Extracellular Vesicles in Smoking-Mediated HIV Pathogenesis and their Potential Role in Biomarker Discovery and Therapeutic Interventions
Abstract
1. Introduction
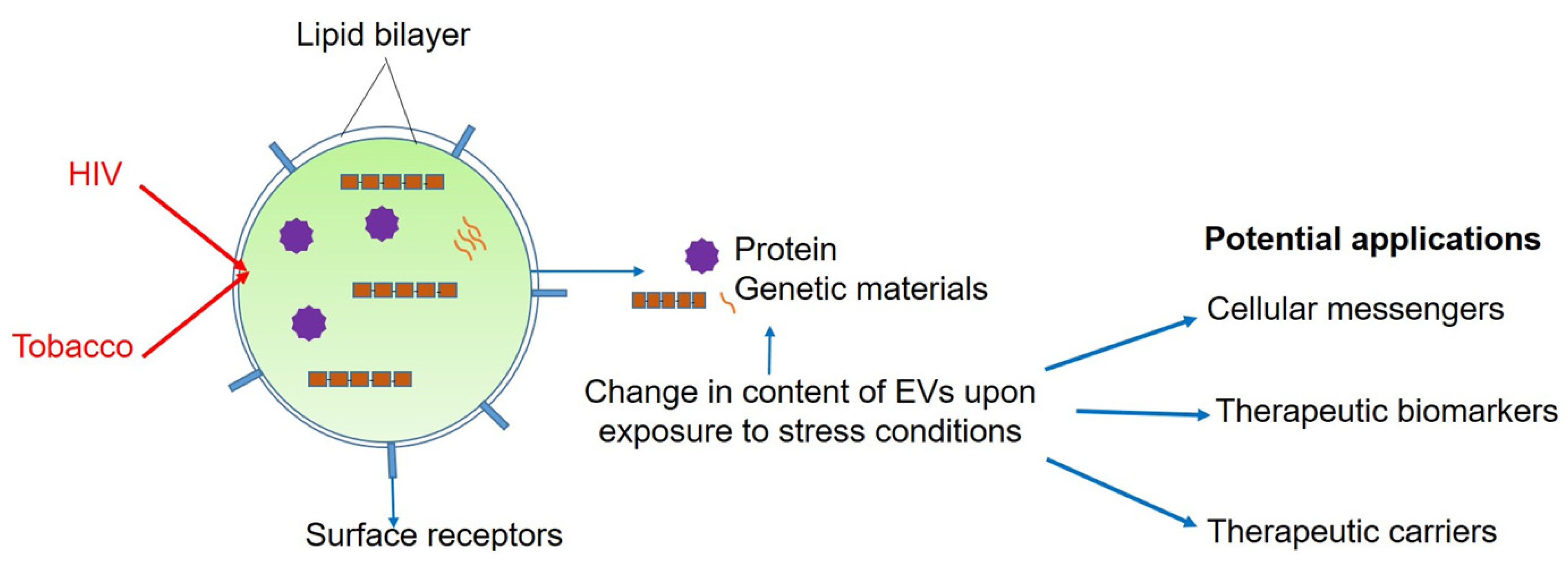
2. Extracellular Vesicles-a Sneak Peek
2.1. Advances Since 2015
2.1.1. Isolation Techniques and Characterization Methods
2.1.2. EVs as Intercellular Messengers
3. Role of EVs in HIV
Advances Since 2015
4. Role of EVs in HIV and Tobacco-Smoking
Advances Since 2015
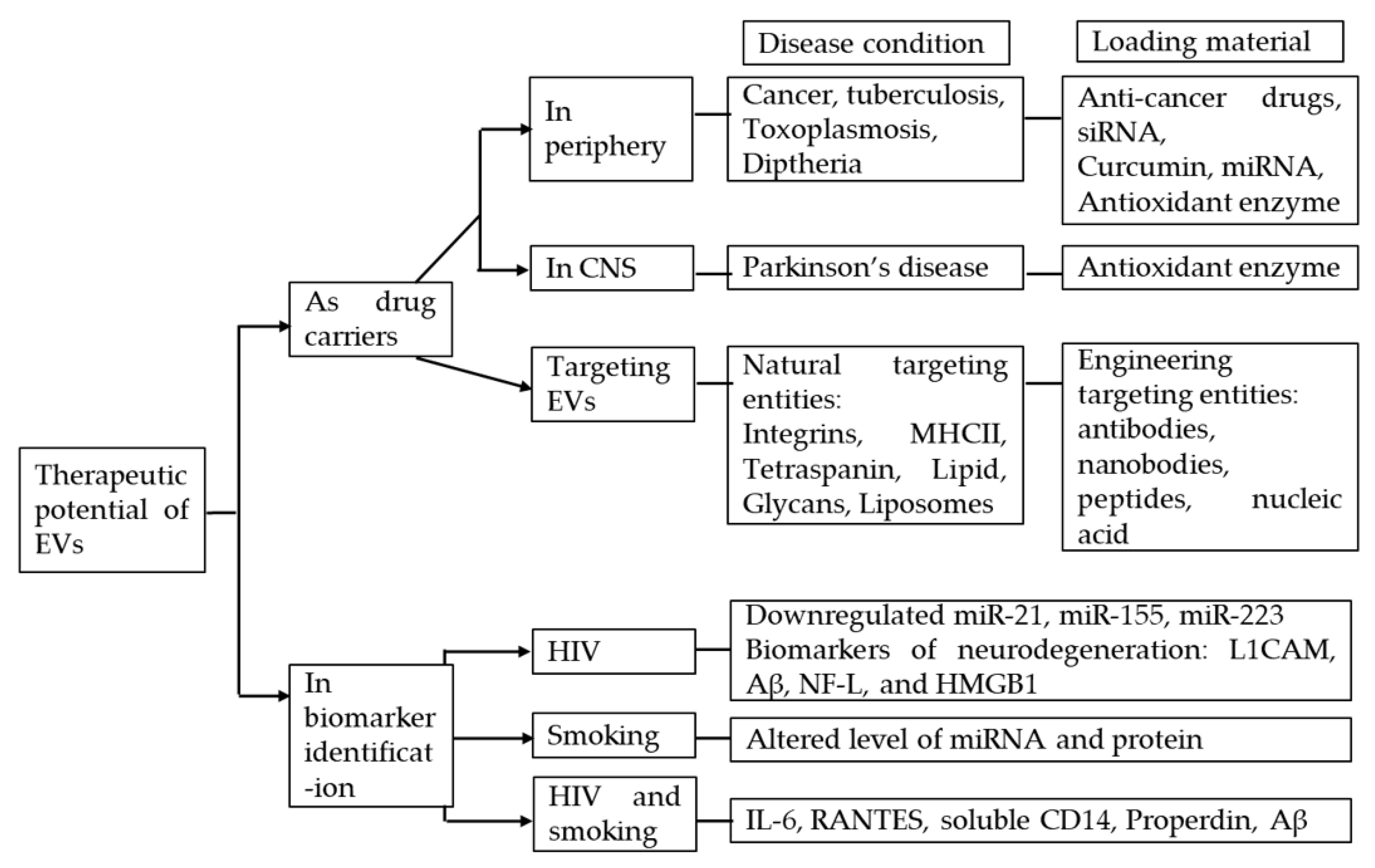
5. Therapeutic Potential of Using EVs in HIV-Tobacco Smoking Comorbidity
5.1. EVs as Drug Carriers
5.1.1. In the Periphery
5.1.2. In CNS
5.1.3. Targeting EVs
5.2. EVs in Biomarker Identification
5.2.1. EVs in HIV
5.2.2. EVs in HIV and Smoking Comorbidity
6. Conclusion and Future Prospects
Funding
Conflicts of Interest
References
- HIV.Gov. The Global HIV/AIDS Epidemic. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 31 March 2020).
- Teeraananchai, S.; Kerr, S.J.; Amin, J.; Ruxrungtham, K.; Law, M.G. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. Hiv Med. 2017, 18, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Logan, R.; Sterne, J.A.; Hernandez-Diaz, S.; Robins, J.M.; Sabin, C.; Bansi, L.; van Sighem, A.; de Wolf, F.; Costagliola, D.; et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids 2010, 24, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Romley, J.A.; Juday, T.; Solomon, M.D.; Seekins, D.; Brookmeyer, R.; Goldman, D.P. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996-2009. Health Aff. 2014, 33, 370–377. [Google Scholar] [CrossRef]
- Reddy, K.P.; Parker, R.A.; Losina, E.; Baggett, T.P.; Paltiel, A.D.; Rigotti, N.A.; Weinstein, M.C.; Freedberg, K.A.; Walensky, R.P. Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. J. Infect. Dis. 2016, 214, 1672–1681. [Google Scholar] [CrossRef]
- Reynolds, N.R. Cigarette smoking and HIV: More evidence for action. Aids Educ. Prev. Off. Publ. Int. Soc. Aids Educ. 2009, 21, 106–121. [Google Scholar] [CrossRef]
- Helleberg, M.; Afzal, S.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Pedersen, C.; Gerstoft, J.; Nordestgaard, B.G.; Obel, N. Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 727–734. [Google Scholar] [CrossRef]
- Helleberg, M.; May, M.T.; Ingle, S.M.; Dabis, F.; Reiss, P.; Fatkenheuer, G.; Costagliola, D.; d’Arminio, A.; Cavassini, M.; Smith, C.; et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. Aids 2015, 29, 221–229. [Google Scholar] [CrossRef]
- Shuter, J.; Bernstein, S.L. Cigarette smoking is an independent predictor of nonadherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2008, 10, 731–736. [Google Scholar] [CrossRef]
- Kariuki, W.; Manuel, J.I.; Kariuki, N.; Tuchman, E.; O’Neal, J.; Lalanne, G.A. HIV and smoking: Associated risks and prevention strategies. Hiv Aids 2016, 8, 17–36. [Google Scholar] [CrossRef]
- Brath, H.; Grabovac, I.; Schalk, H.; Degen, O.; Dorner, T.E. Prevalence and Correlates of Smoking and Readiness to Quit Smoking in People Living with HIV in Austria and Germany. PLoS ONE 2016, 11, e0150553. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Sinha, N.; Kodidela, S.; Kumar, S. Benzo(a)pyrene in Cigarette Smoke Enhances HIV-1 Replication through NF-kappaB Activation via CYP-Mediated Oxidative Stress Pathway. Sci. Rep. 2018, 8, 10394. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Ande, A.; Sinha, N.; Kumar, A.; Kumar, S. Effects of Cigarette Smoke Condensate on Oxidative Stress, Apoptotic Cell Death, and HIV Replication in Human Monocytic Cells. PLoS ONE 2016, 11, e0155791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rao, P.; Sinha, N.; Midde, N.M. Cytochrome P450 and Oxidative Stress as Possible Pathways for Alcohol- and Tobacco-Mediated HIV Pathogenesis and NeuroAIDS. In Neuropathology of Drug Addictions and Substance Misuse; V.R., P., Ed.; 2016; Volume 1, pp. 179–188. [Google Scholar]
- Ranjit, S.; Midde, N.M.; Sinha, N.; Patters, B.J.; Rahman, M.A.; Cory, T.J.; Rao, P.S.; Kumar, S. Effect of Polyaryl Hydrocarbons on Cytotoxicity in Monocytic Cells: Potential Role of Cytochromes P450 and Oxidative Stress Pathways. PLoS ONE 2016, 11, e0163827. [Google Scholar] [CrossRef]
- Ande, A.; McArthur, C.; Ayuk, L.; Awasom, C.; Achu, P.N.; Njinda, A.; Sinha, N.; Rao, P.S.; Agudelo, M.; Nookala, A.R.; et al. Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals. PLoS ONE 2015, 10, e0122402. [Google Scholar] [CrossRef]
- Ande, A.; McArthur, C.; Kumar, A.; Kumar, S. Tobacco smoking effect on HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1453–1464. [Google Scholar] [CrossRef]
- Rumbaugh, J.A.; Tyor, W. HIV-associated neurocognitive disorders: Five new things. Neurology. Clin. Pract. 2015, 5, 224–231. [Google Scholar] [CrossRef]
- Sacktor, N.; McDermott, M.P.; Marder, K.; Schifitto, G.; Selnes, O.A.; McArthur, J.C.; Stern, Y.; Albert, S.; Palumbo, D.; Kieburtz, K.; et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirology 2002, 8, 136–142. [Google Scholar] [CrossRef]
- Cantres-Rosario, Y.M.; Ortiz-Rodriguez, S.C.; Santos-Figueroa, A.G.; Plaud, M.; Negron, K.; Cotto, B.; Langford, D.; Melendez, L.M. HIV Infection Induces Extracellular Cathepsin B Uptake and Damage to Neurons. Sci. Rep. 2019, 9, 8006. [Google Scholar] [CrossRef]
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier. Brain Res. 2011, 1399, 96–115. [Google Scholar] [CrossRef]
- Atluri, V.S.; Hidalgo, M.; Samikkannu, T.; Kurapati, K.R.; Jayant, R.D.; Sagar, V.; Nair, M.P. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: An update. Front. Cell. Neurosci. 2015, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.D.; Dochney, J.A.; Blazekovic, S.; Leone, F.; Metzger, D.; Frank, I.; Gross, R.; Hole, A.; Mounzer, K.; Siegel, S.; et al. The nature and consequences of cognitive deficits among tobacco smokers with HIV: A comparison to tobacco smokers without HIV. J. Neurovirology 2017, 23, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lim, A.; Lau, E.; Alicata, D. Chronic Tobacco-Smoking on Psychopathological Symptoms, Impulsivity and Cognitive Deficits in HIV-Infected Individuals. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2017, 12, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bryant, V.E.; Kahler, C.W.; Devlin, K.N.; Monti, P.M.; Cohen, R.A. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. Aids Care 2013, 25, 1308–1316. [Google Scholar] [CrossRef]
- Wojna, V.; Robles, L.; Skolasky, R.L.; Mayo, R.; Selnes, O.; de la Torre, T.; Maldonado, E.; Nath, A.; Melendez, L.M.; Lasalde-Dominicci, J. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J. Neurovirology 2007, 13, 561–568. [Google Scholar] [CrossRef]
- Tsima, B.; Ratcliffe, S.J.; Schnoll, R.; Frank, I.; Kolson, D.L.; Gross, R. Is Tobacco Use Associated with Neurocognitive Dysfunction in Individuals with HIV? J. Int. Assoc. Provid. Aids Care 2018, 17, 2325958218768018. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. Understanding extracellular vesicle diversity-current status. Expert Rev. Proteom. 2018, 15, 887–910. [Google Scholar] [CrossRef]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewska, P.; Belting, M. Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Stapleton, J.T.; Okeoma, C.M. Vehicles of intercellular communication: Exosomes and HIV-1. J. Gen. Virol. 2019, 100, 350–366. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef]
- Kodidela, S.; Ranjit, S.; Sinha, N.; McArthur, C.; Kumar, A.; Kumar, S. Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS ONE 2018, 13, e0201144. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Patters, B.J.; Gerth, K.A.; Haque, S.; Choudhary, S.; Kumar, S. Potential neuroprotective role of astroglial exosomes against smoking-induced oxidative stress and HIV-1 replication in the central nervous system. Expert Opin. Ther. Targets 2018, 22, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Mussig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kucherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. New Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Vanhamel, J.; Bruggemans, A.; Debyser, Z. Establishment of latent HIV-1 reservoirs: What do we really know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar]
- Kruize, Z.; Kootstra, N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front. Microbiol. 2019, 10, 2828. [Google Scholar] [CrossRef] [PubMed]
- Van Marle, G.; Church, D.L.; van der Meer, F.; Gill, M.J. Combating the HIV reservoirs. Biotechnol. Genet. Eng. Rev. 2018, 34, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Wacleche, V.S.; Tremblay, C.L.; Routy, J.P.; Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.; Margolis, D.M. HIV Persistence on Antiretroviral Therapy and Barriers to a Cure. Adv. Exp. Med. Biol. 2018, 1075, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Chinnappan, M.; Agarwal, S.; Dalvi, P.; Gunewardena, S.; O’Brien-Ladner, A.; Dhillon, N.K. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: Role of altered miRNA cargo in response to HIV infection and substance abuse. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 5174–5185. [Google Scholar] [CrossRef]
- Tang, N.; Sun, B.; Gupta, A.; Rempel, H.; Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3097–3106. [Google Scholar] [CrossRef]
- Kodidela, S.; Wang, Y.; Patters, B.J.; Gong, Y.; Sinha, N.; Ranjit, S.; Gerth, K.; Haque, S.; Cory, T.; McArthur, C.; et al. Proteomic Profiling of Exosomes Derived from Plasma of HIV-Infected Alcohol Drinkers and Cigarette Smokers. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Harding, C.; Stahl, P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun. 1983, 113, 650–658. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Rahman, M.A.; Patters, B.J.; Kodidela, S.; Kumar, S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gheinani, A.H.; Vögeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. Cmls 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Heinemann, M.L.; Ilmer, M.; Silva, L.P.; Hawke, D.H.; Recio, A.; Vorontsova, M.A.; Alt, E.; Vykoukal, J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatography. A 2014, 1371, 125–135. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Cantin, R.; Diou, J.; Belanger, D.; Tremblay, A.M.; Gilbert, C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J. Immunol. Methods 2008, 338, 21–30. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, Chapter 3, Unit 3.22. [Google Scholar] [CrossRef]
- Taylor, D.D.; Zacharias, W.; Gercel-Taylor, C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol. (Cliftonn. J.) 2011, 728, 235–246. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Aras, O.; Shet, A.; Bach, R.R.; Hysjulien, J.L.; Slungaard, A.; Hebbel, R.P.; Escolar, G.; Jilma, B.; Key, N.S. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood 2004, 103, 4545–4553. [Google Scholar] [CrossRef]
- Sharma, S.; Gillespie, B.M.; Palanisamy, V.; Gimzewski, J.K. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir Acs J. Surf. Colloids 2011, 27, 14394–14400. [Google Scholar] [CrossRef]
- Thery, C.; Duban, L.; Segura, E.; Veron, P.; Lantz, O.; Amigorena, S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Thery, C. Exosomes: Secreted vesicles and intercellular communications. F1000 biology reports 2011, 3, 15. [Google Scholar] [CrossRef]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Thery, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Cossetti, C.; Iraci, N.; Mercer, T.R.; Leonardi, T.; Alpi, E.; Drago, D.; Alfaro-Cervello, C.; Saini, H.K.; Davis, M.P.; Schaeffer, J.; et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell 2014, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Klein-Scory, S.; Tehrani, M.M.; Eilert-Micus, C.; Adamczyk, K.A.; Wojtalewicz, N.; Schnolzer, M.; Hahn, S.A.; Schmiegel, W.; Schwarte-Waldhoff, I. New insights in the composition of extracellular vesicles from pancreatic cancer cells: Implications for biomarkers and functions. Proteome Sci. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancre, V.; de Launoit, Y.; Busson, P.; et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl. Cancer Inst. 2015, 107, 363. [Google Scholar] [CrossRef]
- Rolfo, C.; Castiglia, M.; Hong, D.; Alessandro, R.; Mertens, I.; Baggerman, G.; Zwaenepoel, K.; Gil-Bazo, I.; Passiglia, F.; Carreca, A.P.; et al. Liquid biopsies in lung cancer: The new ambrosia of researchers. Biochim. Et Biophys. Acta 2014, 1846, 539–546. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Baj-Krzyworzeka, M.; Baran, J. Characterization and biological role of extracellular vesicles. Postepy Hig. I Med. Dosw. 2014, 68, 1421–1432. [Google Scholar] [CrossRef]
- Vella, L.J. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front. Oncol. 2014, 4, 361. [Google Scholar] [CrossRef]
- Villanueva, M.T. Small containers, important cargo. Nat. Rev. Cancer 2014, 14, 764–765. [Google Scholar] [CrossRef]
- Andaloussi, S.E.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Reviews. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Yu, Q.; He, J.J. Exosome-associated hepatitis C virus in cell cultures and patient plasma. Biochem. Biophys. Res. Commun. 2014, 455, 218–222. [Google Scholar] [CrossRef]
- Narayanan, A.; Iordanskiy, S.; Das, R.; Van Duyne, R.; Santos, S.; Jaworski, E.; Guendel, I.; Sampey, G.; Dalby, E.; Iglesias-Ussel, M.; et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 2013, 288, 20014–20033. [Google Scholar] [CrossRef] [PubMed]
- Sampey, G.C.; Meyering, S.S.; Zadeh, M.A.; Saifuddin, M.; Hakami, R.M.; Kashanchi, F. Exosomes and their role in CNS viral infections. J. Neurovirology 2014, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Patman, G. Hepatitis: Exosomal route of HCV transmission exists in patients. Nat. Reviews. Gastroenterol. Hepatol. 2014, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. London. Ser. Bbiological Sci. 2014, 369. [Google Scholar] [CrossRef]
- Xu, R.; Simpson, R.J.; Greening, D.W. A Protocol for Isolation and Proteomic Characterization of Distinct Extracellular Vesicle Subtypes by Sequential Centrifugal Ultrafiltration. Methods Mol. Biol. 2017, 1545, 91–116. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Welton, J.L.; Webber, J.P.; Botos, L.A.; Jones, M.; Clayton, A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J. Extracell. Vesicles 2015, 4, 27269. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 32. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Juan, T.; Furthauer, M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int. J. Mol. Sci. 2019, 21, 107. [Google Scholar] [CrossRef]
- Shahjin, F.; Guda, R.S.; Schaal, V.L.; Odegaard, K.; Clark, A.; Gowen, A.; Xiao, P.; Lisco, S.J.; Pendyala, G.; Yelamanchili, S.V. Brain-Derived Extracellular Vesicle microRNA Signatures Associated with In Utero and Postnatal Oxycodone Exposure. Cells 2019, 9, 21. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kodidela, S.; Sinha, N.; Haque, S.; Shukla, P.K.; Rao, R.; Kumar, S. Plasma exosomes exacerbate alcohol- and acetaminophen-induced toxicity via CYP2E1 pathway. Sci. Rep. 2019, 9, 6571. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, S.; Tong, X.; Fan, H. Extracellular vesicles and ctDNA in lung cancer: Biomarker sources and therapeutic applications. Cancer Chemother. Pharmacol. 2018, 82, 171–183. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Reviews. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Rowland, A.; Ruanglertboon, W.; van Dyk, M.; Wijayakumara, D.; Wood, L.S.; Meech, R.; Mackenzie, P.I.; Rodrigues, A.D.; Marshall, J.C.; Sorich, M.J. Plasma extracellular nanovesicle (exosome)-derived biomarkers for drug metabolism pathways: A novel approach to characterize variability in drug exposure. Br. J. Clin. Pharmacol. 2019, 85, 216–226. [Google Scholar] [CrossRef]
- Dias, M.V.S.; Costa, C.S.; daSilva, L.L.P. The Ambiguous Roles of Extracellular Vesicles in HIV Replication and Pathogenesis. Front. Microbiol. 2018, 9, 2411. [Google Scholar] [CrossRef]
- Li, M.; Aliotta, J.M.; Asara, J.M.; Tucker, L.; Quesenberry, P.; Lally, M.; Ramratnam, B. Quantitative proteomic analysis of exosomes from HIV-1-infected lymphocytic cells. Proteomics 2012, 12, 2203–2211. [Google Scholar] [CrossRef]
- Soares, H. HIV-1 Intersection with CD4 T Cell Vesicle Exocytosis: Intercellular Communication Goes Viral. Front. Immunol. 2014, 5, 454. [Google Scholar] [CrossRef][Green Version]
- Qiao, X.; He, B.; Chiu, A.; Knowles, D.M.; Chadburn, A.; Cerutti, A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 2006, 7, 302–310. [Google Scholar] [CrossRef]
- Aquaro, S.; Scopelliti, F.; Pollicita, M.; Perno, C.F. Oxidative stress and HIV infection: Target pathways for novel therapies? Future Hiv Ther. 2008, 2, 327–338. [Google Scholar] [CrossRef]
- Gil, L.; Martinez, G.; Gonzalez, I.; Tarinas, A.; Alvarez, A.; Giuliani, A.; Molina, R.; Tapanes, R.; Perez, J.; Leon, O.S. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol. Res. 2003, 47, 217–224. [Google Scholar] [CrossRef]
- Elbim, C.; Pillet, S.; Prevost, M.H.; Preira, A.; Girard, P.M.; Rogine, N.; Matusani, H.; Hakim, J.; Israel, N.; Gougerot-Pocidalo, M.A. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: Relationship with viral load. J. Virol. 1999, 73, 4561–4566. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bazdar, D.A.; Lederman, M.M.; Funderburg, N.; Sieg, S.F. Decreased IL-7 responsiveness is related to oxidative stress in HIV disease. PLoS ONE 2013, 8, e58764. [Google Scholar] [CrossRef]
- Aukrust, P.; Luna, L.; Ueland, T.; Johansen, R.F.; Muller, F.; Froland, S.S.; Seeberg, E.C.; Bjoras, M. Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood 2005, 105, 4730–4735. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, H.; Zhang, H.; Shi, Y.; Wei, F.; Liu, D.; Liu, K.; Chen, D. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012, 1458, 1–11. [Google Scholar] [CrossRef]
- Hubert, A.; Subra, C.; Jenabian, M.A.; Tremblay Labrecque, P.F.; Tremblay, C.; Laffont, B.; Provost, P.; Routy, J.P.; Gilbert, C. Elevated Abundance, Size, and MicroRNA Content of Plasma Extracellular Vesicles in Viremic HIV-1+ Patients: Correlations With Known Markers of Disease Progression. Journal of acquired immune deficiency syndromes (1999) 2015, 70, 219–227. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 7227. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.M.; Xu, Y.M.; Huang, L.F.; Wang, X.Z. Exosomes: Novel biomarkers for clinical diagnosis. TheScientificWorldJournal 2015, 2015, 657086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gupta, D.; Shankar, S.; Srivastava, R.K. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget 2015, 6, 3280–3291. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.D.; Suzuki, K.; Law, M.; Trebicka, J.; Neuhaus Nordwall, J.; Johnson, M.; Vjecha, M.J.; Kelleher, A.D.; Emery, S. Circulating miR-122 and miR-200a as biomarkers for fatal liver disease in ART-treated, HIV-1-infected individuals. Sci. Rep. 2017, 7, 10934. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; Gerth, K.; Haque, S.; Gong, Y.; Ismael, S.; Singh, A.; Tauheed, I.; Kumar, S. Extracellular Vesicles: A Possible Link between HIV and Alzheimer’s Disease-Like Pathology in HIV Subjects? Cells 2019, 8, 968. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirology 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. Aids (Lond. Engl.) 2017, 31, F9–F17. [Google Scholar] [CrossRef]
- Buhl, R.; Jaffe, H.A.; Holroyd, K.J.; Wells, F.B.; Mastrangeli, A.; Saltini, C.; Cantin, A.M.; Crystal, R.G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet (Lond. Engl.) 1989, 2, 1294–1298. [Google Scholar] [CrossRef]
- Morris, A.; George, M.P.; Crothers, K.; Huang, L.; Lucht, L.; Kessinger, C.; Kleerup, E.C. HIV and chronic obstructive pulmonary disease: Is it worse and why? Proc. Am. Thorac. Soc. 2011, 8, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.B.; Langkamp-Henken, B.; Bender, B.S.; Findley, K.; Herrlinger-Garcia, K.A.; Uphold, C.R. Oxidative stress and antioxidant capacity in smoking and nonsmoking men with HIV/acquired immunodeficiency syndrome. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2005, 20, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, F.; Zhang, Y.; Elbourkadi, N.; Wang, Z.; Yu, C.; Taylor, E.W. Mechanisms and genes involved in enhancement of HIV infectivity by tobacco smoke. Toxicology 2010, 278, 242–248. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Eldh, M.; Ekstrom, K.; Valadi, H.; Sjostrand, M.; Olsson, B.; Jernas, M.; Lotvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef]
- Steel, H.C.; Venter, W.D.F.; Theron, A.J.; Anderson, R.; Feldman, C.; Kwofie, L.; Cronje, T.; Arullapan, N.; Rossouw, T.M. Effects of Tobacco Usage and Antiretroviral Therapy on Biomarkers of Systemic Immune Activation in HIV-Infected Participants. Mediat. Inflamm. 2018, 2018, 8357109. [Google Scholar] [CrossRef]
- Rossouw, T.M.; Anderson, R.; Feldman, C. Impact of HIV infection and smoking on lung immunity and related disorders. Eur. Respir. J. 2015, 46, 1781–1795. [Google Scholar] [CrossRef]
- Agus Somia, I.K.; Merati, T.P.; Bakta, I.M.; Putra Manuaba, I.B.; Yasa, W.P.S.; Sukrama, I.D.M.; Suryana, K.; Wisaksana, R. High levels of serum IL-6 and serum hepcidin and low CD4 cell count were risk factors of anemia of chronic disease in HIV patients on the combination of antiretroviral therapy. Hiv/Aids 2019, 11, 133–139. [Google Scholar] [CrossRef]
- Borges, A.H.; O’Connor, J.L.; Phillips, A.N.; Ronsholt, F.F.; Pett, S.; Vjecha, M.J.; French, M.A.; Lundgren, J.D. Factors Associated With Plasma IL-6 Levels During HIV Infection. J. Infect. Dis. 2015, 212, 585–595. [Google Scholar] [CrossRef]
- Cioe, P.A.; Baker, J.; Hammer, J.; Kojic, E.M.; Onen, N.; Patel, P.; Kahler, C.W. Soluble CD14 and D-dimer are associated with smoking and heavy alcohol use in HIV-Infected dults. In Proceedings of Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 3–6 March 2014. [Google Scholar]
- Theron, A.J.; Anderson, R.; Rossouw, T.M.; Steel, H.C. The Role of Transforming Growth Factor Beta-1 in the Progression of HIV/AIDS and Development of Non-AIDS-Defining Fibrotic Disorders. Front. Immunol. 2017, 8, 1461. [Google Scholar] [CrossRef]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lasser, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lotvall, J. Endosomal signalling via exosome surface TGFbeta-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef]
- Hu, G.; Niu, F.; Liao, K.; Periyasamy, P.; Sil, S.; Liu, J.; Dravid, S.M.; Buch, S. HIV-1 Tat-Induced Astrocytic Extracellular Vesicle miR-7 Impairs Synaptic Architecture. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef]
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2018, 13, 330–344. [Google Scholar] [CrossRef]
- Ojha, C.R.; Lapierre, J.; Rodriguez, M.; Dever, S.M.; Zadeh, M.A.; DeMarino, C.; Pleet, M.L.; Kashanchi, F.; El-Hage, N. Interplay between Autophagy, Exosomes and HIV-1 Associated Neurological Disorders: New Insights for Diagnosis and Therapeutic Applications. Viruses 2017, 9, 176. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sinica. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. Acs Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Jiang, X.C.; Gao, J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Pascucci, L.; Cocce, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Vigano, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Cocce, V.; Franze, S.; Brini, A.T.; Gianni, A.B.; Pascucci, L.; Ciusani, E.; Alessandri, G.; Farronato, G.; Cavicchini, L.; Sordi, V.; et al. In Vitro Anticancer Activity of Extracellular Vesicles (EVs) Secreted by Gingival Mesenchymal Stromal Cells Primed with Paclitaxel. Pharmaceutics 2019, 11, 61. [Google Scholar] [CrossRef]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2018, 283, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. Ccs 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; De, L.K.T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yuan, M.; Zhang, T.; Wei, H.; Xu, S.; Jiang, N.; Zheng, N.; Wu, Z. Extracellular vesicles expressing a single-chain variable fragment of an HIV-1 specific antibody selectively target Env(+) tissues. Theranostics 2019, 9, 5657–5671. [Google Scholar] [CrossRef]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: Similarities and differences. Adv. Drug Deliv. Rev. 2009, 61, 746–759. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Therapy. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef]
- Shahjin, F.; Chand, S.; Yelamanchili, S.V. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, N.; Gerth, K.A.; Rahman, M.A.; Yallapu, M.M.; Midde, N.M. Specific packaging and circulation of cytochromes P450, especially 2E1 isozyme, in human plasma exosomes and their implications in cellular communications. Biochem. Biophys. Res. Commun. 2017, 491, 675–680. [Google Scholar] [CrossRef]
- Sharma, B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr. Hiv Res. 2014, 12, 13–21. [Google Scholar] [CrossRef]
- Akay, C.; Cooper, M.; Odeleye, A.; Jensen, B.K.; White, M.G.; Vassoler, F.; Gannon, P.J.; Mankowski, J.; Dorsey, J.L.; Buch, A.M.; et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirology 2014, 20, 39–53. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release Off. J. Control. Release Soc. 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Mallegol, J.; Van Niel, G.; Lebreton, C.; Lepelletier, Y.; Candalh, C.; Dugave, C.; Heath, J.K.; Raposo, G.; Cerf-Bensussan, N.; Heyman, M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 2007, 132, 1866–1876. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zoller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Et Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Ruiz-de-Leon, M.J.; Jimenez-Sousa, M.A.; Moreno, S.; Garcia, M.; Gutierrez-Rivas, M.; Leon, A.; Montero-Alonso, M.; Gonzalez-Garcia, J.; Resino, S.; Rallon, N.; et al. Lower expression of plasma-derived exosome miR-21 levels in HIV-1 elite controllers with decreasing CD4 T cell count. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi 2019, 52, 667–671. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, J.; Zeng, Y. Therapeutic Potential of Anti-HIV RNA-loaded Exosomes. Biomed. Environ. Sci. Bes 2018, 31, 215–226. [Google Scholar] [CrossRef]
- Hu, G.; Yelamanchili, S.; Kashanchi, F.; Haughey, N.; Bond, V.C.; Witwer, K.W.; Pulliam, L.; Buch, S. Proceedings of the 2017 ISEV symposium on "HIV, NeuroHIV, drug abuse, & EVs". J. Neurovirology 2017, 23, 935–940. [Google Scholar] [CrossRef]
- Dimov, I.; Jankovic Velickovic, L.; Stefanovic, V. Urinary exosomes. TheScientificWorldJournal 2009, 9, 1107–1118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aethlon. Available online: https://www.aethlonmedical.com/ (accessed on 31 March 2020).
- Ryu, A.R.; Kim, D.H.; Kim, E.; Lee, M.Y. The Potential Roles of Extracellular Vesicles in Cigarette Smoke-Associated Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4692081. [Google Scholar] [CrossRef] [PubMed]
| HIV-Associated EV Component | HIV + Smoking-Associated Component | ||
|---|---|---|---|
| Type | Specific | Type | Specific |
| Viral proteins | Nef, Tat [104] | Cytokines | IL-6 10, MCP-1 11, RANTES 12 [37,128,131] |
| Viral entry receptors | CCR5 1 [103] | Others | Properdin [49], soluble CD144, TGF-β1 13 [129] |
| Oxidative stress markers | cystine, oxidized cys-gly, Catalase, PRDX1 2, PRDX2 2, and TXN 3 [115] | ||
| Anti-inflammatory marker | PUFA [115] | ||
| Immune activation markers | CD14 4, CRP 5, HLA-A, and HLA-B 6 [115] | ||
| NeurodegenerationMarkers | Aβ 7, HMGB1 8 and NF-L 9 [119,120,121] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, S.; Kodidela, S.; Gerth, K.; Hatami, E.; Verma, N.; Kumar, S. Extracellular Vesicles in Smoking-Mediated HIV Pathogenesis and their Potential Role in Biomarker Discovery and Therapeutic Interventions. Cells 2020, 9, 864. https://doi.org/10.3390/cells9040864
Haque S, Kodidela S, Gerth K, Hatami E, Verma N, Kumar S. Extracellular Vesicles in Smoking-Mediated HIV Pathogenesis and their Potential Role in Biomarker Discovery and Therapeutic Interventions. Cells. 2020; 9(4):864. https://doi.org/10.3390/cells9040864
Chicago/Turabian StyleHaque, Sanjana, Sunitha Kodidela, Kelli Gerth, Elham Hatami, Neha Verma, and Santosh Kumar. 2020. "Extracellular Vesicles in Smoking-Mediated HIV Pathogenesis and their Potential Role in Biomarker Discovery and Therapeutic Interventions" Cells 9, no. 4: 864. https://doi.org/10.3390/cells9040864
APA StyleHaque, S., Kodidela, S., Gerth, K., Hatami, E., Verma, N., & Kumar, S. (2020). Extracellular Vesicles in Smoking-Mediated HIV Pathogenesis and their Potential Role in Biomarker Discovery and Therapeutic Interventions. Cells, 9(4), 864. https://doi.org/10.3390/cells9040864






