Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor
Abstract
1. Introduction
2. A Brief History of Hyaluronan Mediated Motility Receptor (HMMR)
What’s in a name? That which we call a rose By any other word would smell as sweet.Romeo and Juliet, 2, 2, 45-46
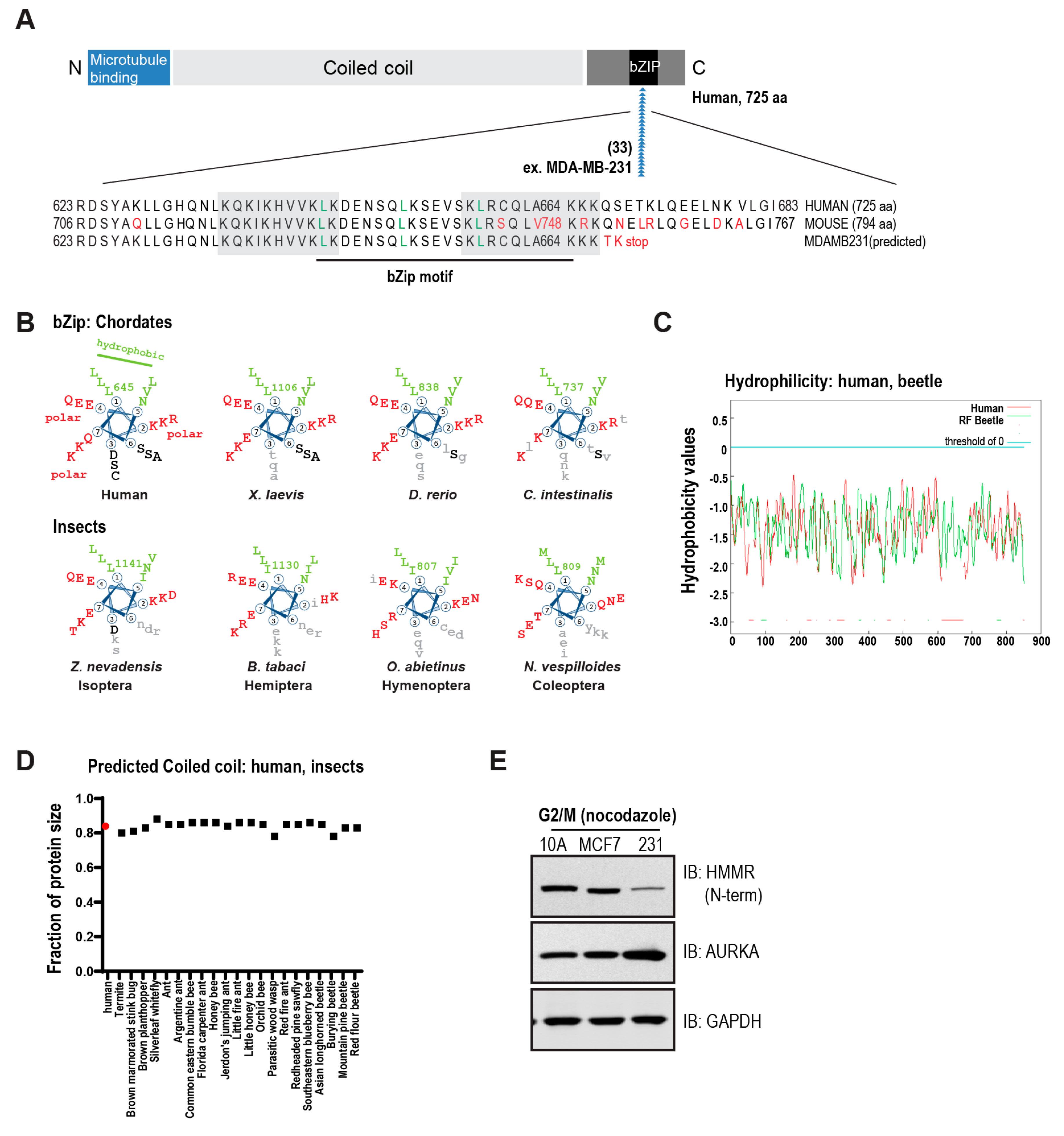
3. The Conserved Basic C-Terminal Domain in HMMR Is a Leucine Zipper Motif
3.1. Structural Domains in HMMR
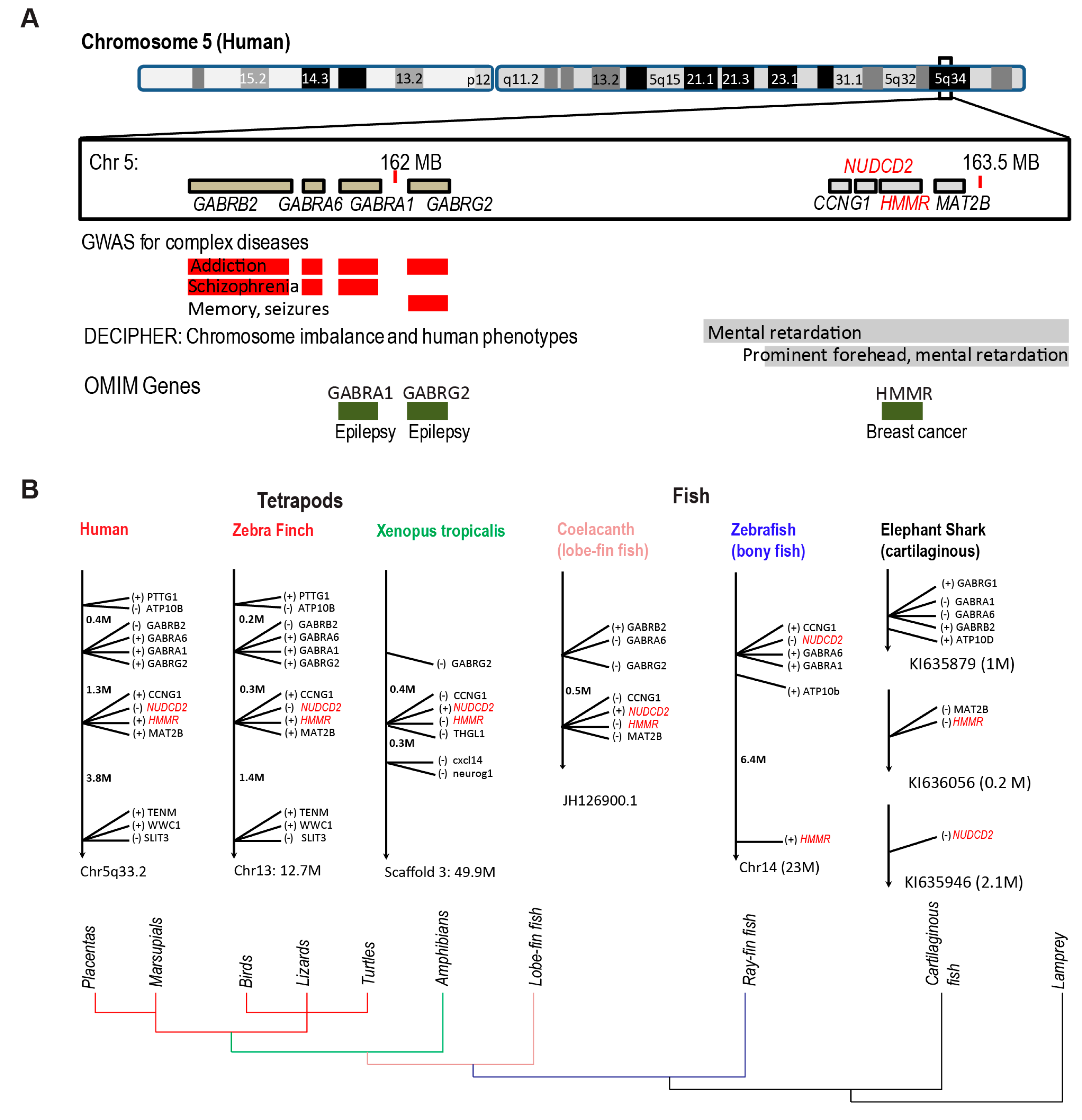
3.2. Evolution of HMMR
3.3. Conserved HMMR-NudC Domain-Containing Protein 2 (NUDCD2) Gene Cluster
4. HMMR Functions as a Homeostasis, Mitosis, and Meiosis Regulator
4.1. HMMR Is Needed for Tissue Homeostasis and Neural Development
4.2. HMMR Regulates Spindle Assembly in Mitotic Cells and Meiotic Extracts.
5. HMMR Associates with Breast Cancer risk, Cancer Prognosis, and Progression
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caon, I.; Bartolini, B.; Parnigoni, A.; Carava, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Karalis, T.; Heldin, P. Intracellular hyaluronan: Importance for cellular functions. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, C.; Hoare, K.; Owens, R.; Hohn, H.P.; Hook, M.; Moore, D.; Cripps, V.; Austen, L.; Nance, D.M.; Turley, E.A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992, 117, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Assmann, V.; Fieber, C.; Sleeman, J.P.; Moll, J.; Ponta, H.; Hart, I.R.; Herrlich, P. Problems with RHAMM: A new link between surface adhesion and oncogenesis? Cell 1998, 95, 591–592. [Google Scholar] [CrossRef][Green Version]
- Connell, M.; Chen, H.; Jiang, J.; Kuan, C.W.; Fotovati, A.; Chu, T.L.; He, Z.; Lengyell, T.C.; Li, H.; Kroll, T.; et al. HMMR acts in the PLK1-dependent spindle positioning pathway and supports neural development. eLife 2017, 6, e28672. [Google Scholar] [CrossRef]
- Fieber, C.; Plug, R.; Sleeman, J.; Dall, P.; Ponta, H.; Hofmann, M. Characterisation of the murine gene encoding the intracellular hyaluronan receptor IHABP (RHAMM). Gene 1999, 226, 41–50. [Google Scholar] [CrossRef]
- Hofmann, M.; Fieber, C.; Assmann, V.; Gottlicher, M.; Sleeman, J.; Plug, R.; Howells, N.; von Stein, O.; Ponta, H.; Herrlich, P. Identification of IHABP, a 95 kDa intracellular hyaluronate binding protein. J. Cell Sci. 1998, 111, 1673–1684. [Google Scholar]
- Jiang, J.; Casalegno-Garduno, R.; Chen, H.; Schmitt, A.; Schmitt, M.; Maxwell, C.A. Multifunctional proteins bridge mitosis with motility and cancer with inflammation and arthritis. Sci. World J. 2010, 10, 1244–1257. [Google Scholar] [CrossRef]
- Maxwell, C.A.; McCarthy, J.; Turley, E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? J. Cell Sci. 2008, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Goetinck, P.F.; Stirpe, N.S.; Tsonis, P.A.; Carlone, D. The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J. Cell Biol. 1987, 105, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, L.; Turley, E.A. Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J. Biol. Chem. 1993, 268, 8617–8623. [Google Scholar]
- Yang, B.; Hall, C.L.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a novel heparin binding domain in RHAMM and evidence that it modifies HA mediated locomotion of ras-transformed cells. J. Cell. Biochem. 1994, 56, 455–468. [Google Scholar] [CrossRef]
- Csoka, A.B.; Stern, R. Hypotheses on the evolution of hyaluronan: A highly ironic acid. Glycobiology 2013, 23, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A.; Keats, J.J.; Crainie, M.; Sun, X.; Yen, T.; Shibuya, E.; Hendzel, M.; Chan, G.; Pilarski, L.M. RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol. Biol. Cell 2003, 14, 2262–2276. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Rape, M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol. Cell 2010, 38, 369–382. [Google Scholar] [CrossRef]
- Dunsch, A.K.; Hammond, D.; Lloyd, J.; Schermelleh, L.; Gruneberg, U.; Barr, F.A. Dynein light chain 1 and a spindle-associated adaptor promote dynein asymmetry and spindle orientation. J. Cell Biol. 2012, 198, 1039–1054. [Google Scholar] [CrossRef]
- Assmann, V.; Jenkinson, D.; Marshall, J.F.; Hart, I.R. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J. Cell Sci. 1999, 112, 3943–3954. [Google Scholar]
- Fulcher, L.J.; He, Z.; Mei, L.; Macartney, T.J.; Wood, N.T.; Prescott, A.R.; Whigham, A.J.; Varghese, J.; Gourlay, R.; Ball, G.; et al. FAM83D directs protein kinase CK1alpha to the mitotic spindle for proper spindle positioning. EMBO Rep. 2019, 20, e47495. [Google Scholar] [CrossRef]
- Li, J.; Shima, H.; Nishizawa, H.; Ikeda, M.; Brydun, A.; Matsumoto, M.; Kato, H.; Saiki, Y.; Liu, L.; Watanabe-Matsui, M.; et al. Phosphorylation of BACH1 switches its function from transcription factor to mitotic chromosome regulator and promotes its interaction with HMMR. Biochem. J. 2018, 475, 981–1002. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.L.H.; Connell, M.; Zhou, L.; He, Z.; Won, J.; Chen, H.; Rahavi, S.M.R.; Mohan, P.; Nemirovsky, O.; Fotovati, A.; et al. Cell Cycle-Dependent Tumor Engraftment and Migration Are Enabled by Aurora-A. Mol. Cancer Res. 2018, 16, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Nousiainen, M.; Sillje, H.H.; Sauer, G.; Nigg, E.A.; Korner, R. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA 2006, 103, 5391–5396. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Nashchekin, D.; Wheatley, L.; Irion, U.; Dahlgaard, K.; Montague, T.G.; Hall, J.; Johnston, D.S. Anterior-posterior axis specification in Drosophila oocytes: Identification of novel bicoid and oskar mRNA localization factors. Genetics 2011, 188, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Ikeshima-Kataoka, H.; Skeath, J.B.; Nabeshima, Y.; Doe, C.Q.; Matsuzaki, F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature 1997, 390, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.P.; Jan, L.Y.; Jan, Y.N. Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell 1997, 90, 449–458. [Google Scholar] [CrossRef]
- Irion, U.; Adams, J.; Chang, C.W.; Johnston, D.S. Miranda couples oskar mRNA/Staufen complexes to the bicoid mRNA localization pathway. Dev. Biol. 2006, 297, 522–533. [Google Scholar] [CrossRef]
- Wittmann, T.; Boleti, H.; Antony, C.; Karsenti, E.; Vernos, I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 1998, 143, 673–685. [Google Scholar] [CrossRef]
- Boleti, H.; Karsenti, E.; Vernos, I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell 1996, 84, 49–59. [Google Scholar] [CrossRef]
- Garrido, G.; Vernos, I. Non-centrosomal TPX2-Dependent Regulation of the Aurora A Kinase: Functional Implications for Healthy and Pathological Cell Division. Front. Oncol. 2016, 6, 88. [Google Scholar] [CrossRef]
- Maxwell, C.A.; Keats, J.J.; Belch, A.R.; Pilarski, L.M.; Reiman, T. Receptor for hyaluronan-mediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res. 2005, 65, 850–860. [Google Scholar] [PubMed]
- Groen, A.C.; Cameron, L.A.; Coughlin, M.; Miyamoto, D.T.; Mitchison, T.J.; Ohi, R. XRHAMM functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr. Biol. 2004, 14, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mohan, P.; Jiang, J.; Nemirovsky, O.; He, D.; Fleisch, M.C.; Niederacher, D.; Pilarski, L.M.; Lim, C.J.; Maxwell, C.A. Spatial regulation of Aurora A activity during mitotic spindle assembly requires RHAMM to correctly localize TPX2. Cell Cycle 2014, 13, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Scrofani, J.; Sardon, T.; Meunier, S.; Vernos, I. Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr. Biol. 2015, 25, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Connell, M.; Mei, L.; Reid, G.S.D.; Maxwell, C.A. The non-motor adaptor HMMR dampens Eg5-mediated forces to preserve the kinetics and integrity of chromosome segregation. Mol. Biol. Cell 2018, 29, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Eibes, S.; Gallisa-Sune, N.; Rosas-Salvans, M.; Martinez-Delgado, P.; Vernos, I.; Roig, J. Nek9 Phosphorylation Defines a New Role for TPX2 in Eg5-Dependent Centrosome Separation before Nuclear Envelope Breakdown. Curr. Biol. 2018, 28, 121–129. [Google Scholar] [CrossRef]
- Stern, R. Go Fly a Chitin: The Mystery of Chitin and Chitinases in Vertebrate Tissues. Front. Biosci. 2017, 22, 580–595. [Google Scholar] [CrossRef]
- Casini, P.; Nardi, I.; Ori, M. RHAMM mRNA expression in proliferating and migrating cells of the developing central nervous system. Gene Expr. Patterns 2010, 10, 93–97. [Google Scholar] [CrossRef]
- Lindwall, C.; Olsson, M.; Osman, A.M.; Kuhn, H.G.; Curtis, M.A. Selective expression of hyaluronan and receptor for hyaluronan mediated motility (Rhamm) in the adult mouse subventricular zone and rostral migratory stream and in ischemic cortex. Brain Res. 2013, 1503, 62–77. [Google Scholar] [CrossRef]
- Li, H.; Kroll, T.; Moll, J.; Frappart, L.; Herrlich, P.; Heuer, H.; Ploubidou, A. Spindle Misorientation of Cerebral and Cerebellar Progenitors Is a Mechanistic Cause of Megalencephaly. Stem Cell Rep. 2017, 9, 1071–1080. [Google Scholar] [CrossRef]
- Prager, A.; Hagenlocher, C.; Ott, T.; Schambony, A.; Feistel, K. hmmr mediates anterior neural tube closure and morphogenesis in the frog Xenopus. Dev. Biol. 2017, 430, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Vallee, R.B.; Tsai, J.W. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006, 20, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Toba, S.; Hirotsune, S. A unique role of dynein and nud family proteins in corticogenesis. Neuropathology 2012, 32, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Yamada, M.; Mimori-Kiyosue, Y.; Shirai, Y.; Suzuki, A.; Ohno, S.; Saya, H.; Wynshaw-Boris, A.; Hirotsune, S. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat. Cell Biol. 2009, 11, 1057–1068. [Google Scholar] [CrossRef]
- Mori, D.; Yano, Y.; Toyo-oka, K.; Yoshida, N.; Yamada, M.; Muramatsu, M.; Zhang, D.; Saya, H.; Toyoshima, Y.Y.; Kinoshita, K.; et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol. Cell. Biol. 2007, 27, 352–367. [Google Scholar] [CrossRef]
- Yamada, M.; Toba, S.; Yoshida, Y.; Haratani, K.; Mori, D.; Yano, Y.; Mimori-Kiyosue, Y.; Nakamura, T.; Itoh, K.; Fushiki, S.; et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 2008, 27, 2471–2483. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Cai, Y.; Lu, Y.; Si, J.; Zhou, T. NudC-like protein 2 regulates the LIS1/dynein pathway by stabilizing LIS1 with Hsp90. Proc. Natl. Acad. Sci. USA 2010, 107, 3499–3504. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Zhang, J.; Liu, M.; Wang, W.; Gao, Y.; Sun, Q.; Zhang, J.; Lu, Y.; Wang, F.; et al. NudC-like protein 2 restrains centriole amplification by stabilizing HERC2. Cell Death Dis. 2019, 10, 628. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Li, M.; Gao, Y.; Zhang, W.; Huang, Y.; Zhuo, W.; Yan, X.; Liu, W.; Wang, F.; et al. NudCL2 is an Hsp90 cochaperone to regulate sister chromatid cohesion by stabilizing cohesin subunits. Cell. Mol. Life Sci. 2019, 76, 381–395. [Google Scholar] [CrossRef]
- Li, H.; Frappart, L.; Moll, J.; Winkler, A.; Kroll, T.; Hamann, J.; Kufferath, I.; Groth, M.; Taudien, S.; Schutte, M.; et al. Impaired Planar Germ Cell Division in the Testis, Caused by Dissociation of RHAMM from the Spindle, Results in Hypofertility and Seminoma. Cancer Res. 2016, 76, 6382–6395. [Google Scholar] [CrossRef]
- Li, H.; Moll, J.; Winkler, A.; Frappart, L.; Brunet, S.; Hamann, J.; Kroll, T.; Verlhac, M.H.; Heuer, H.; Herrlich, P.; et al. RHAMM deficiency disrupts folliculogenesis resulting in female hypofertility. Biol. Open 2015, 4, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M.; Thomas, L.; Bequignon, E.; Schmitt, A.; Stouvenel, L.; Montantin, G.; Tissier, S.; Duquesnoy, P.; Copin, B.; Chantot, S.; et al. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am. J. Hum. Genet. 2019, 105, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Ellenbecker, M.; Osterli, E.; Wang, X.; Day, N.J.; Baumgarten, E.; Hickey, B.; Voronina, E. Dynein Light Chain DLC-1 Facilitates the Function of the Germline Cell Fate Regulator GLD-1 in Caenorhabditis elegans. Genetics 2019, 211, 665–681. [Google Scholar] [CrossRef]
- Wen, Q.; Tang, E.I.; Lui, W.Y.; Lee, W.M.; Wong, C.K.C.; Silvestrini, B.; Cheng, C.Y. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E924–E948. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; Groen, A.C.; Prokhorova, T.; Gerson, R.; White, E.; Rodriguez, A.; Walter, J.C.; Livingston, D.M. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 2006, 127, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, A.; Torre, D.; Keenan, A.B.; Jagodnik, K.M.; Lee, H.J.; Wang, L.; Silverstein, M.C.; Ma’ayan, A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.; Whigham, A.; Clarke, R.; Brenes-Murillo, A.J.; Estes, B.; Madhessian, D.; Lundberg, E.; Wadsworth, P.; Lamond, A.I. Proteomic analysis of cell cycle progression in asynchronous cultures, including mitotic subphases, using PRIMMUS. Elife 2017, 6, e27574. [Google Scholar] [CrossRef]
- Neumann, B.; Walter, T.; Heriche, J.K.; Bulkescher, J.; Erfle, H.; Conrad, C.; Rogers, P.; Poser, I.; Held, M.; Liebel, U.; et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 2010, 464, 721–727. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, H.; Zhang, Y.; Mei, L.; Fang, X.; Zhang, X.; Zhang, F.; Chen, H.; Liu, Y.; et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl. Acad. Sci. USA 2014, 111, E89–E98. [Google Scholar] [CrossRef]
- Sohr, S.; Engeland, K. RHAMM is differentially expressed in the cell cycle and downregulated by the tumor suppressor p53. Cell Cycle 2008, 7, 3448–3460. [Google Scholar] [CrossRef]
- Pujana, M.A.; Han, J.D.; Starita, L.M.; Stevens, K.N.; Tewari, M.; Ahn, J.S.; Rennert, G.; Moreno, V.; Kirchhoff, T.; Gold, B.; et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat. Genet. 2007, 39, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Kannan, N.; Nemirovsky, O.; Chen, H.; Connell, M.; Taylor, B.; Jiang, J.; Pilarski, L.M.; Fleisch, M.C.; Niederacher, D.; et al. BRCA1 controls the cell division axis and governs ploidy and phenotype in human mammary cells. Oncotarget 2017, 8, 32461–32475. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A.; Benitez, J.; Gomez-Baldo, L.; Osorio, A.; Bonifaci, N.; Fernandez-Ramires, R.; Costes, S.V.; Guino, E.; Chen, H.; Evans, G.J.; et al. Interplay between BRCA1 and RHAMM regulates epithelial apicobasal polarization and may influence risk of breast cancer. PLoS Biol. 2011, 9, e1001199. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Meghani, K.; Caron, M.C.; Yang, C.; Ronato, D.A.; Bian, J.; Sharma, A.; Moore, J.; Niraj, J.; Detappe, A.; et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 2018, 563, 522–526. [Google Scholar] [CrossRef]
- Liu, S.; Ginestier, C.; Charafe-Jauffret, E.; Foco, H.; Kleer, C.G.; Merajver, S.D.; Dontu, G.; Wicha, M.S. BRCA1 regulates human mammary stem/progenitor cell fate. Proc. Natl. Acad. Sci. USA 2008, 105, 1680–1685. [Google Scholar] [CrossRef]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef]
- Ding, L.; Su, Y.; Fassl, A.; Hinohara, K.; Qiu, X.; Harper, N.W.; Huh, S.J.; Bloushtain-Qimron, N.; Jovanovic, B.; Ekram, M.; et al. Perturbed myoepithelial cell differentiation in BRCA mutation carriers and in ductal carcinoma in situ. Nat. Commun. 2019, 10, 4182. [Google Scholar] [CrossRef]
- Assmann, V.; Gillett, C.E.; Poulsom, R.; Ryder, K.; Hart, I.R.; Hanby, A.M. The pattern of expression of the microtubule-binding protein RHAMM/IHABP in mammary carcinoma suggests a role in the invasive behaviour of tumour cells. J. Pathol. 2001, 195, 191–196. [Google Scholar] [CrossRef]
- Zlobec, I.; Baker, K.; Terracciano, L.M.; Lugli, A. RHAMM, p21 combined phenotype identifies microsatellite instability-high colorectal cancers with a highly adverse prognosis. Clin. Cancer Res. 2008, 14, 3798–3806. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Guo, L.; Li, J.W.; Liu, N.; Qi, R.; Liu, J. Expression of hyaluronan receptors CD44 and RHAMM in stomach cancers: Relevance with tumor progression. Int. J. Oncol. 2000, 17, 927–932. [Google Scholar] [CrossRef]
- Rein, D.T.; Roehrig, K.; Schondorf, T.; Lazar, A.; Fleisch, M.; Niederacher, D.; Bender, H.G.; Dall, P. Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J. Cancer Res. Clin. Oncol. 2003, 129, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Gust, K.M.; Hofer, M.D.; Perner, S.R.; Kim, R.; Chinnaiyan, A.M.; Varambally, S.; Moller, P.; Rinnab, L.; Rubin, M.A.; Greiner, J.; et al. RHAMM (CD168) is overexpressed at the protein level and may constitute an immunogenic antigen in advanced prostate cancer disease. Neoplasia 2009, 11, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A.; Rasmussen, E.; Zhan, F.; Keats, J.J.; Adamia, S.; Strachan, E.; Crainie, M.; Walker, R.; Belch, A.R.; Pilarski, L.M.; et al. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood 2004, 104, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, K.K.; Spurlock, G.; Kluwe, L.; Chuzhanova, N.; Ferner, R.E.; Frayling, I.M.; Dumanski, J.P.; Guha, A.; Mautner, V.; Upadhyaya, M. High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin. Cancer Res. 2008, 14, 1015–1024. [Google Scholar] [CrossRef]
- Mohan, P.; Castellsague, J.; Jiang, J.; Allen, K.; Chen, H.; Nemirovsky, O.; Spyra, M.; Hu, K.; Kluwe, L.; Pujana, M.A.; et al. Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to aurora kinase inhibition. Oncotarget 2013, 4, 80–93. [Google Scholar] [CrossRef]
- Blanco, I.; Kuchenbaecker, K.; Cuadras, D.; Wang, X.; Barrowdale, D.; de Garibay, G.R.; Librado, P.; Sanchez-Gracia, A.; Rozas, J.; Bonifaci, N.; et al. Assessing associations between the AURKA-HMMR-TPX2-TUBG1 functional module and breast cancer risk in BRCA1/2 mutation carriers. PLoS ONE 2015, 10, e0120020. [Google Scholar] [CrossRef]
- Kalmyrzaev, B.; Pharoah, P.D.; Easton, D.F.; Ponder, B.A.; Dunning, A.M.; Team, S. Hyaluronan-mediated motility receptor gene single nucleotide polymorphisms and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3618–3620. [Google Scholar] [CrossRef][Green Version]
- Venables, J.P.; Klinck, R.; Bramard, A.; Inkel, L.; Dufresne-Martin, G.; Koh, C.; Gervais-Bird, J.; Lapointe, E.; Froehlich, U.; Durand, M.; et al. Identification of alternative splicing markers for breast cancer. Cancer Res. 2008, 68, 9525–9531. [Google Scholar] [CrossRef]
- Du, Y.C.; Chou, C.K.; Klimstra, D.S.; Varmus, H. Receptor for hyaluronan-mediated motility isoform B promotes liver metastasis in a mouse model of multistep tumorigenesis and a tail vein assay for metastasis. Proc. Natl. Acad. Sci. USA 2011, 108, 16753–16758. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Mei, L.; Connell, M.; Maxwell, C.A. Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor. Cells 2020, 9, 819. https://doi.org/10.3390/cells9040819
He Z, Mei L, Connell M, Maxwell CA. Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor. Cells. 2020; 9(4):819. https://doi.org/10.3390/cells9040819
Chicago/Turabian StyleHe, Zhengcheng, Lin Mei, Marisa Connell, and Christopher A. Maxwell. 2020. "Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor" Cells 9, no. 4: 819. https://doi.org/10.3390/cells9040819
APA StyleHe, Z., Mei, L., Connell, M., & Maxwell, C. A. (2020). Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor. Cells, 9(4), 819. https://doi.org/10.3390/cells9040819




