scholar] [CrossRef]
- Stanga, S.; Brambilla, L.; Tasiaux, B.; Dang, A.H.; Ivanoiu, A.; Octave, J.N.; Rossi, D.; Van Pesch, V.; Kienlen-Campard, P. A Role for GDNF and Soluble APP as Biomarkers of Amyotrophic Lateral Sclerosis Pathophysiology. Front. Neurol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Thomsen, G.M.; Avalos, P.; Ma, A.A.; Alkaslasi, M.; Cho, N.; Wyss, L.; Vit, J.P.; Godoy, M.; Suezaki, P.; Shelest, O.; et al. Transplantation of Neural Progenitor Cells Expressing Glial Cell Line-Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis. Stem Cells 2018, 36, 1122–1131. [Google Scholar] [CrossRef]
- Gross, S.K.; Shim, B.S.; Bartus, R.T.; Deaver, D.; McEachin, Z.; Betourne, A.; Boulis, N.M.; Maragakis, N.J. Focal and dose-dependent neuroprotection in ALS mice following AAV2-neurturin delivery. Exp. Neurol. 2020, 323, 113091. [Google Scholar] [CrossRef]
- Trupp, M.; Ryden, M.; Jornvall, H.; Funakoshi, H.; Timmusk, T.; Arenas, E.; Ibanez, C.F. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J. Cell Biol. 1995, 130, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Widenfalk, J.; Nosrat, C.; Tomac, A.; Westphal, H.; Hoffer, B.; Olson, L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 1997, 17, 8506–8519. [Google Scholar] [PubMed]
- Nakayama, S.; Iida, K.; Tsuzuki, T.; Iwashita, T.; Murakami, H.; Asai, N.; Iwata, Y.; Ichihara, M.; Ito, S.; Kawai, K.; et al. Implication of expression of GDNF/Ret signalling components in differentiation of bone marrow haemopoietic cells. Br. J. Haematol. 1999, 105, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.W.; Klein, R.D.; Farinas, I.; Sauer, H.; Armanini, M.; Phillips, H.; Reichardt, L.F.; Ryan, A.M.; Carver-Moore, K.; Rosenthal, A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996, 382, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, A.; D’Agati, V.; Larsson-Blomberg, L.; Costantini, F.; Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994, 367, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Araki, T.; Jackman, A.; Heuckeroth, R.O.; Snider, W.D.; Johnson, E.M., Jr.; Milbrandt, J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 1998, 21, 317–324. [Google Scholar] [CrossRef]
- Rossi, J.; Herzig, K.H.; Voikar, V.; Hiltunen, P.H.; Segerstrale, M.; Airaksinen, M.S. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor alpha2. J. Clin. Investig. 2003, 112, 707–716. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Lampe, P.A.; Johnson, E.M.; Milbrandt, J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev. Biol. 1998, 200, 116–129. [Google Scholar] [CrossRef]
- Bhang, D.H.; Kim, B.J.; Kim, B.G.; Schadler, K.; Baek, K.H.; Kim, Y.H.; Hsiao, W.; Ding, B.S.; Rafii, S.; Weiss, M.J.; et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat. Commun. 2018, 9, 4379. [Google Scholar] [CrossRef]
- Rossi, J.; Luukko, K.; Poteryaev, D.; Laurikainen, A.; Sun, Y.F.; Laakso, T.; Eerikainen, S.; Tuominen, R.; Lakso, M.; Rauvala, H.; et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron 1999, 22, 243–252. [Google Scholar] [CrossRef]
- Song, X.J.; Li, D.Q.; Farley, W.; Luo, L.H.; Heuckeroth, R.O.; Milbrandt, J.; Pflugfelder, S.C. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest. Ophthalmol Vis. Sci. 2003, 44, 4223–4229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindfors, P.H.; Lindahl, M.; Rossi, J.; Saarma, M.; Airaksinen, M.S. Ablation of persephin receptor glial cell line-derived neurotrophic factor family receptor alpha4 impairs thyroid calcitonin production in young mice. Endocrinology 2006, 147, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Tomac, A.C.; Agulnick, A.D.; Haughey, N.; Chang, C.F.; Zhang, Y.; Backman, C.; Morales, M.; Mattson, M.P.; Wang, Y.; Westphal, H.; et al. Effects of cerebral ischemia in mice deficient in Persephin. Proc. Natl. Acad. Sci. USA 2002, 99, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Verity, A.N.; Wyatt, T.L.; Lee, W.; Hajos, B.; Baecker, P.A.; Eglen, R.M.; Johnson, R.M. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J. Neurosci. Res. 1999, 55, 187–197. [Google Scholar] [CrossRef]
- Esseghir, S.; Todd, S.K.; Hunt, T.; Poulsom, R.; Plaza-Menacho, I.; Reis-Filho, J.S.; Isacke, C.M. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor up-regulation in breast cancer. Cancer Res. 2007, 67, 11732–11741. [Google Scholar] [CrossRef]
- Tanabe, K.; Nishimura, K.; Dohi, S.; Kozawa, O. Mechanisms of interleukin-1beta-induced GDNF release from rat glioma cells. Brain Res. 2009, 1274, 11–20. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nitta, A.; Fukumitsu, H.; Nomoto, H.; Shen, L.; Furukawa, S. Involvement of glial cell line-derived neurotrophic factor in activation processes of rodent macrophages. J. Neurosci. Res. 2005, 79, 476–487. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ni, J.; Jiang, H.; Hsu, T.A.; Dugich-Djordjevic, M.; Feng, L.; Zhang, M.; Mei, L.; Gentz, R.; Lu, B. Cloning and characterization of glial cell line-derived neurotrophic factor receptor-B: A novel receptor for members of glial cell line-derived neurotrophic factor family of neurotrophic factors. Neuroscience 1998, 83, 7–14. [Google Scholar] [CrossRef]
- Yang, C.; Hutto, D.; Sah, D.W. Distribution of GDNF family receptor alpha3 and RET in rat and human non-neural tissues. J. Mol. Histol. 2006, 37, 69–77. [Google Scholar] [CrossRef]
- Kondo, S.; Kishi, H.; Tokimitsu, Y.; Muraguchi, A. Possible involvement of glial cell line-derived neurotrophic factor and its receptor, GFRalpha1, in survival and maturation of thymocytes. Eur. J. Immunol. 2003, 33, 2233–2240. [Google Scholar] [CrossRef]
- Gattei, V.; Celetti, A.; Cerrato, A.; Degan, M.; De Iuliis, A.; Rossi, F.M.; Chiappetta, G.; Consales, C.; Improta, S.; Zagonel, V.; et al. Expression of the RET receptor tyrosine kinase and GDNFR-alpha in normal and leukemic human hematopoietic cells and stromal cells of the bone marrow microenvironment. Blood 1997, 89, 2925–2937. [Google Scholar]
- Fonseca-Pereira, D.; Arroz-Madeira, S.; Rodrigues-Campos, M.; Barbosa, I.A.; Domingues, R.G.; Bento, T.; Almeida, A.R.; Ribeiro, H.; Potocnik, A.J.; Enomoto, H.; et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 2014, 514, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Leal, V.; Bruno, R.; Derfuss, T.; Krumbholz, M.; Hohlfeld, R.; Meinl, E. Expression and function of glial cell line-derived neurotrophic factor family ligands and their receptors on human immune cells. J. Immunol. 2005, 175, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Arroz-Madeira, S.; Fonseca-Pereira, D.; Ribeiro, H.; Lasrado, R.; Pachnis, V.; Veiga-Fernandes, H. RET/GFRalpha signals are dispensable for thymic T cell development in vivo. PLoS ONE 2012, 7, e52949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rusmini, M.; Griseri, P.; Lantieri, F.; Matera, I.; Hudspeth, K.L.; Roberto, A.; Mikulak, J.; Avanzini, S.; Rossi, V.; Mattioli, G.; et al. Induction of RET dependent and independent pro-inflammatory programs in human peripheral blood mononuclear cells from Hirschsprung patients. PLoS ONE 2013, 8, e59066. [Google Scholar] [CrossRef]
- Almeida, A.R.; Fonseca-Pereira, D.; Arroz-Madeira, S.; Ribeiro, H.; Labao-Almeida, C.; Veiga-Fernandes, H. The neurotrophic factor receptor RET regulates IL-10 production by in vitro polarised T helper 2 cells. Eur. J. Immunol. 2014, 44, 3605–3613. [Google Scholar] [CrossRef]
- Rusmini, M.; Griseri, P.; Matera, I.; Pontarini, E.; Ravazzolo, R.; Mavilio, D.; Ceccherini, I. Expression variability and function of the RET gene in adult peripheral blood mononuclear cells. J. Cell Physiol. 2014, 229, 2027–2037. [Google Scholar] [CrossRef]
- Ibiza, S.; Garcia-Cassani, B.; Ribeiro, H.; Carvalho, T.; Almeida, L.; Marques, R.; Misic, A.M.; Bartow-McKenney, C.; Larson, D.M.; Pavan, W.J.; et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 2016, 535, 440–443. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Coles, M.C.; Foster, K.E.; Patel, A.; Williams, A.; Natarajan, D.; Barlow, A.; Pachnis, V.; Kioussis, D. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 2007, 446, 547–551. [Google Scholar] [CrossRef]
- Cavel, O.; Shomron, O.; Shabtay, A.; Vital, J.; Trejo-Leider, L.; Weizman, N.; Krelin, Y.; Fong, Y.; Wong, R.J.; Amit, M.; et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012, 72, 5733–5743. [Google Scholar] [CrossRef]
- Tanaka, F.; Tominaga, K.; Fujikawa, Y.; Nagami, Y.; Kamata, N.; Yamagami, H.; Tanigawa, T.; Shiba, M.; Watanabe, T.; Fujiwara, Y.; et al. Concentration of Glial Cell Line-Derived Neurotrophic Factor Positively Correlates with Symptoms in Functional Dyspepsia. Dig. Dis. Sci. 2016, 61, 3478–3485. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, P.E.; Liberatore, G.T.; Wong, J.Y.; Porritt, M.J.; Frerichs, F.; Donnan, G.A.; Howells, D.W. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999, 19, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Kerschensteiner, M.; Stadelmann, C.; Dechant, G.; Wekerle, H.; Hohlfeld, R. Neurotrophic cross-talk between the nervous and immune systems: Implications for neurological diseases. Ann. Neurol. 2003, 53, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Oh-hashi, K.; Ito, M.; Shitara, H.; Hirata, Y.; Kiuchi, K. Identification of a novel GDNF mRNA induced by LPS in immune cell lines. Neurosci. Res. 2008, 61, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rickert, U.; Grampp, S.; Wilms, H.; Spreu, J.; Knerlich-Lukoschus, F.; Held-Feindt, J.; Lucius, R. Glial Cell Line-Derived Neurotrophic Factor Family Members Reduce Microglial Activation via Inhibiting p38MAPKs-Mediated Inflammatory Responses. J. Neurodegener Dis. 2014, 2014, 369468. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, J.; Merkel, L.; Heckers, S.; Gudi, V.; Schwab, M.H.; Stangel, M. Investigation of Neuregulin-1 and Glial Cell-Derived Neurotrophic Factor in Rodent Astrocytes and Microglia. J. Mol. Neurosci. 2019, 67, 484–493. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Flamant, M.; Aubert, P.; Rolli-Derkinderen, M.; Bourreille, A.; Neunlist, M.R.; Mahe, M.M.; Meurette, G.; Marteyn, B.; Savidge, T.; Galmiche, J.P.; et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: A role for S-nitrosoglutathione. Gut 2011, 60, 473–484. [Google Scholar] [CrossRef]
- Neunlist, M.; Aubert, P.; Bonnaud, S.; Van Landeghem, L.; Coron, E.; Wedel, T.; Naveilhan, P.; Ruhl, A.; Lardeux, B.; Savidge, T.; et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G231–G241. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, W.; Chen, W.; Sun, L.; Li, X.; Zhang, C.; Yang, H. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol. Neurobiol. 2014, 50, 274–289. [Google Scholar] [CrossRef]
- Zhang, D.K.; He, F.Q.; Li, T.K.; Pang, X.H.; Cui, D.J.; Xie, Q.; Huang, X.L.; Gan, H.T. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 2010, 222, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Flemming, S.; Burkard, N.; Bergauer, L.; Metzger, M.; Germer, C.T.; Schlegel, N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 309, G613–G624. [Google Scholar] [CrossRef] [PubMed]
- Von Boyen, G.B.; Steinkamp, M.; Geerling, I.; Reinshagen, M.; Schafer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: A key to the regulation of epithelial apoptosis in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shine, H.D.; Li, D.Q.; De Paiva, C.S.; Farley, W.J.; Jones, D.B.; Pflugfelder, S.C. Glial cell-derived neurotrophic factor gene delivery enhances survival of human corneal epithelium in culture and the overexpression of GDNF in bioengineered constructs. Exp. Eye Res. 2008, 87, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Qi, H.; Ma, P.; Zhang, L.; Yoon, K.C.; Pflugfelder, S.C.; Li, D.Q. An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor. Stem Cells 2010, 28, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.P.; DeMaro, J.A.; Osborne, P.A.; Milbrandt, J.; Johnson, E.M., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp. Neurol. 1999, 158, 504–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Cao, X.; Feng, X.; Wang, X. Serum biomarker analysis in patients with premature ovarian insufficiency. Cytokine 2020, 126, 154876. [Google Scholar] [CrossRef]
- Malin, S.A.; Molliver, D.C.; Koerber, H.R.; Cornuet, P.; Frye, R.; Albers, K.M.; Davis, B.M. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J. Neurosci. 2006, 26, 8588–8599. [Google Scholar] [CrossRef]
- Ikeda-Miyagawa, Y.; Kobayashi, K.; Yamanaka, H.; Okubo, M.; Wang, S.; Dai, Y.; Yagi, H.; Hirose, M.; Noguchi, K. Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons. Mol. Pain 2015, 11, 8. [Google Scholar] [CrossRef]
- Amaya, F.; Shimosato, G.; Nagano, M.; Ueda, M.; Hashimoto, S.; Tanaka, Y.; Suzuki, H.; Tanaka, M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur. J. Neurosci. 2004, 20, 2303–2310. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Na, S.; An, B.; Yang, S.R.; Kim, W.J.; Ha, K.S.; Han, E.T.; Park, W.S.; Lee, C.M.; Lee, J.Y.; et al. Paracrine influence of human perivascular cells on the proliferation of adenocarcinoma alveolar epithelial cells. Korean J. Physiol. Pharmacol. 2017, 21, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Alevy, Y.G.; Patel, A.C.; Romero, A.G.; Patel, D.A.; Tucker, J.; Roswit, W.T.; Miller, C.A.; Heier, R.F.; Byers, D.E.; Brett, T.J.; et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Investig. 2012, 122, 4555–4568. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Theresine, M.; Poli, A.; Domingues, O.; Ammerlaan, W.; Brons, N.H.; Hentges, F.; Zimmer, J. Increased th2 cytokine secretion, eosinophilic airway inflammation, and airway hyperresponsiveness in neurturin-deficient mice. J. Immunol. 2011, 186, 6497–6504. [Google Scholar] [CrossRef]
- Mauffray, M.; Domingues, O.; Hentges, F.; Zimmer, J.; Hanau, D.; Michel, T. Neurturin influences inflammatory responses and airway remodeling in different mouse asthma models. J. Immunol. 2015, 194, 1423–1433. [Google Scholar] [CrossRef]
- Pan, J.X.; Deng, F.L.; Zeng, B.H.; Zheng, P.; Liang, W.W.; Yin, B.M.; Wu, J.; Dong, M.X.; Luo, Y.Y.; Wang, H.Y.; et al. Absence of gut microbiota during early life affects anxiolytic Behaviors and monoamine neurotransmitters system in the hippocampal of mice. J. Neurol. Sci. 2019, 400, 160–168. [Google Scholar] [CrossRef]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef]
- Brun, P.; Gobbo, S.; Caputi, V.; Spagnol, L.; Schirato, G.; Pasqualin, M.; Levorato, E.; Palu, G.; Giron, M.C.; Castagliuolo, I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol. Cell Neurosci. 2015, 68, 24–35. [Google Scholar] [CrossRef]
- Romeo, G.; Ronchetto, P.; Luo, Y.; Barone, V.; Seri, M.; Ceccherini, I.; Pasini, B.; Bocciardi, R.; Lerone, M.; Kaariainen, H.; et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature 1994, 367, 377–378. [Google Scholar] [CrossRef]
- Edery, P.; Lyonnet, S.; Mulligan, L.M.; Pelet, A.; Dow, E.; Abel, L.; Holder, S.; Nihoul-Fekete, C.; Ponder, B.A.; Munnich, A. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature 1994, 367, 378–380. [Google Scholar] [CrossRef]
- Bordeaux, M.C.; Forcet, C.; Granger, L.; Corset, V.; Bidaud, C.; Billaud, M.; Bredesen, D.E.; Edery, P.; Mehlen, P. The RET proto-oncogene induces apoptosis: A novel mechanism for Hirschsprung disease. EMBO J. 2000, 19, 4056–4063. [Google Scholar] [CrossRef] [PubMed]
- Porokuokka, L.L.; Virtanen, H.T.; Linden, J.; Sidorova, Y.; Danilova, T.; Lindahl, M.; Saarma, M.; Andressoo, J.O. Gfra1 Underexpression Causes Hirschsprung’s Disease and Associated Enterocolitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 655–678. [Google Scholar] [CrossRef] [PubMed]
- Okragly, A.J.; Niles, A.L.; Saban, R.; Schmidt, D.; Hoffman, R.L.; Warner, T.F.; Moon, T.D.; Uehling, D.T.; Haak-Frendscho, M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J. Urol. 1999, 161, 438–441. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wang, Y.; Zhang, X.; Chen, L.; Sun, D. GDNF enhances the anti-inflammatory effect of human adipose-derived mesenchymal stem cell-based therapy in renal interstitial fibrosis. Stem. Cell. Res. 2019, 41, 101605. [Google Scholar] [CrossRef]
- Trevaskis, J.L.; Sacramento, C.B.; Jouihan, H.; Ali, S.; Le Lay, J.; Oldham, S.; Bhagroo, N.; Boland, B.B.; Cann, J.; Chang, Y.; et al. Neurturin and a GLP-1 Analogue Act Synergistically to Alleviate Diabetes in Zucker Diabetic Fatty Rats. Diabetes 2017, 66, 2007–2018. [Google Scholar] [CrossRef]
- Anitha, M.; Gondha, C.; Sutliff, R.; Parsadanian, A.; Mwangi, S.; Sitaraman, S.V.; Srinivasan, S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Investig. 2006, 116, 344–356. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Y.; Wang, Z.; Cui, W.; Peng, Y.; Li, R. Expression of glial cell line-derived neurotrophic factor and its receptors in cultured retinal Muller cells under high glucose circumstance. Anat. Rec. (Hoboken) 2012, 295, 532–539. [Google Scholar] [CrossRef]
- Lieu, T.; Kollarik, M.; Myers, A.C.; Undem, B.J. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L790–L798. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Lieu, T.M.; Myers, A.C.; Meeker, S.; Undem, B.J. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L941–L948. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Z.; Wang, H.; Li, T.; He, W.; Lv, W.; Zhang, J. Transcriptome Analysis Reveals Distinct Gene Expression Profiles in Eosinophilic and Noneosinophilic Chronic Rhinosinusitis with Nasal Polyps. Sci. Rep. 2016, 6, 26604. [Google Scholar] [CrossRef] [PubMed]
- Zissler, U.M.; Jakwerth, C.A.; Guerth, F.M.; Pechtold, L.; Aguilar-Pimentel, J.A.; Dietz, K.; Suttner, K.; Piontek, G.; Haller, B.; Hajdu, Z.; et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. EBioMedicine 2018, 36, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mendoza, S.; Macedo-Ramos, H.; Santos, F.A.; Quadros-de-Souza, L.C.; Paiva, M.M.; Pinto, T.C.; Teixeira, L.M.; Baetas-da-Cruz, W. Streptococcus pneumoniae infection regulates expression of neurotrophic factors in the olfactory bulb and cultured olfactory ensheathing cells. Neuroscience 2016, 317, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Sanders, K.M.; Youssef, M.R.; Yanushefski, K.M.; Jensen, L.E.; Yosipovitch, G.; Akiyama, T. Role of neurturin in spontaneous itch and increased nonpeptidergic intraepidermal fiber density in a mouse model of psoriasis. Pain 2017, 158, 2196–2202. [Google Scholar] [CrossRef]
- Hidaka, T.; Ogawa, E.; Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Fujimura, T.; Aiba, S.; Nakayama, K.; Okuyama, R.; et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017, 18, 64–73. [Google Scholar] [CrossRef]
- Edamitsu, T.; Taguchi, K.; Kobayashi, E.H.; Okuyama, R.; Yamamoto, M. Aryl Hydrocarbon Receptor Directly Regulates Artemin Gene Expression. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- Murota, H.; Izumi, M.; Abd El-Latif, M.I.; Nishioka, M.; Terao, M.; Tani, M.; Matsui, S.; Sano, S.; Katayama, I. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 671–682. [Google Scholar] [CrossRef]
- Otsuki, K.; Uchida, S.; Watanuki, T.; Wakabayashi, Y.; Fujimoto, M.; Matsubara, T.; Funato, H.; Watanabe, Y. Altered expression of neurotrophic factors in patients with major depression. J. Psychiatr Res. 2008, 42, 1145–1153. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Korn, C.; Garcia-Fernandez, M.; Domingues, O.; Villadiego, J.; Martin-Perez, D.; Isern, J.; Bejarano-Garcia, J.A.; Zimmer, J.; Perez-Simon, J.A.; et al. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood 2019, 133, 224–236. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B.M. Can Stopping Nerves, Stop Cancer? Trends Neurosci. 2016, 39, 880–889. [Google Scholar] [CrossRef]
- Fielder, G.C.; Yang, T.W.; Razdan, M.; Li, Y.; Lu, J.; Perry, J.K.; Lobie, P.E.; Liu, D.X. The GDNF Family: A Role in Cancer? Neoplasia 2018, 20, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Leider-Trejo, L.; Binenbaum, Y.; Kulish, N.; Fridman, E.; Shabtai-Orbach, A.; Wong, R.J.; Gil, Z. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene 2017, 36, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Eibl, G.; Duffy, J.P.; Reber, H.A.; Hines, O.J. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery 2003, 134, 293–299. [Google Scholar] [CrossRef]
- Meng, L.X.; Chi, Y.H.; Wang, X.X.; Ding, Z.J.; Fei, L.C.; Zhang, H.; Mou, L.; Cui, W.; Xue, Y.J. Neurotrophic artemin promotes motility and invasiveness of MIA PaCa-2 pancreatic cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qian, P.; Wu, Z.S.; Ren, X.; Steiner, M.; Bougen, N.M.; Liu, S.; Liu, D.X.; Zhu, T.; Lobie, P.E. Artemin stimulates radio-and chemo-resistance by promoting TWIST1-BCL-2-dependent cancer stem cell-like behavior in mammary carcinoma cells. J. Biol. Chem. 2012, 287, 42502–42515. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Chen, T.S.; Chiu, C.M.; Chang, L.K.; Liao, K.F.; Tan, H.M.; Yeh, W.L.; Chang, G.R.; Wang, M.Y.; Lu, D.Y. GDNF increases cell motility in human colon cancer through VEGF-VEGFR1 interaction. Endocr. Relat. Cancer 2014, 21, 73–84. [Google Scholar] [CrossRef]
- Jimenez, A.; Lopez-Ornelas, A.; Estudillo, E.; Gonzalez-Mariscal, L.; Gonzalez, R.O.; Segovia, J. A soluble form of GAS1 inhibits tumor growth and angiogenesis in a triple negative breast cancer model. Exp. Cell Res. 2014, 327, 307–317. [Google Scholar] [CrossRef]
- Andreucci, E.; Francica, P.; Fearns, A.; Martin, L.A.; Chiarugi, P.; Isacke, C.M.; Morandi, A. Targeting the receptor tyrosine kinase RET in combination with aromatase inhibitors in ER positive breast cancer xenografts. Oncotarget 2016, 7, 80543–80553. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Q.; Hou, J.; Gu, Y.; Zhang, Y.; Chen, Z.; Fan, J.; Zhou, W.; Qiu, S.; Zhang, Y.; et al. Tumor-Induced Generation of Splenic Erythroblast-like Ter-Cells Promotes Tumor Progression. Cell 2018, 173, 634–648. [Google Scholar] [CrossRef]
- Grieco, M.; Santoro, M.; Berlingieri, M.T.; Melillo, R.M.; Donghi, R.; Bongarzone, I.; Pierotti, M.A.; Della Porta, G.; Fusco, A.; Vecchio, G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 1990, 60, 557–563. [Google Scholar] [CrossRef]
- Hofstra, R.M.; Landsvater, R.M.; Ceccherini, I.; Stulp, R.P.; Stelwagen, T.; Luo, Y.; Pasini, B.; Hoppener, J.W.; Van Amstel, H.K.; Romeo, G.; et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994, 367, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Drilon, A.; Poirier, J.T. RET mutations in neuroendocrine tumors: Including small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; McCusker, M.G.; Russo, A.; Scilla, K.A.; Gittens, A.; Arensmeyer, K.; Mehra, R.; Adamo, V.; Rolfo, C. RET fusions in solid tumors. Cancer Treat. Rev. 2019, 81, 101911. [Google Scholar] [CrossRef]
- Nencini, S.; Ringuet, M.; Kim, D.H.; Greenhill, C.; Ivanusic, J.J. GDNF, Neurturin, and Artemin Activate and Sensitize Bone Afferent Neurons and Contribute to Inflammatory Bone Pain. J. Neurosci. 2018, 38, 4899–4911. [Google Scholar] [CrossRef]
- Malin, S.; Molliver, D.; Christianson, J.A.; Schwartz, E.S.; Cornuet, P.; Albers, K.M.; Davis, B.M. TRPV1 and TRPA1 function and modulation are target tissue dependent. J. Neurosci. 2011, 31, 10516–10528. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, B.S.; Roy, S.; Hingtgen, C.M. Glial cell line-derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons. Neuroscience 2009, 161, 148–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lippoldt, E.K.; Elmes, R.R.; McCoy, D.D.; Knowlton, W.M.; McKemy, D.D. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J. Neurosci. 2013, 33, 12543–12552. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.P.; Mohammadi, E.; Ligon, C.O.; Johnson, A.C.; Gershon, M.D.; Rao, M.; Shen, Y.; Chan, C.C.; Eidam, H.S.; DeMartino, M.P.; et al. Exploring the Potential of RET Kinase Inhibition for Irritable Bowel Syndrome: A Preclinical Investigation in Rodent Models of Colonic Hypersensitivity. J. Pharmacol. Exp. Ther. 2019, 368, 299–307. [Google Scholar] [CrossRef]
- DeBerry, J.J.; Saloman, J.L.; Dragoo, B.K.; Albers, K.M.; Davis, B.M. Artemin Immunotherapy Is Effective in Preventing and Reversing Cystitis-Induced Bladder Hyperalgesia via TRPA1 Regulation. J. Pain. 2015, 16, 628–636. [Google Scholar] [CrossRef]
- Merighi, A. Targeting the glial-derived neurotrophic factor and related molecules for controlling normal and pathologic pain. Expert Opin. Ther. Targets 2016, 20, 193–208. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Saarma, M. Glial cell line-derived neurotrophic factor family ligands and their therapeutic potential. Mol. Biol. (Mosk) 2016, 50, 589–598. [Google Scholar] [CrossRef] [PubMed]
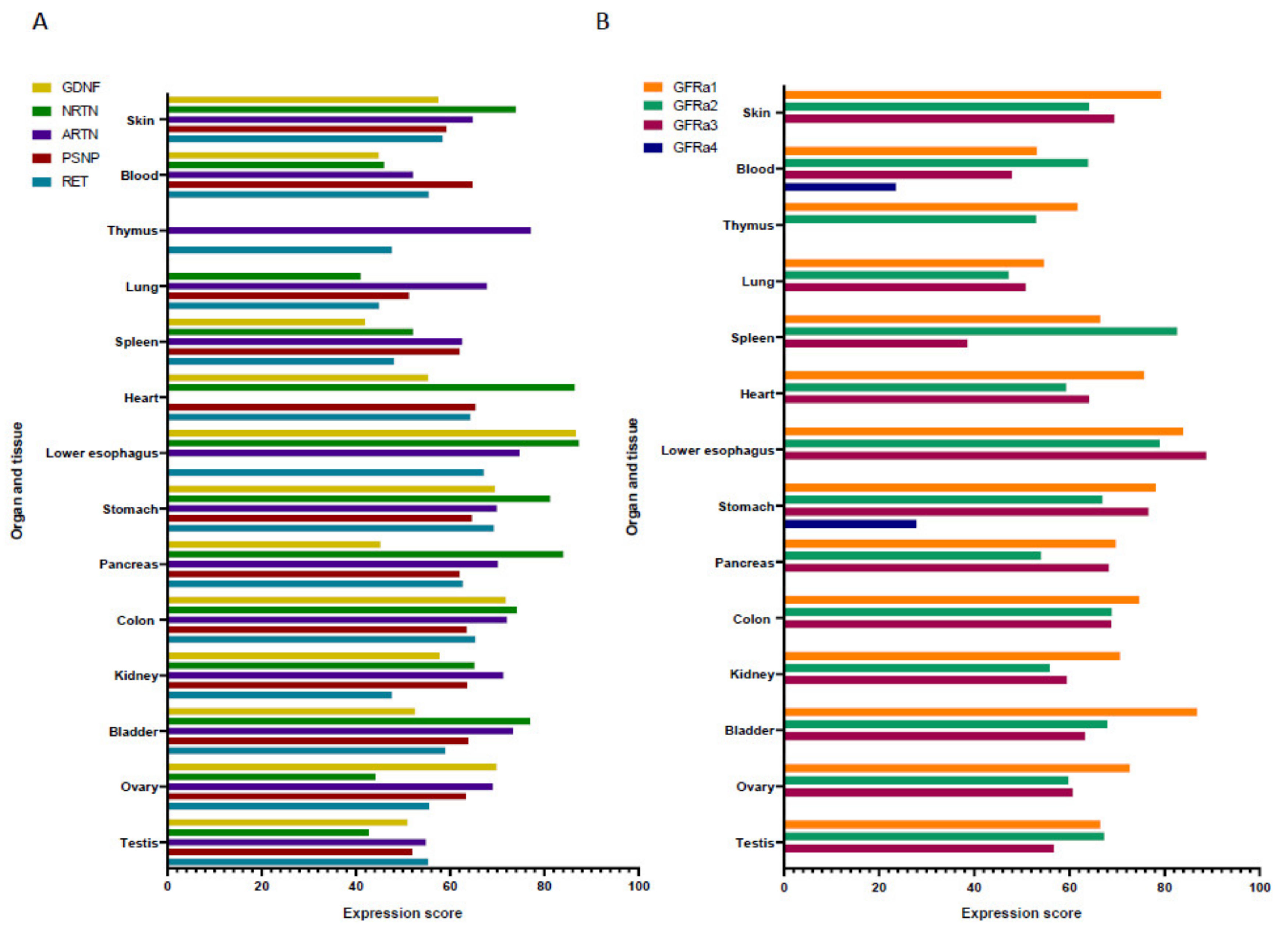
| Pathology | GFL/Receptor Involved | Species | Expression Status or Related Mechanism of Action |
|---|---|---|---|
| Hirschsprung’s disease | RET | Human | Loss-of-function mutation |
| GFRα1 | Mouse | Reduction of expression | |
| Crohn’s disease | GDNF | Human | Upregulation |
| Colitis | GDNF | Mouse | Expressed by enteric glial cells, regulates intestinal epithelial barrier integrity |
| Interstitial cystitis | GDNF | Human | Upregulation |
| Renal interstitial fibrosis | GDNF | Mouse | Expressed by adipose-derived mesenchymal stem cells, regulates macrophage activity |
| Diabetes | NRTN | Rat | Used for injections, prevents hyperglycemia |
| GDNF | Mouse | Prevents enteric neuronal apoptosis via PI3K/Akt signaling activation | |
| Diabetic retinopathy | GDNF and GFRα1 | Rat’s cultured Müller cells | Expressed under high glucose conditions, protecting role |
| Asthma | GDNF | Guinea pig | Allergen sensitization induces its expression in airway mucosa and tracheal epithelium |
| NRTN | Mouse | Inactivation of the molecule increases inflammation and airway remodelling markers | |
| Eosinophilic chronic rhinosinusitis | GFRα2 and NCAM | Human | Downregulation in nasal polyps |
| Grass-pollen allergy | GFRα1–4, GDNF and NCAM | Human | Downregulation in nasal samples from patients under allergen-specific immunotherapy |
| Streptococcus pneumoniae infection | GDNF | Mouse | Downregulation in the olfactory bulb |
| Psoriasis | NRTN | Human | Upregulation in the skin |
| NRTN and GFRα2 | Mouse | Blocking the pathway reduces nonpeptidergic nerve density | |
| Atopic dermatitis | ARTN | Human | Upregulation in epidermis via activation of AhR |
| Accumulates in dermal fibroblasts and induces epidermal hyper-innervation | |||
| Mood disorder | GDNF and ARTN | Human | Downregulation in blood |
| Cancer | All GFL’s | Human | Upregulation in a variety of cancer cells of epithelial origin, associated with malignant progression and poor prognosis |
| GDNF, ARTN, GFRα1 and RET | Stimulates radio and chemoresistance via autophagy, mitogenesis and neutralizing apoptosis | ||
| Pancreatic cancer | GDNF | Mouse | Inhibition of its expression from endoneurial macrophages reduces perineural invasion |
| All GFL’s | Human | Enhances integrin expression and the upregulation of MMP | |
| Breast cancer | ARTN | Human cell lines | Promotes angiogenesis and metastasis via TWIST1-VEGF-A signaling |
| Colon cancer | GDNF | Human | Increases cancer cell migration via VEGF-VEGFR interaction |
| Breast cancer and glioma | GDNF and RET | Human cell lines | Blocking of the pathway leads to the impairment of tumor growth |
| Hepatocellular carcinoma | GFRα3, RET and ARTN | Human | Upregulation, correlates with poor prognosis |
| ARTN | Mouse | Expressed by tumor-inducible erythroblast-like cells, promotes HCC survival and invasion | |
| Neuroendocrine tumors | RET | Human | Loss- of-function mutation leads to papillary, medullary thyroid carcinoma and neuroendocrine small cell lung cancers |
| Pain sensitivity | GDNF, NRTN, ARTN and GFRa3 | Mouse | Sensitivity to heat and cold via TRPV1 signaling |
| Inflammatory bone pain | GDNF, NRTN, GFRα1–2 | Rat | Via activation and sensitization of nonpeptidergic neurons |
| Abdominal pain (IBS) | RET | Rat | Inhibition attenuates the number of abdominal contractions via visceral nociception |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morel, L.; Domingues, O.; Zimmer, J.; Michel, T. Revisiting the Role of Neurotrophic Factors in Inflammation. Cells 2020, 9, 865. https://doi.org/10.3390/cells9040865
Morel L, Domingues O, Zimmer J, Michel T. Revisiting the Role of Neurotrophic Factors in Inflammation. Cells. 2020; 9(4):865. https://doi.org/10.3390/cells9040865
Chicago/Turabian StyleMorel, Lucas, Olivia Domingues, Jacques Zimmer, and Tatiana Michel. 2020. "Revisiting the Role of Neurotrophic Factors in Inflammation" Cells 9, no. 4: 865. https://doi.org/10.3390/cells9040865
APA StyleMorel, L., Domingues, O., Zimmer, J., & Michel, T. (2020). Revisiting the Role of Neurotrophic Factors in Inflammation. Cells, 9(4), 865. https://doi.org/10.3390/cells9040865





