Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Canine Intestinal Organoids
2.2. Gene Expression Analysis
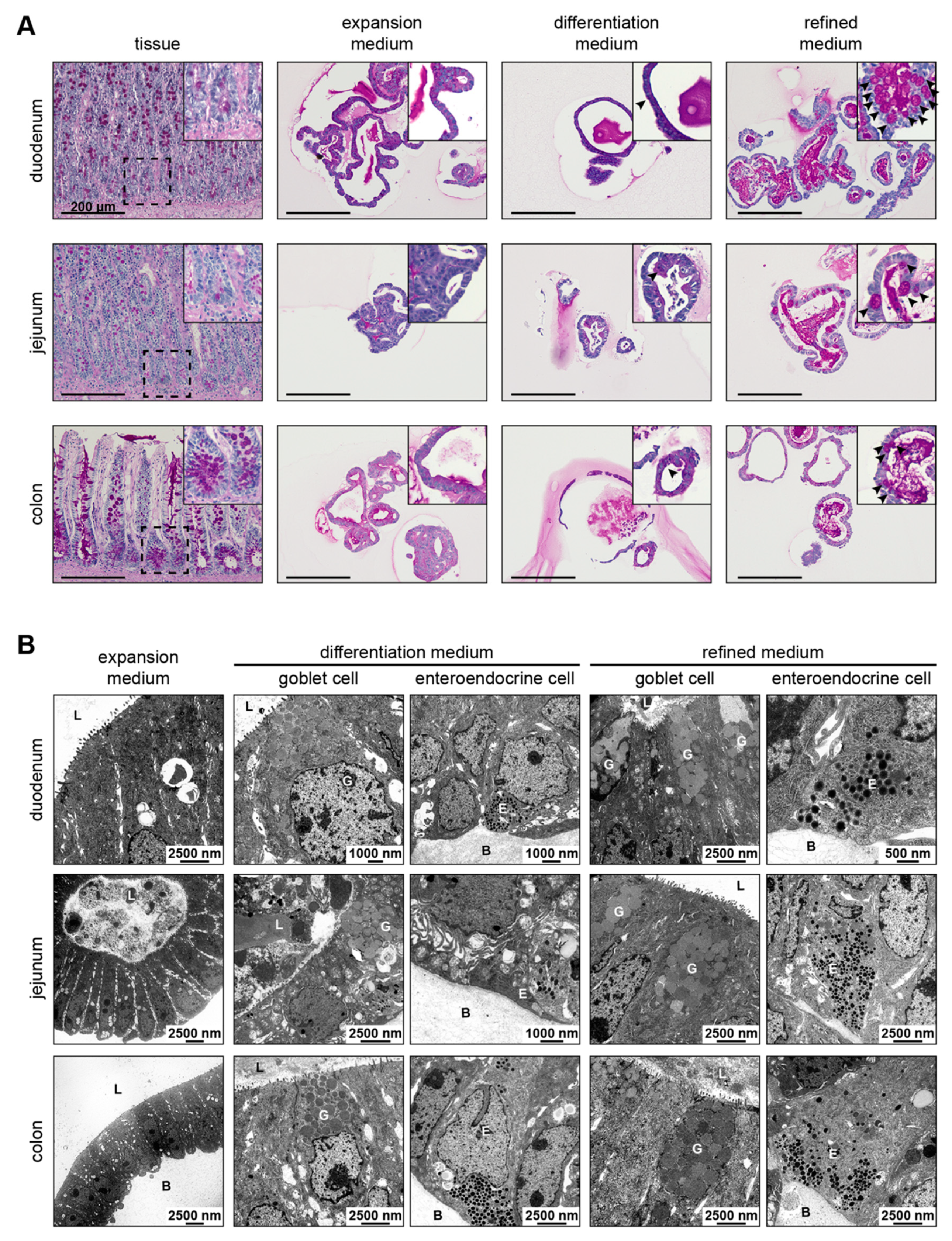
2.3. Periodic Acid-Schiff Reaction of Organoid and Tissue Sections
2.4. Transmission Electron Microscopy of Organoids
2.5. Proliferation Assay
2.6. Viability and Apoptosis Assays
2.7. Statistical Evaluation
3. Results
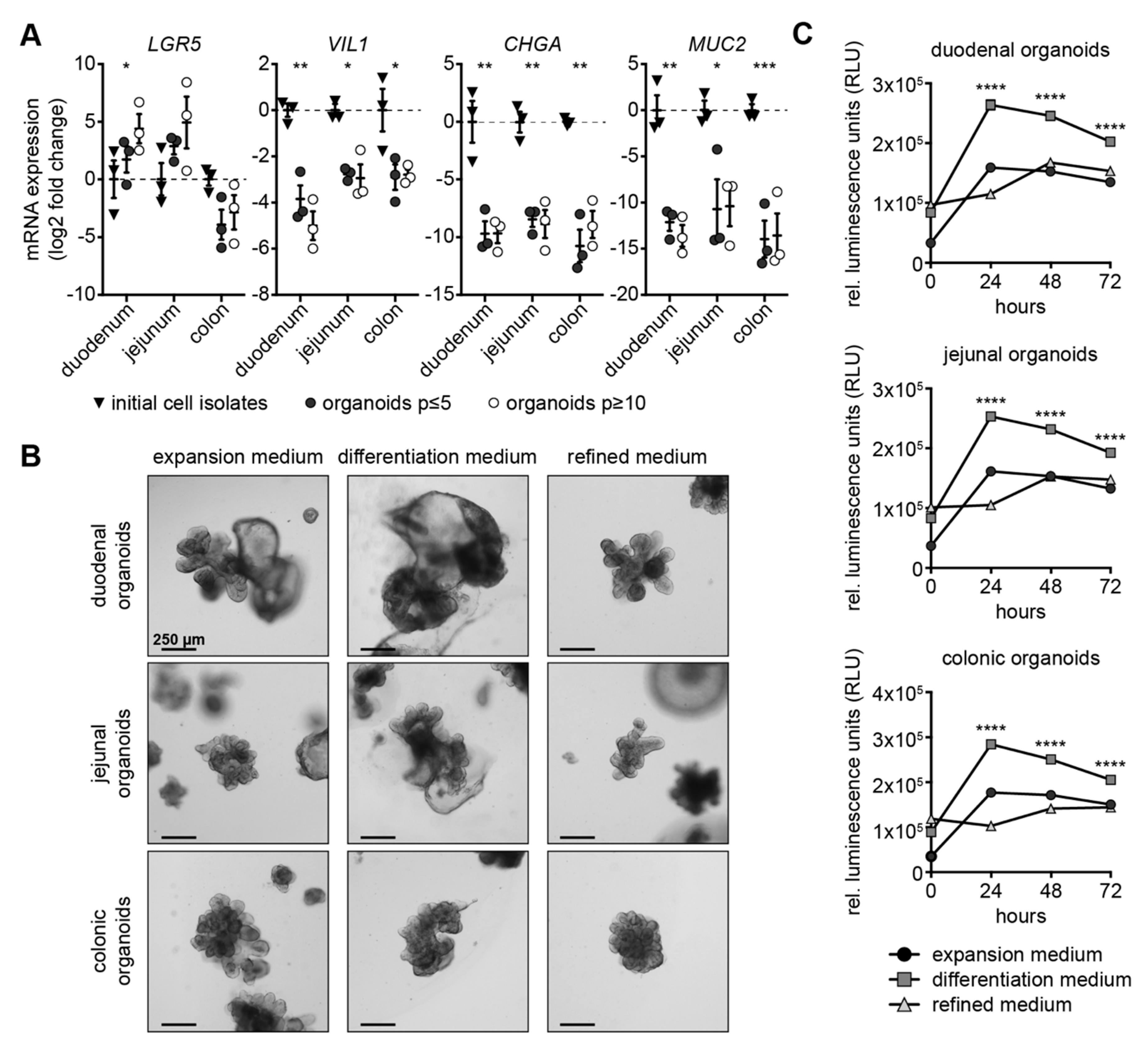
3.1. Expansion Medium Does Not Support Expression of Secretory Lineage Differentiation Markers
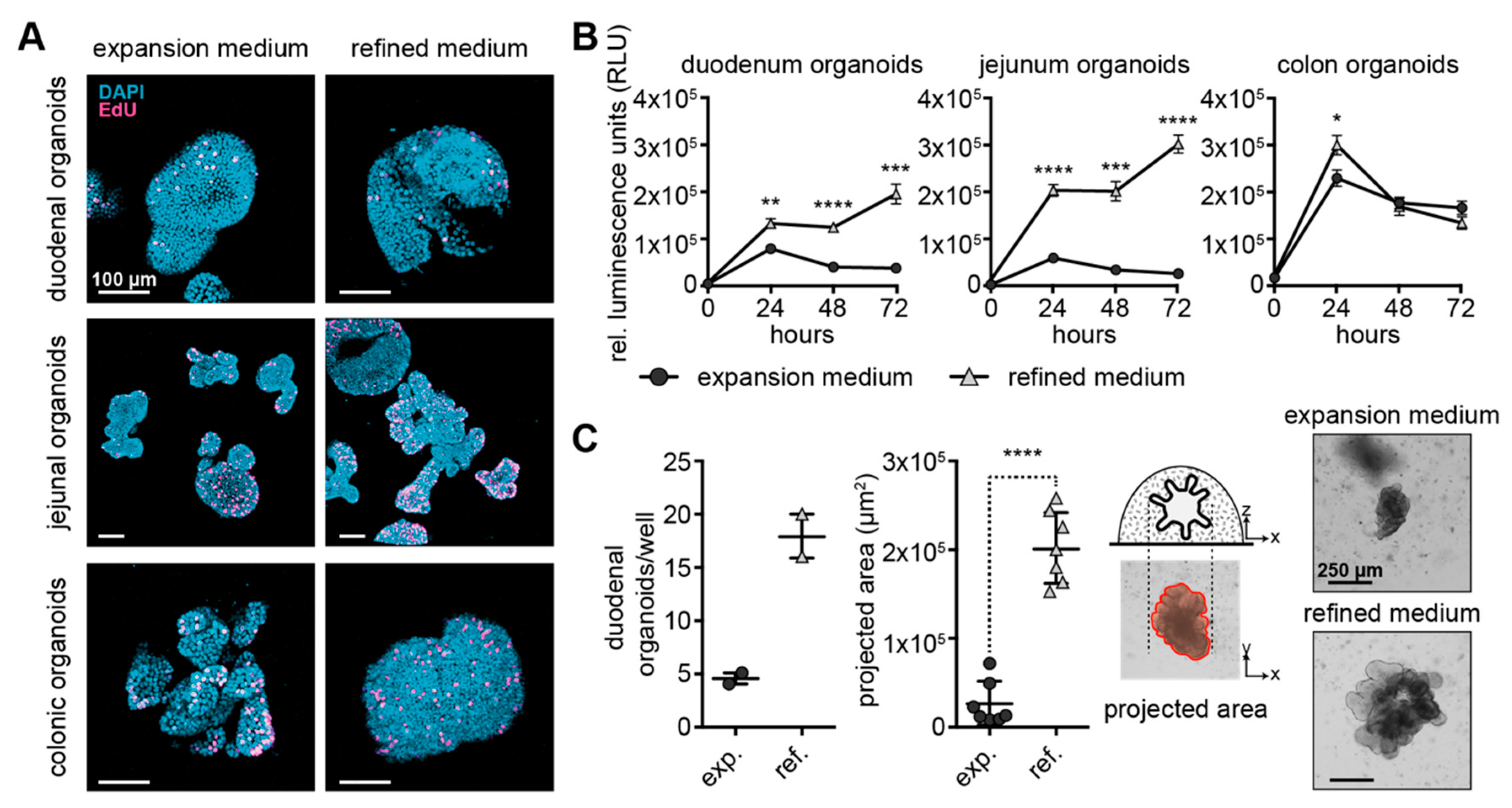
3.2. Canine Intestinal Organoids Grow Efficiently in Refined Medium
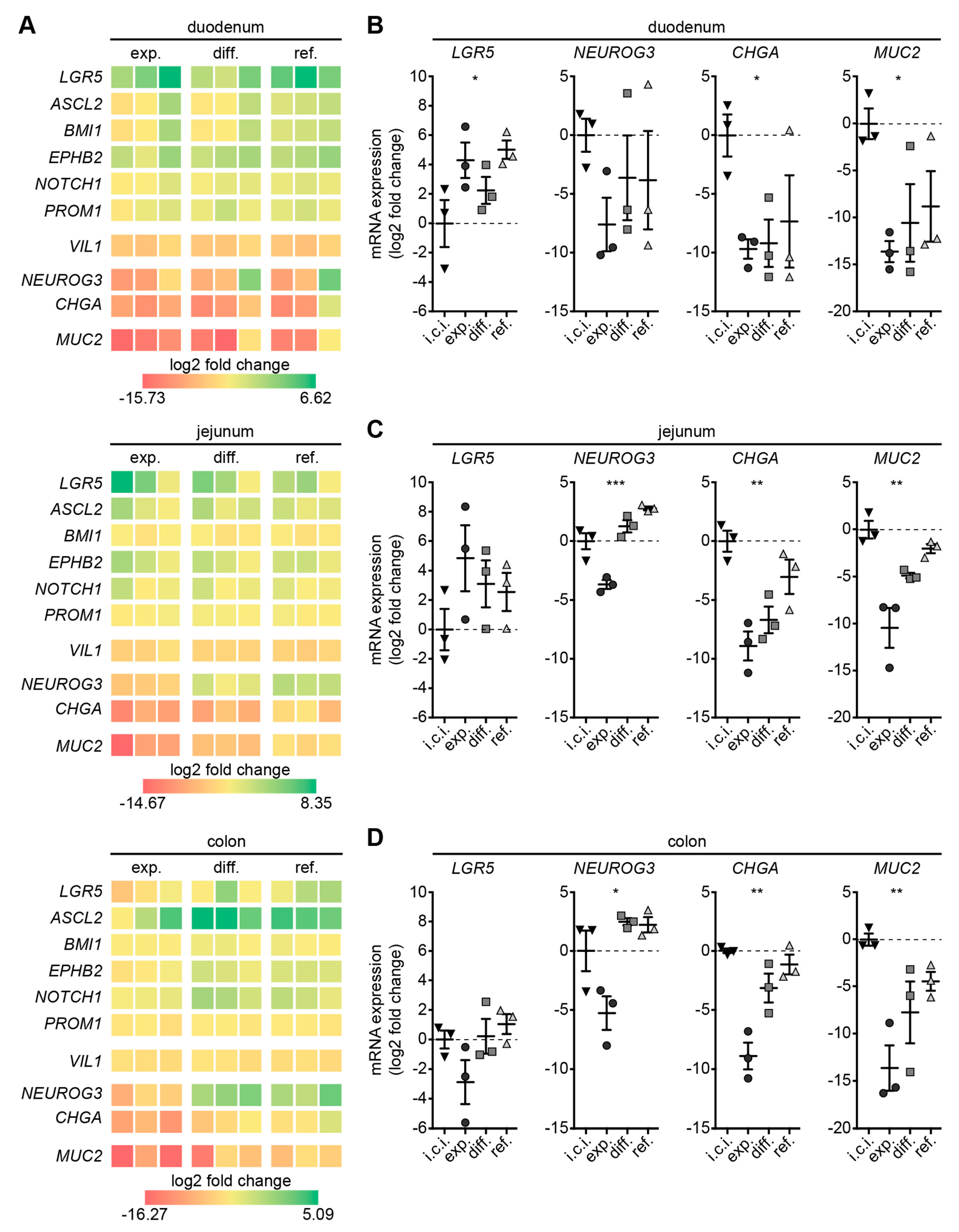
3.3. Refined Medium Promotes Expression of Cell Differentiation Markers in Canine Intestinal Organoids
3.4. Refined and Differentiation Medium Both Support Organoid Differentiation
3.5. Refined Medium Supports Continuous Growth and Derivation of Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.P.; Crockett, S.; Shaheen, N.J. The Burden of Gastrointestinal and Liver Disease around the World. In GI Epidemiology; Wiley: Oxford, UK, 2014; pp. 1–13. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.I. Infectious Diseases of the GI Tract. Available online: https://www.msdvetmanual.com/digestive-system/digestive-system-introduction/infectious-diseases-of-the-gi-tract (accessed on 19 September 2019).
- Albani, S.; Colomb, J.; Prakken, B. Translational Medicine 2.0: From Clinical Diagnosis–Based to Molecular-Targeted Therapies in the Era of Globalization. Clin. Pharmacol. Ther. 2010, 87, 642–645. [Google Scholar] [CrossRef]
- Albani, S.; Prakken, B. The advancement of translational medicine—From regional challenges to global solutions. Nat. Med. 2009, 15, 1006–1009. [Google Scholar] [CrossRef]
- Scherzer, M.; Kramer, N.; Unger, C.; Walzl, A.; Walter, S.; Stadler, M.; Hengstschläger, M.; Dolznig, H. Preclinical Cancer Models with the Potential to Predict Clinical Response. In Drug Discovery in Cancer Epigenetics; Elsevier BV: Oxford, UK, 2016; pp. 97–122. [Google Scholar]
- Browning, T.H.; Trier, J.S. Organ culture of mucosal biopsies of human small intestine. J. Clin. Investig. 1969, 48, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kedinger, M.; Haffen, K.; Simon-Assmann, P. Intestinal tissue and cell cultures. Differentiation 1987, 36, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.S.; Flint, N.; Somers, A.S.; Eyden, B.; Potten, C.S. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 1992, 101. [Google Scholar]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef]
- Wang, X.; Yamamoto, Y.; Wilson, L.H.; Zhang, T.; Howitt, B.; Farrow, M.A.; Kern, F.; Ning, G.; Hong, Y.; Khor, C.C.; et al. Cloning and variation of ground state intestinal stem cells. Nature 2015, 522, 173–178. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Date, S.; Sato, T. Mini-Gut Organoids: Reconstitution of the Stem Cell Niche. Annu. Rev. Cell Dev. Boil. 2015, 31, 269–289. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Brink, S.V.D.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.M.; Siersema, P.D.; et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, R.; Gunasekara, D.B.; Reed, M.I.; Disalvo, M.; Nguyen, D.L.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. Formation of Human Colonic Crypt Array by Application of Chemical Gradients Across a Shaped Epithelial Monolayer. Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.H.; Behnke, M. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Boil. Open 2017, 6, 698–705. [Google Scholar] [CrossRef] [PubMed]
- 2017 ACVIM Forum Research Abstract Program. J. Veter- Intern. Med. 2017, 31, 1225–1361. [CrossRef]
- Nantasanti, S.; Spee, B.; Kruitwagen, H.S.; Chen, C.; Geijsen, N.; Oosterhoff, L.A.; Van Wolferen, M.E.; Peláez, N.; Fieten, H.; Wubbolts, R.W.; et al. Disease Modeling and Gene Therapy of Copper Storage Disease in Canine Hepatic Organoids. Stem Cell Rep. 2015, 5, 895–907. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Oosterhoff, L.A.; Vernooij, I.G.; Schrall, I.M.; Van Wolferen, M.E.; Bannink, F.; Roesch, C.; Van Uden, L.; Molenaar, M.; Helms, J.B.; et al. Long-Term Adult Feline Liver Organoid Cultures for Disease Modeling of Hepatic Steatosis. Stem Cell Rep. 2017, 8, 822–830. [Google Scholar] [CrossRef]
- Derricott, H.; Luu, L.; Fong, W.Y.; Hartley, C.S.; Johnston, L.; Armstrong, S.D.; Randle, N.; Duckworth, C.A.; Campbell, B.J.; Wastling, J.M.; et al. Developing a 3D intestinal epithelium model for livestock species. Cell Tissue Res. 2018, 375, 409–424. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Young, R.; Jayaraman, S.; Sehgal, A.; Paxton, E.; Thomson, S.; Katzer, F.; Hope, J.; Innes, E.A.; Morrison, L.; et al. Development of in vitro enteroids derived from bovine small intestinal crypts. Veter- Res. 2018, 49, 54. [Google Scholar] [CrossRef]
- Pierzchalska, M.; Grabacka, M.; Michalik, M.; Zyla, K.; Pierzchalski, P. Prostaglandin E2 supports growth of chicken embryo intestinal organoids in Matrigel matrix. Biotechniques 2012, 52, 307–315. [Google Scholar] [CrossRef]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Shearin, A.L.; Ostrander, E.A. Leading the way: Canine models of genomics and disease. Dis. Model. Mech. 2010, 3, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Muraro, M.J.; Boisset, J.-C.; Wiebrands, K.; Lyubimova, A.; Dharmadhikari, G.; Born, M.V.D.; Van Es, J.; Jansen, E.; Clevers, H.; et al. De Novo Prediction of Stem Cell Identity using Single-Cell Transcriptome Data. Cell Stem Cell 2016, 19, 266–277. [Google Scholar] [CrossRef]
- Audie, J.P.; Janin, A.; Porchet, N.; Copin, M.C.; Gosselin, B.; Aubert, J.P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 1993, 41, 1479–1485. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Born, M.V.D.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Munoz, J.; Stange, D.; Schepers, A.G.; Van De Wetering, M.; Koo, B.-K.; Itzkovitz, S.; Volckmann, R.; Kung, K.S.; Koster, J.; Radulescu, S.; et al. The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012, 31, 3079–3091. [Google Scholar] [CrossRef]
- Jenny, M.; Uhl, C.; Roche, C.; Duluc, I.; Guillermin, V.; Guillemot, F.; Jensen, J.; Kedinger, M.; Gradwohl, G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002, 21, 6338–6347. [Google Scholar] [CrossRef]
- Lopez-Diaz, L.; Jain, R.N.; Keeley, T.M.; VanDussen, K.L.; Brunkan, C.S.; Gumucio, D.L.; Samuelson, L.C. Intestinal Neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev. Boil. 2007, 309, 298–305. [Google Scholar] [CrossRef]
- Kingsbury, D.; Sun, L.; Qi, Y.; Fredericks, J.; Wang, Q.; Wannemuehler, M.; Jergens, A.; Allenspach, K. DDW Abstract: Optimizing the Development and Characterization of Canine Small Intestinal Crypt Organoids as a Research Model. Gastroenterology 2017, 152 (Suppl. 1). [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Boil. 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, K.; He, Y.; Koo-McCoy, S.; Kumaraswamy, P.; Nie, B.; Shaw, K.; Chan, P.; Leadbetter, M.; He, L.; Lewis, J.G.; et al. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Rep. 2017, 9, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Wallach, T.E.; Bayrer, J.R. Intestinal Organoids. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 180–185. [Google Scholar] [CrossRef]
- Meng, Y.; Ren, Z.; Xu, F.; Zhou, X.; Song, C.; Wang, V.Y.-F.; Liu, W.; Lu, L.; Thomson, J.A.; Chen, G. Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 1347–1356. [Google Scholar] [CrossRef]
- Uchida, R.; Saito, Y.; Nogami, K.; Kajiyama, Y.; Suzuki, Y.; Kawase, Y.; Nakaoka, T.; Muramatsu, T.; Kimura, M.; Saito, H. Epigenetic silencing of Lgr5 induces senescence of intestinal epithelial organoids during the process of aging. NPJ Aging Mech. Dis. 2018, 4, 1. [Google Scholar] [CrossRef]
- Heikal, A.A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef]
| Reagent | Expansion Medium | Differentiation Medium | Refined Medium |
|---|---|---|---|
| basal medium | + | + | + |
| N2 | 1x | 1x | − |
| EGF | 50 ng/mL | 50 ng/mL | − a |
| Noggin | 100 ng/mL | 100 ng/mL | 100 ng/mL |
| Rspo1 | 10% v/v | 10% v/v | 10% v/v |
| Wnt3a | 43% v/v | 43% v/v | 50% v/v |
| Nicotinamide | 10 mM | − | − |
| Gastrin | 10 nM | 10 nM | 10 nM |
| A83-01 | 500 nM | 500 nM | 500 nM |
| SB202190 | 10 µM | − | − |
| HGF | 50 ng/mL | 50 ng/mL | 50 ng/mL |
| IGF1 | − | − | 100 ng/mL |
| FGF2 | − | − | 50 ng/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, N.; Pratscher, B.; Meneses, A.M.C.; Tschulenk, W.; Walter, I.; Swoboda, A.; Kruitwagen, H.S.; Schneeberger, K.; Penning, L.C.; Spee, B.; et al. Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells 2020, 9, 822. https://doi.org/10.3390/cells9040822
Kramer N, Pratscher B, Meneses AMC, Tschulenk W, Walter I, Swoboda A, Kruitwagen HS, Schneeberger K, Penning LC, Spee B, et al. Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells. 2020; 9(4):822. https://doi.org/10.3390/cells9040822
Chicago/Turabian StyleKramer, Nina, Barbara Pratscher, Andre M. C. Meneses, Waltraud Tschulenk, Ingrid Walter, Alexander Swoboda, Hedwig S. Kruitwagen, Kerstin Schneeberger, Louis C. Penning, Bart Spee, and et al. 2020. "Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine" Cells 9, no. 4: 822. https://doi.org/10.3390/cells9040822
APA StyleKramer, N., Pratscher, B., Meneses, A. M. C., Tschulenk, W., Walter, I., Swoboda, A., Kruitwagen, H. S., Schneeberger, K., Penning, L. C., Spee, B., Kieslinger, M., Brandt, S., & Burgener, I. A. (2020). Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells, 9(4), 822. https://doi.org/10.3390/cells9040822







