The Blood Circulating Rare Cell Population. What Is It and What Is It Good for?
Abstract
1. Introduction
2. Megakaryocytes
2.1. Megakaryocyte General Background
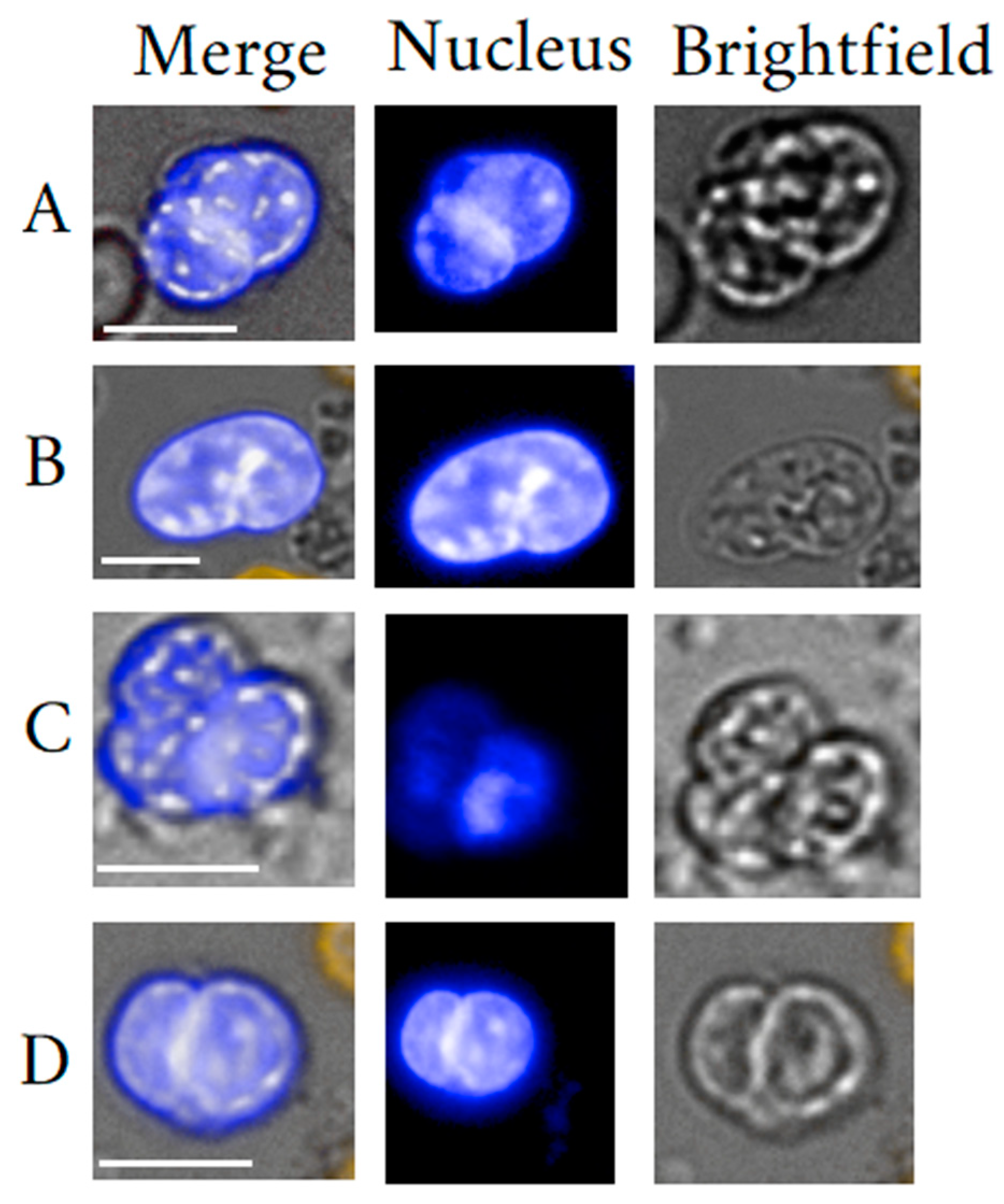
2.2. Circulating Megakaryocytes
2.3. Megakaryocyte Isolation
2.4. MKC Clinical Usefulness
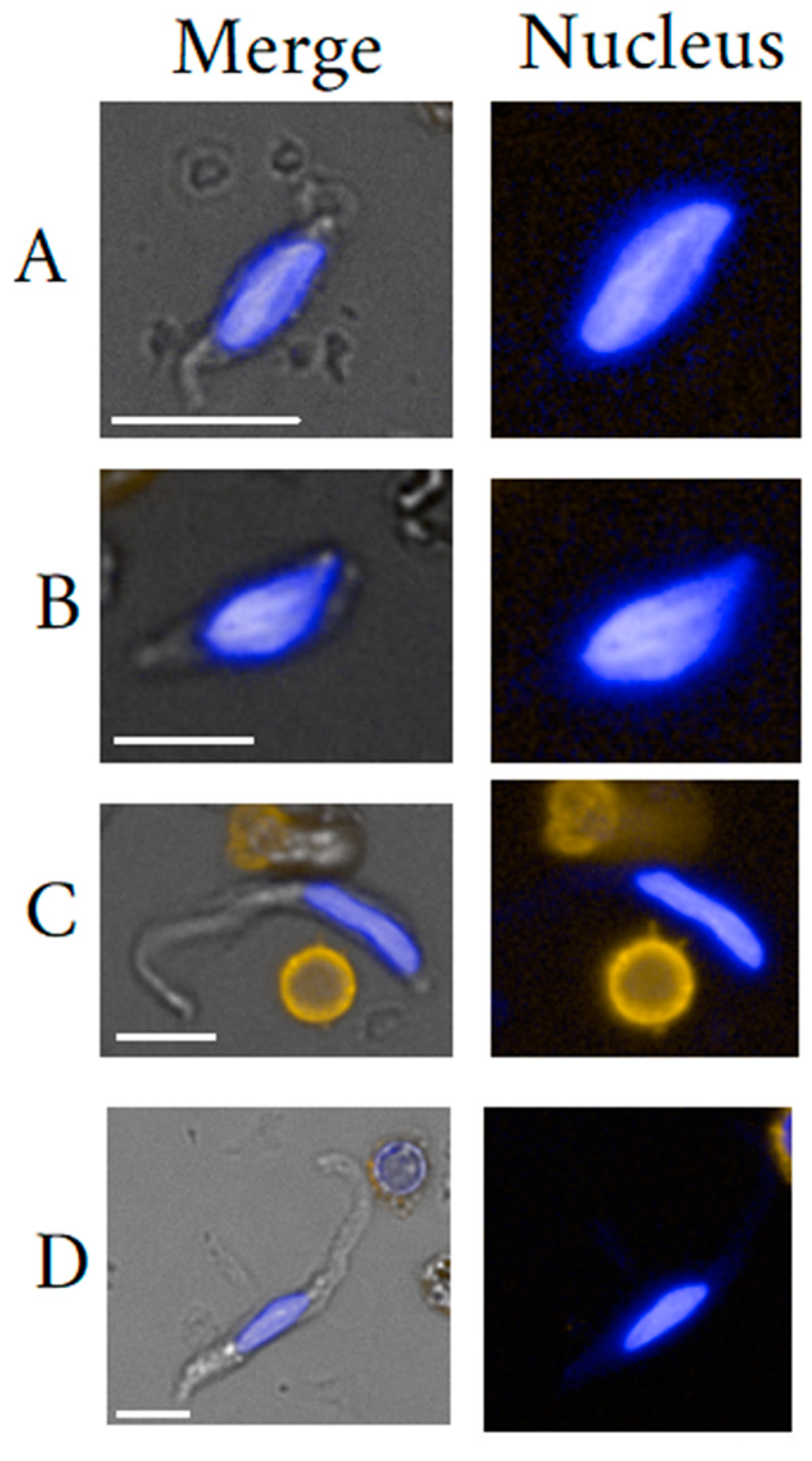
3. Endothelial Cells
3.1. Endothelial Cell General Background
3.2. Circulating Endothelial Cells
3.3. Endothelial Cell Isolation
3.4. Endothelial Cell Clinical Use
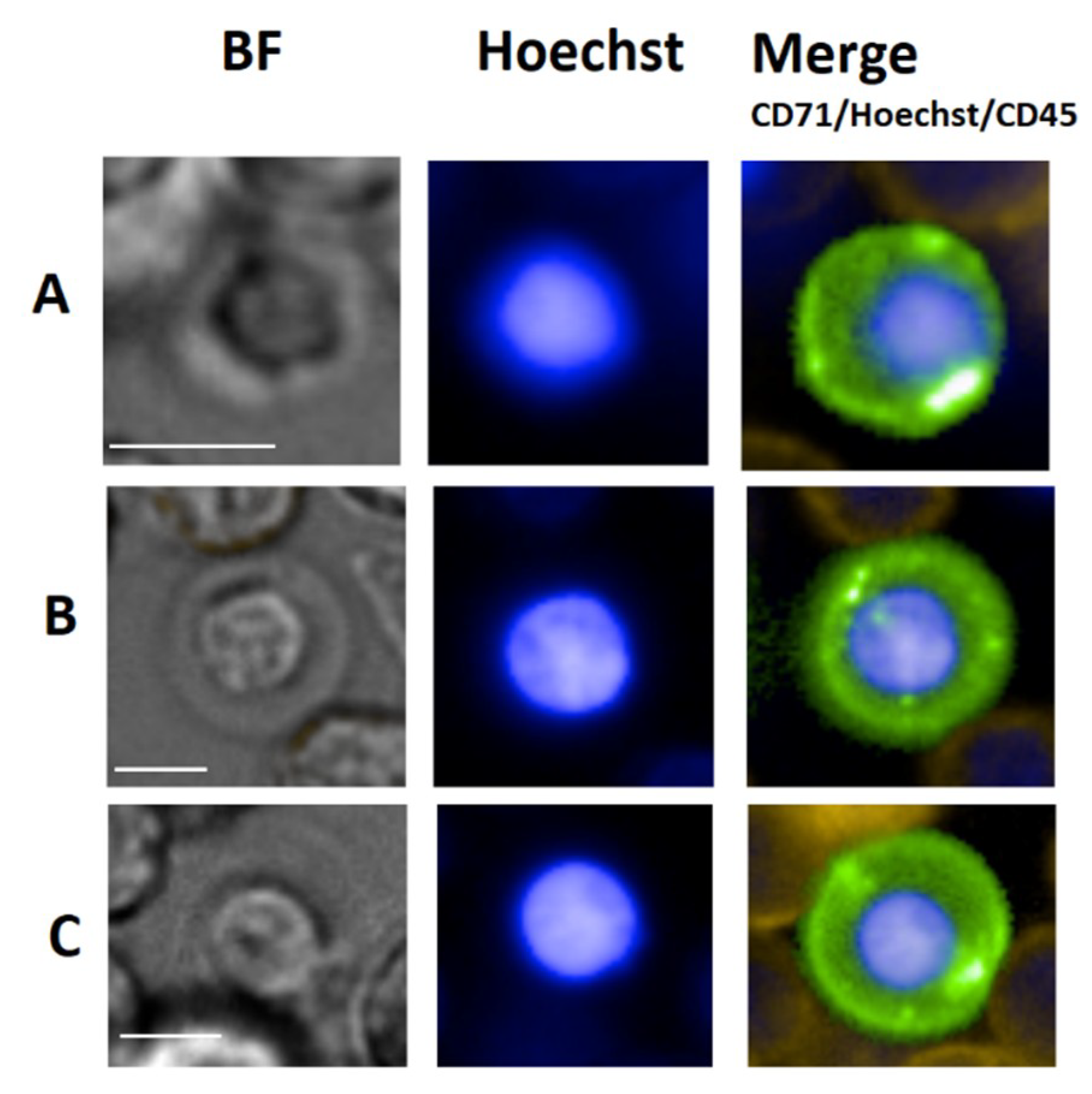
4. Erythroblasts
4.1. Erythroblast General Background
4.2. Circulating Erythroblasts
4.3. Erythroblast Isolation
4.4. Erythroblast Clinical Use
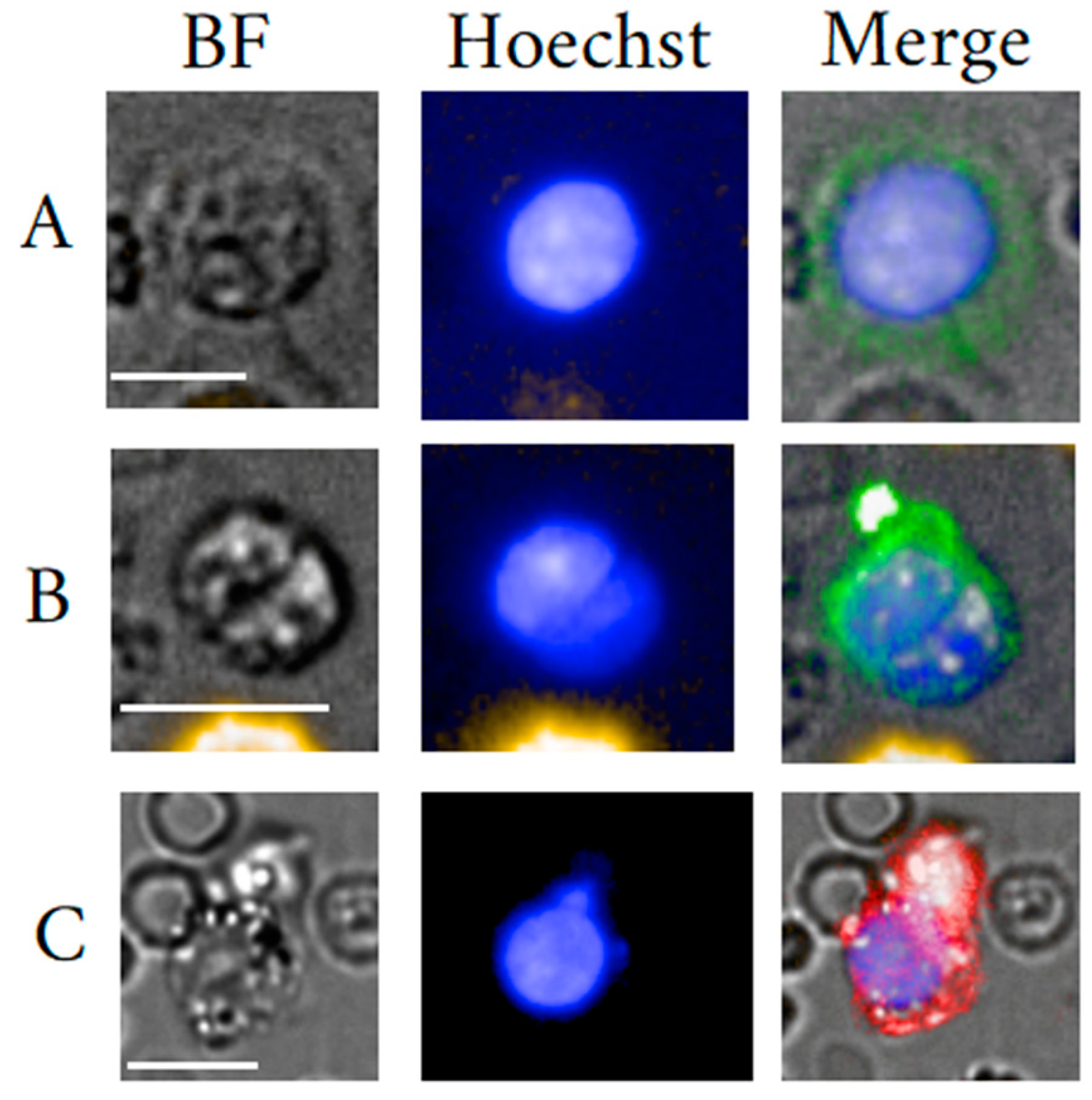
5. Fibroblast-like Cells
5.1. Fibrocyte General Background
5.2. Mesenchymal Stem Cells General Background
5.3. Circulating Fibroblast-Like Cells Clinical Usefulness
6. Very Small Embryonic Stem Cells
6.1. Very Small Embryonic Stem Cell General Background
6.2. VSEL Stem Cell Clinical Usefulness
7. Epithelial Cells
7.1. Epithelial Cell General Background
7.2. Circulating Epithelial Cells
7.3. Circulating EC Usefulness
8. Miscellaneous
9. Circulating Rare Cell Population Concentrations
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Allard, W.J. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients with Nonmalignant Diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 10936. [Google Scholar] [CrossRef] [PubMed]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and Migratory Activity of Circulating Endothelial Progenitor Cells Inversely Correlate with Risk Factors for Coronary Artery Disease. Circ. Res. 2001, 89, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Gilpin, S.; Ask, K.; Cox, G.; Cook, D.; Gauldie, J.; Margetts, P.J.; Farkas, L.; Dobranowski, J.; Boylan, C.; et al. Circulating Fibrocytes Are an Indicator of Poor Prognosis in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Holmén, C.; Elsheikh, E.; Stenvinkel, P.; Qureshi, A.R.; Pettersson, E.; Jalkanen, S.; Sumitran-Holgersson, S. Circulating Inflammatory Endothelial Cells Contribute to Endothelial Progenitor Cell Dysfunction in Patients with Vasculitis and Kidney Involvement. J. Am. Soc. Nephrol. 2005, 16, 3110–3120. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Avogaro, A. It Is All in the Blood: The Multifaceted Contribution of Circulating Progenitor Cells in Diabetic Complications. Exp. Diabetes Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, A.; Robak, T.; Krawczyńska, A.; Pluta, A.; Cebula, B.; Smolewski, P.; Wrzesien-Kus, A. Circulating endothelial cells in patients with acute myeloid leukemia. Eur. J. Haematol. 2005, 75, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Valerio, D.; Aiello, R.; Altieri, V.; Malato, A.P.; Fortunato, A.; Canazio, A. Culture of Fetal Erythroid Progenitor Cells from Maternal Blood for Non-Invasive Prenatal Genetic Diagnosis. Prenat. Diagn. 1996, 16, 1073–1082. [Google Scholar] [CrossRef]
- Wei, X.; Cai, B.; Chen, K.; Cheng, L.; Zhu, Y.; Wang, Z.; Sun, Y.; Liu, W.; Guo, S.-S.; Zhang, Y.; et al. Enhanced isolation and release of fetal nucleated red blood cells using multifunctional nanoparticle-based microfluidic device for non-invasive prenatal diagnostics. Sens. Actuators B: Chem. 2019, 281, 131–138. [Google Scholar] [CrossRef]
- Schreier, S.; Borwornpinyo, S.; Udomsangpetch, R.; Triampo, W. An update of circulating rare cell types in healthy adult peripheral blood: Findings of immature erythroid precursors. Ann. Transl. Med. 2018, 6, 406. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, X.; Guo, T.; Chan, P.Y.; Shaw, G.; Hines, J.; Stankiewicz, E.; Wang, Y.; Oliver, R.T.D.; Ahmad, A.S.; et al. The Novel Association of Circulating Tumor Cells and Circulating Megakaryocytes with Prostate Cancer Prognosis. Clin. Cancer Res. 2017, 23, 5112–5122. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulou, T. Bone marrow homing: The players, the playfield, and their evolving roles. Curr. Opin. Hematol. 2003, 10, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, E.; Claassen, M. Sensitive detection of rare disease-associated cell subsets via representation learning. Nat. Commun. 2017, 8, 14825. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Weiss, M.J.; Poncz, M. Megakaryocyte biology and related disorders. J. Clin. Investig. 2005, 115, 3332–3338. [Google Scholar] [CrossRef] [PubMed]
- Camarata, T.; Topczewski, J.; Simon, H.-G. Lmp4 regulates Tbx5 during zebrafish heart development. Dev. Boil. 2006, 295, 460–461. [Google Scholar] [CrossRef][Green Version]
- Redmond, J.; Kantor, R.S.; Auerbach, H.E.; Spiritos, M.D.; Moore, J.T. Extramedullary hematopoiesis during therapy with granulocyte colony-stimulating factor. Arch. Pathol. Lab. Med. 1994, 118, 1014–1015. [Google Scholar]
- Jackson, W.; Chandler, P.; Sosnoski, D.M.; Ohanessian, S.E.; Mobley, A.; Meisel, K.D.; Mastro, A.M. Role of Megakaryocytes in Breast Cancer Metastasis to Bone. Cancer Res. 2017, 77, 1942–1954. [Google Scholar] [CrossRef]
- Cunin, P.; Nigrovic, P. Megakaryocytes as immune cells. J. Leukoc. Boil. 2019, 105, 1111–1121. [Google Scholar] [CrossRef]
- Frydman, G.; Ellett, F.; Jorgensen, J.; Marand, A.; Zukerberg, L.; Selig, M.; Tessier, S.; Wong, K.; Olaleye, D.; Vanderburg, C.; et al. 1686. Crit. Care Med. 2020, 48, 818. [Google Scholar] [CrossRef]
- Maroni, P. Megakaryocytes in Bone Metastasis: Protection or Progression? Cells 2019, 8, 134. [Google Scholar] [CrossRef]
- Levine, R.F.; Eldor, A.; Shoff, P.K.; Kirwin, S.; Tenza, D.; Cramer, E.M. Circulating megakaryocytes: Delivery of large numbers of intact, mature megakaryocytes to the lungs. Eur. J. Haematol. 2009, 51, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Zucker-Franklin, D.; Philipp, C.S. Platelet Production in the Pulmonary Capillary Bed. Am. J. Pathol. 2000, 157, 69–74. [Google Scholar] [CrossRef]
- Ravid, K.; Lu, J.; Zimmet, J.M.; Jones, M.R. Roads to polyploidy: The megakaryocyte example. J. Cell. Physiol. 2002, 190, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Mattia, G.; Vulcano, F.; Milazzo, L.; Barca, A.; Macioce, G.; Giampaolo, A.; Hassan, H.J. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood 2002, 99, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.M.; Airo, R.; Pollack, S.; Crosby, W.H. Circulating Megakaryocytes and Platelet Release in the Lung. Blood 1965, 26, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.R.; Cliffton, E.E.; Mercer, C.; Koss, L.G. The Megakaryocyte Blood Count. Am. J. Med. Sci. 1966, 252, 301. [Google Scholar] [CrossRef]
- Schreier, S.; Sawaisorn, P.; Udomsangpetch, R.; Triampo, W. Advances in rare cell isolation: An optimization and evaluation study. J. Transl. Med. 2017, 15, 6. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, Z.Y.; Groopman, J.E. The α-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood 1998, 92, 756–764. [Google Scholar] [CrossRef]
- Dejima, H.; Nakanishi, H.; Kuroda, H.; Yoshimura, M.; Sakakura, N.; Ueda, N.; Ohta, Y.; Tanaka, R.; Mori, S.; Yoshida, T.; et al. Detection of abundant megakaryocytes in pulmonary artery blood in lung cancer patients using a microfluidic platform. Lung Cancer 2018, 125, 128–135. [Google Scholar] [CrossRef]
- Hansen, M.; Pedersen, N.T. Circulating Megakaryocytes in Blood from the Antecubital Vein in Healthy, Adult Humans. Scand. J. Haematol. 2009, 20, 371–376. [Google Scholar] [CrossRef]
- Anand, M.; Hazarika, B.; Kumar, L.; Kumar, R.; Chopra, A. High abundance of circulating megakaryocytic cells in chronic myeloid leukemia in Indian patients. Revisiting George Minot to re-interpret megakaryocytic maturation. Blood Cells Mol. Dis. 2016, 60, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Gupta, R.J.; Kumar, S. Megakaryocytes in Peripheral Blood Smears. Turk. J. Hematol. 2019, 36, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A.; Harker, L.A.; Burstein, S.A. Purification of human megakaryocytes by fluorescence-activated cell sorting. Blood 1987, 70, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.C.; Suriyaphol, P.; Thaicharoen, P.; Bhakdi, S.C.; Komoltri, C.; Chaiyaprasithi, B.; Charnkaew, K. Accuracy of Tumour-Associated Circulating Endothelial Cells as a Screening Biomarker for Clinically Significant Prostate Cancer. Cancers 2019, 11, 1064. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.G.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef]
- Nurden, A.T. Qualitative disorders of platelets and megakaryocytes. J. Thromb. Haemost. 2005, 3, 1773–1782. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellstrom-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Ku, N.K.; Rashidi, H. Unusual finding of a megakaryocyte in a peripheral blood smear. Blood 2017, 130, 2573. [Google Scholar] [CrossRef]
- Hume, R.; West, J.T.; Malmgren, R.A.; Chu, E.A. Quantitative Observations of Circulating Megakaryocytes in the Blood of Patients with Cancer. N. Engl. J. Med. 1964, 270, 111–117. [Google Scholar] [CrossRef]
- Leversha, M.A.; Han, J.; Asgari, Z.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Anand, A.; Lilja, H.; Heller, G.; Fleisher, M.; et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 2009, 15, 2091–2097. [Google Scholar] [CrossRef]
- Kamal, M.; Leslie, M.; Horton, C.; Hills, N.; Davis, R.; Nguyen, R.; Razaq, M.; Moxley, K.; Hofman, P.; Hofman, R.; et al. Cytopathologic identification of circulating tumor cells (CTCs) in breast cancer: Application of size-based enrichment. Clin. Diagn. Pathol. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Winkelmann, M.; Stöckler, J.; Grassmuck, J.; Pfitzer, P.; Schneider, W. Ploidy Pattern of Megakaryocytes in Patients with Metastatic Tumors with and without Paraneoplastic Thrombosis and in Controls. Pathophysiol. Haemost. Thromb. 1984, 14, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, A.; Baek, K.-H.; Lynch, R.C.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.E.; Ryeom, S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood 2010, 115, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, J.A.J.; Fuller, K.; Mirzai, B.; Kavanagh, S.; So, C.-C.; Ip, H.-W.; Guo, B.; Forsyth, C.; Howman, R.; Erber, W. Dysregulation of the intrinsic apoptotic pathway mediates megakaryocytic hyperplasia in myeloproliferative neoplasms. J. Clin. Pathol. 2016, 69, 1017–1024. [Google Scholar] [CrossRef]
- Papadantonakis, N.; Matsuura, S.; Ravid, K. Megakaryocyte pathology and bone marrow fibrosis: The lysyl oxidase connection. Blood 2012, 120, 1774–1781. [Google Scholar] [CrossRef]
- Wang, M.; Feng, R.; Zhang, J.-M.; Xu, L.-L.; Feng, F.-E.; Wang, C.-C.; Wang, Q.-M.; Zhu, X.-L.; He, Y.; Xue, J.; et al. Dysregulated megakaryocyte distribution associated with nestin+ mesenchymal stem cells in immune thrombocytopenia. Blood Adv. 2019, 3, 1416–1428. [Google Scholar] [CrossRef]
- Cho, J. A paradigm shift in platelet transfusion therapy. Blood 2015, 125, 3523–3525. [Google Scholar] [CrossRef]
- Pulecio, J.; Alejo-Valle, O.; Capellera-Garcia, S.; Vitaloni, M.; Rio, P.; Mejia-Ramirez, E.; Caserta, I.; Bueren, J.; Flygare, J.; Raya, A. Direct Conversion of Fibroblasts to Megakaryocyte Progenitors. Cell Rep. 2016, 17, 671–683. [Google Scholar] [CrossRef]
- Martinez, A.F.; Miller, W.M. Enabling Large-Scale Ex Vivo Production of Megakaryocytes from CD34+Cells Using Gas-Permeable Surfaces. Stem Cells Transl. Med. 2019, 8, 658–670. [Google Scholar] [CrossRef]
- Sim, X.; Poncz, M.; Gadue, P.; French, D.L. Understanding platelet generation from megakaryocytes: Implications for in vitro–derived platelets. Blood 2016, 127, 1227–1233. [Google Scholar] [CrossRef]
- Choi, K.; Kennedy, M.; Kazarov, A.; Papadimitriou, J.C.; Keller, G. A common precursor for hematopoietic and endothelial cells. Development 1998, 125, 725–732. [Google Scholar]
- Blancas, A.A.; Wong, L.E.; Glaser, D.E.; McCloskey, K.E. Specialized Tip/Stalk-Like and Phalanx-Like Endothelial Cells from Embryonic Stem Cells. Stem Cells Dev. 2012, 22, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Calleri, A.; Cassi, C.; Gobbi, A.; Capillo, M.; Pruneri, G.; Martinelli, G.; Bertolini, F. Circulating endothelial cells as a novel marker of angiogenesis. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Boston, MA, USA, 2003; Volume 522, pp. 83–97. [Google Scholar]
- Strijbos, M.H.; Gratama, J.W.; Kraan, J.; Lamers, C.H.; Bakker, M.D.; Sleijfer, S. Circulating endothelial cells in oncology: Pitfalls and promises. Br. J. Cancer 2008, 98, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Khakoo, A.Y.; Finkel, T. Endothelial progenitor cells. Annu. Rev. Med. 2005, 56, 79–101. [Google Scholar] [CrossRef]
- Richardson, M.R.; Yoder, M.C. Endothelial progenitor cells: Quo Vadis? J. Mol. Cell. Cardiol. 2010, 50, 266–272. [Google Scholar] [CrossRef]
- Fadini, G.P.; Losordo, U.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef]
- Ali, A.M.; Ueno, T.; Tanaka, S.; Takada, M.; Ishiguro, H.; Abdellah, A.Z.; Toi, M. Determining circulating endothelial cells using CellSearch system during preoperative systemic chemotherapy in breast cancer patients. Eur. J. Cancer 2011, 47, 2265–2272. [Google Scholar] [CrossRef]
- Kraan, J.; Strijbos, M.H.; Sieuwerts, A.M.; Foekens, J.A.; Bakker, M.A.D.; Verhoef, C.; Sleijfer, S.; Gratama, J.W. A new approach for rapid and reliable enumeration of circulating endothelial cells in patients. J. Thromb. Haemost. 2012, 10, 931–939. [Google Scholar] [CrossRef]
- Shih, I. The role of CD146 (Mel-CAM) in biology and pathology. J. Pathol. 1999, 189, 4–11. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Gootjes, E.C.; Kraan, J.; Buffart, T.E.; Bakkerus, L.; Zonderhuis, B.M.; Verhoef, C.; Verheul, H.M.; Sleijfer, S. CD276-Positive Circulating Endothelial Cells Do Not Predict Response to Systemic Therapy in Advanced Colorectal Cancer. Cells 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Bearzi, C.; Leri, A.; Monaco, F.L.; Rota, M.; Gonzalez, A.; Hosoda, T.; Pepe, M.; Qanud, K.; Ojaimi, C.; Bardelli, S.; et al. Identification of a coronary vascular progenitor cell in the human heart. Proc. Natl. Acad. Sci. USA 2009, 106, 15885–15890. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, G.C.; Awad, O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2003, 276, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Hatch, A.; Antontsev, V.G.; Murthy, S.K.; Melero-Martin, J.M. Microfluidic Capture of Endothelial Colony-Forming Cells from Human Adult Peripheral Blood: Phenotypic and Functional Validation In Vivo. Tissue Eng. Part C Methods 2014, 21, 274–283. [Google Scholar] [CrossRef]
- Bethel, K.; Luttgen, M.S.; Damani, S.; Kolatkar, A.; Lamy, R.; Sabouri-Ghomi, M.; Topol, S.; Topol, E.J.; Kuhn, P. Fluid phase biopsy for detection and characterization of circulating endothelial cells in myocardial infarction. Phys. Boil. 2014, 11, 016002. [Google Scholar] [CrossRef]
- Bouvier, C.A. Circulating endothelium as an indicator of vascular injury. Thromb. Diath. Haemorrh. 1970, 40, 163. [Google Scholar]
- Katayama, Y.; Battista, M.; Kao, W.-M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Investig. 2000, 105, 71–77. [Google Scholar] [CrossRef]
- Mund, J.A.; Estes, M.L.; Yoder, M.C.; Ingram, D.A.; Case, J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arter. Thromb. Vasc. Boil. 2012, 32, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Plouffe, B.; Hatch, A.; Von Gise, A.; Sallmon, H.; Zamanian, R.T.; Murthy, S.K. Design and validation of an endothelial progenitor cell capture chip and its application in patients with pulmonary arterial hypertension. J. Mol. Med. 2011, 89, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Matos, R.; Morais, S.; Campos, M.; Lima, M. Soluble endothelial cell molecules and circulating endothelial cells in patients with venous thromboembolism. Blood Coagul. Fibrinolysis 2017, 28, 589–595. [Google Scholar] [CrossRef]
- Alessio, A.M.; Beltrame, M.P.; Nascimento, M.C.F.; Vicente, C.P.; Ap De Godoy, J.; Silva, J.C.S.; Bittar, L.F.; Lorand-Metze, I.G.; De Paula, E.; Annichino-Bizzacchi, J.M. Circulating Progenitor and Mature Endothelial Cells in Deep Vein Thrombosis. Int. J. Med. Sci. 2013, 10, 1746–1754. [Google Scholar] [CrossRef]
- Dignat-George, F.; Sampol, J.; Lip, G.; Blann, A.D. Circulating Endothelial Cells: Realities and Promises in Vascular Disorders. Pathophysiol. Haemost. Thromb. 2003, 33, 495–499. [Google Scholar] [CrossRef]
- Ilié, M.; Long, E.; Hofman, V.; Selva, E.; Bonnetaud, C.; Boyer, J.; Venissac, N.; Sanfiorenzo, C.; Ferrua, B.; Marquette, C.-H.; et al. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br. J. Cancer 2014, 110, 1236–1243. [Google Scholar] [CrossRef]
- Goon, P.; Lip, G.; Boos, C.; Stonelake, P.; Blann, A.D. Circulating Endothelial Cells, Endothelial Progenitor Cells, and Endothelial Microparticles in Cancer. Neoplasia 2006, 8, 79–88. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, W.; Lu, D.; Wu, Z.; Duan, H.; Luo, Y.; Feng, J.; Yang, N.; Fu, L.; Yan, X. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2011, 109, 1127–1132. [Google Scholar] [CrossRef]
- Kristiansen, G.; Yu, Y.; Schlüns, K.; Sers, C.; Dietel, M.; Petersen, I. Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal. Cell. Pathol. 2003, 25, 77–81. [Google Scholar] [CrossRef]
- Oka, S.; Uramoto, H.; Chikaishi, Y.; Tanaka, F. The expression of CD146 predicts a poor overall survival in patients with adenocarcinoma of the lung. Anticancer. Res. 2012, 32, 861–864. [Google Scholar]
- Rahbari, N.N.; Schölch, S.; Bork, U.; Kahlert, C.; Schneider, M.; Rahbari, M.; Büchler, M.W.; Weitz, J.; Reissfelder, C. Prognostic value of circulating endothelial cells in metastatic colorectal cancer. Oncotarget 2017, 8, 37491–37501. [Google Scholar] [CrossRef] [PubMed]
- Kawaishi, M.; Fujiwara, Y.; Fukui, T.; Kato, T.; Yamada, K.; Ohe, Y.; Kunitoh, H.; Sekine, I.; Yamamoto, N.; Nokihara, H.; et al. Circulating Endothelial Cells in Non-small Cell Lung Cancer Patients Treated with Carboplatin and Paclitaxel. J. Thorac. Oncol. 2009, 4, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-Q.; Ding, H.; Garfield, D.H.; Gu, A.; Pei, J.; Du, W.-D. Can Determination of Circulating Endothelial Cells and Serum Caspase-Cleaved CK18 Predict for Response and Survival in Patients with Advanced Non–Small-Cell Lung Cancer Receiving Endostatin and Paclitaxel–Carboplatin Chemotherapy? A Retrospective Study. J. Thorac. Oncol. 2012, 7, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, J.; Wei, X.; Wang, L.; Lin, L.; Liu, Z.; Wang, X.; Sun, B.; Li, K. Circulating endothelial cells and tumor blood volume as predictors in lung cancer. Cancer Sci. 2013, 104, 445–452. [Google Scholar] [CrossRef]
- Lin, P.P.; Gires, O.; Wang, D.D.; Li, L.; Wang, H.-X. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci. Rep. 2017, 7, 9789. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wang, Z.; Gu, M.; Li, Y.; Wang, D.D.; Li, W.; Lin, P.P. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2019, 469, 355–366. [Google Scholar] [CrossRef]
- Nichols, W.W.; Buynak, E.B.; Bradt, C.; Hill, R.; Aronson, M.; Jarrell, B.E.; Mueller, S.N.; Levine, E.M. Cytogenetic evaluation of human endothelial cell cultures. J. Cell. Physiol. 1987, 132, 453–462. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I.; Larsen, G.; Hallstrom, A.; McAnulty, J.; Pinski, S.; et al. Endothelial Cell Senescence in Human Atherosclerosis. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef]
- Borradaile, N.M.; Pickering, J.G. Polyploidy impairs human aortic endothelial cell function and is prevented by nicotinamide phosphoribosyltransferase. Am. J. Physiol. Physiol. 2010, 298, C66–C74. [Google Scholar] [CrossRef]
- Rhone, P.; Ruszkowska-Ciastek, B.; Celmer, M.; Brkic, A.; Bielawski, K.; Boinska, J.; Zarychta, E.; Rosc, D. Increased number of endothelial progenitors in peripheral blood as a possible early marker of tumour growth in post-menopausal breast cancer patients. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2017, 68, 139–148. [Google Scholar]
- Zahran, A.M.; Abdel-Rahim, M.H.; Refaat, A.; Sayed, M.; Othman, M.M.; Khalak, L.M.R.; Hetta, H.F. Circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in hepatocellular carcinoma patients: Contribution to diagnosis and prognosis. Acta Oncol. 2019, 59, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Solomon, M.A.; McCoy, J.P. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytom. Part B: Clin. Cytom. 2005, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dignat-George, F.; Blann, A.; Sampol, J. Circulating endothelial cells in acute coronary syndromes. Blood 2000, 95, 728. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, I.; Dimitrakopoulou, N.; Kafasi, N.; Tentolouris, A.; Dimitrakopoulou, A.; Anastasiou, I.A.; Mourouzis, I.; Jude, E.; Tentolouris, N. Endothelial progenitor cells and peripheral neuropathy in subjects with type 2 diabetes mellitus. J. Diabetes Complicat. 2020, 34, 107517. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, J.H.; Cha, R.R.; Kim, W.S.; Lee, J.M.; Lee, S.S.; Kim, H.J.; Lee, C.M.; Kim, H.J.; Ha, C.Y.; et al. Clinical significance of endothelial progenitor cells in patients with liver cirrhosis with or without hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2020, 32, 87–94. [Google Scholar] [CrossRef]
- Farinacci, M.; Krahn, T.; Dinh, W.; Volk, H.-D.; Düngen, H.-D.; Wagner, J.; Konen, T.; Von Ahsen, O. Circulating endothelial cells as biomarker for cardiovascular diseases. Res. Pract. Thromb. Haemost. 2018, 3, 49–58. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Rössig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kämper, U.; Dimmeler, S.; Zeiher, A.M. Reduced Number of Circulating Endothelial Progenitor Cells Predicts Future Cardiovascular Events. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chang, L.-T.; Tung, W.-C.; Chen, Y.-L.; Chang, C.-L.; Leu, S.; Sun, C.-K.; Tsai, T.-H.; Tsai, I.-T.; Chang, H.-W.; et al. Levels and values of circulating endothelial progenitor cells, soluble angiogenic factors, and mononuclear cell apoptosis in liver cirrhosis patients. J. Biomed. Sci. 2012, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Sabulski, A.; Aunins, B.; Abdullah, S.; Luebbering, N.; Davies, S.M. Circulating Endothelial Cells As a Marker of Vascular Injury in Pediatric Patients. Boil. Blood Marrow Transplant. 2020, 26, S144–S145. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; Khan, Z.A.; Picard, A.; Wu, X.; Paruchuri, S.; Bischoff, J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 2007, 109, 4761–4768. [Google Scholar] [CrossRef] [PubMed]
- Moonen, J.R.A.J.; Krenning, G.; Brinker, M.G.L.; Koerts, J.A.; Van Luyn, M.J.; Harmsen, M.C. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc. Res. 2010, 86, 506–515. [Google Scholar] [CrossRef]
- Schatteman, G.C.; Hanlon, H.D.; Jiao, C.; Dodds, S.; Christy, B.A. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J. Clin. Investig. 2000, 106, 571–578. [Google Scholar] [CrossRef]
- Michaud, S. Élise; Dussault, S.; Haddad, P.; Groleau, J.; Rivard, A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis 2006, 187, 423–432. [Google Scholar] [CrossRef]
- Magalhães, F.D.C.; Aguiar, P.F.; Tossige-Gomes, R.; Magalhães, S.M.; Ottone, V.D.O.; Fernandes, T.; De Oliveira, E.M.; Dias-Peixoto, M.F.; Rocha-Vieira, E.; Amorim, F.T. High-intensity interval training followed by postexercise cold-water immersion does not alter angiogenic circulating cells, but increases circulating endothelial cells. Appl. Physiol. Nutr. Metab. 2020, 45, 101–111. [Google Scholar] [CrossRef]
- McGrath, K.E.; Bushnell, T.; Palis, J. Multispectral imaging of hematopoietic cells: Where flow meets morphology. J. Immunol. Methods 2008, 336, 91–97. [Google Scholar] [CrossRef]
- Dong, H.Y.; Wilkes, S.; Yang, H. CD71 is Selectively and Ubiquitously Expressed at High Levels in Erythroid Precursors of All Maturation Stages. Am. J. Surg. Pathol. 2011, 35, 723–732. [Google Scholar] [CrossRef]
- Choolani, M.; Casagrandi, D.; Bearfield, C.; Geary, J.; Redman, C.; Muttukrishna, S. Characterization of first trimester fetal erythroblasts for non-invasive prenatal diagnosis. Mol. Hum. Reprod. 2003, 9, 227–235. [Google Scholar] [CrossRef]
- Yurchenko, V.; Constant, S.; Eisenmesser, E.; Bukrinsky, M. Cyclophilin–CD147 interactions: A new target for anti-inflammatory therapeutics. Clin. Exp. Immunol. 2010, 160, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Stachon, A.; Kempf, R.; Holland-Letz, T.; Friese, J.; Becker, A.; Krieg, M. Daily monitoring of nucleated red blood cells in the blood of surgical intensive care patients. Clin. Chim. Acta 2006, 366, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tong, X.; Wu, L.; He, G.; Ding, B.; Yao, L.; Liu, Y. A Comparison of in vitro Culture of Fetal Nucleated Erythroblasts from Fetal Chorionic Villi and Maternal Peripheral Blood for Noninvasive Prenatal Diagnosis. Fetal Diagn. Ther. 2012, 32, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Griffin, D.K.; Jestice, K.; Hackett, G.; Cooper, J.; Ferguson-Smith, M.A. Evaluating the culture of fetal erythroblasts from maternal blood for non-invasive prenatal diagnosis. Prenat. Diagn. 1998, 18, 883–892. [Google Scholar] [CrossRef]
- Kuert, S.; Holland-Letz, T.; Friese, J.; Stachon, A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin. Chem. Lab. Med. 2011, 49, 257–263. [Google Scholar] [CrossRef]
- Paul, M.S.; Paolucci, S.; Barjesteh, N.; Wood, R.D.; Sharif, S. Chicken erythrocytes respond to Toll-like receptor ligands by up-regulating cytokine transcripts. Res. Veter- Sci. 2013, 95, 87–91. [Google Scholar] [CrossRef]
- Troeger, C.; Zhong, X.; Burgemeister, R.; Minderer, S.; Tercanli, S.; Holzgreve, W.; Hahn, S. Approximately half of the erythroblasts in maternal blood are of fetal origin. Mol. Hum. Reprod. 1999, 5, 1162–1165. [Google Scholar] [CrossRef]
- Kwon, K.H.; Jeon, Y.J.; Hwang, H.S.; Lee, K.A.; Kim, Y.J.; Chung, H.W.; Pang, M.-G. A high yield of fetal nucleated red blood cells isolated using optimal osmolality and a double-density gradient system. Prenat. Diagn. 2007, 27, 1245–1250. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Carter, N.P.; Price, C.M.; Colman, S.M.; Milton, P.J.; Hackett, G.A.; Greaves, M.F.; Ferguson-Smith, M.A. Prenatal diagnosis from maternal blood: Simultaneous immunophenotyping and FISH of fetal nucleated erythrocytes isolated by negative magnetic cell sorting. J. Med. Genet. 1993, 30, 1051–1056. [Google Scholar] [CrossRef]
- Sneha Rao, A.R.; Rao, P.S.; Sandhya, I.; Shalini, P. Spectrum of Diseases with Nucleated RBCs. APALM 2019, 6. [Google Scholar] [CrossRef]
- Da Rin, G.; Vidali, M.; Balboni, F.; Benegiamo, A.; Borin, M.; Ciardelli, M.L.; Dima, F.; Di Fabio, A.; Fanelli, A.; Fiorini, F.; et al. Performance evaluation of the automated nucleated red blood cell count of five commercial hematological analyzers. Int. J. Lab. Hematol. 2017, 39, 663–670. [Google Scholar] [CrossRef]
- May, J.E.; Marques, M.B.; Reddy, V.V.; Gangaraju, R. Three neglected numbers in the CBC: The RDW, MPV, and NRBC count. Clevel. Clin. J. Med. 2019, 86, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Buoro, S.; Manenti, B.; Seghezzi, M. Which clinical significance has automatic detection of very low levels of nucleated red blood cells in the peripheral blood? Ann. Transl. Med. 2016, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Rowan, R.M. The clinical relevance of nucleated red blood cell counts. Sysmex J. Internat. 2000, 10, 59–63. [Google Scholar]
- Harrison, C.N.; McLornan, D.P. Current treatment algorithm for the management of patients with myelofibrosis, JAK inhibitors, and beyond. Hematology 2017, 2017, 489–497. [Google Scholar] [CrossRef]
- Phan, T.-T.; Vy, H.T.; Ho, T.T.; Tran, V.T.; Tran, T.T.; Pho, S.P.; Pham, T.T.B.; Le, T.T.; Nguyen, S.T. Emergence role of nucleated red blood cells in molecular response evaluation for chronic myeloid leukemia. Int. J. Gen. Med. 2019, 12, 333–341. [Google Scholar] [CrossRef]
- Desai, S.; Jones, S.L.; Turner, K.L.; Hall, J.; Moore, L.J. Nucleated Red Blood Cells Are Associated with a Higher Mortality Rate in Patients with Surgical Sepsis. Surg. Infect. 2012, 13, 360–365. [Google Scholar] [CrossRef]
- Menk, M.; Giebelhäuser, L.; Vorderwülbecke, G.; Gassner, M.; Graw, J.A.; Weiss, B.; Zimmermann, M.; Wernecke, K.-D.; Weber-Carstens, S. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): An observational study. Ann. Intensiv. Care 2018, 8, 42. [Google Scholar] [CrossRef]
- Monteiro Júnior, J.G.D.M.; Torres, D.D.O.C.; Da Silva, M.C.F.C.; Príncipe, T.R.N.; De Vasconcelos, R.B.; De Brito, M.E.C.; Limeira, M.A.A.; Dos Santos, A.C.O.; Montarroyos, U.R.; Filho, D.C.S. Performance of a Hematological Scoring System in Predicting All-Cause Mortality in Patients with Acute Myocardial Infarction. Int. J. Cardiovasc. Sci. 2019. [Google Scholar] [CrossRef]
- Stachon, A.; Segbers, E.; Holland-Letz, T.; Kempf, R.; Hering, S.; Krieg, M. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: A prospective cohort study. Crit. Care 2007, 11, R62. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R.; Spiegel, L.A.; Chesney, J.; Hogan, M.; Cerami, A. Circulating Fibrocytes Define a New Leukocyte Subpopulation That Mediates Tissue Repair. Mol. Med. 1994, 1, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Rennert, R.C.; Rodrigues, M.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Fujiwara, T.; Longaker, M.T.; Gurtner, G.C. Tracking the elusive fibrocyte: Identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014, 32, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Cowper, S.; Wu, S.-P.; Bockenstedt, L.K.; Bucala, R. Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int. J. Biochem. Cell Boil. 2004, 36, 598–606. [Google Scholar] [CrossRef]
- Inoue, T.; Plieth, D.; Venkov, C.D.; Xu, C.; Neilson, E.G. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005, 67, 2488–2493. [Google Scholar] [CrossRef]
- Choi, Y.H.; Burdick, M.D.; Strieter, R.M. Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. Int. J. Biochem. Cell Boil. 2009, 42, 662–671. [Google Scholar] [CrossRef]
- Heukels, P.; Van Hulst, J.A.C.; Van Nimwegen, M.; Boorsma, C.E.; Melgert, B.N.; Toorn, L.M.V.D.; Boomars, K.A.T.; Wijsenbeek, M.S.; Hoogsteden, H.; Von Der Thusen, J.H.; et al. Fibrocytes are increased in lung and peripheral blood of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 90. [Google Scholar] [CrossRef]
- Galligan, C.L.; Fish, E.N. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum. 2012, 64, 3583–3593. [Google Scholar] [CrossRef]
- Kao, H.-K.; Chen, B.; Murphy, G.F.; Li, Q.; Orgill, D.; Guo, L. Peripheral Blood Fibrocytes. Ann. Surg. 2011, 254, 1066–1074. [Google Scholar] [CrossRef]
- Skinner, B.; Johnson, E.E.P. Nuclear morphologies: Their diversity and functional relevance. Chromosomes 2016, 126, 195–212. [Google Scholar] [CrossRef]
- Herzog, E.L.; Bucala, R. Fibrocytes in health and disease. Exp. Hematol. 2010, 38, 548–556. [Google Scholar] [CrossRef]
- Phillips, R.J.; Burdick, M.D.; Hong, K.; Lutz, M.A.; Murray, L.A.; Xue, Y.Y.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Investig. 2004, 114, 438–446. [Google Scholar] [CrossRef]
- Ishii, G.; Sangai, T.; Sugiyama, K.; Ito, T.; Hasebe, T.; Endoh, Y.; Magae, J.; Ochiai, A. In Vivo Characterization of Bone Marrow-Derived Fibroblasts Recruited into Fibrotic Lesions. Stem Cells 2005, 23, 699–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pilling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R.H. Identification of Markers that Distinguish Monocyte-Derived Fibrocytes from Monocytes, Macrophages, and Fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb. Haemost. 2009, 101, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Juarez, C.F.; Dedhia, P.H.; Jin, S.; Ruiz-Vega, R.; Ma, D.; Liu, Y.; Yamaga, K.; Shestova, O.; Gay, D.L.; Yang, Z.; et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat. Commun. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Siddiqui, J.; Pienta, K.J.; Getzenberg, R.H. Circulating fibroblast-like cells in men with metastatic prostate cancer. Prostate 2012, 73, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.B.; Kolodsick, J.E.; Thannickal, V.J.; Cooke, K.; Moore, B.B.; Hogaboam, C.; Wilke, C.A.; Toews, G.B. CCR2-Mediated Recruitment of Fibrocytes to the Alveolar Space after Fibrotic Injury. Am. J. Pathol. 2005, 166, 675–684. [Google Scholar] [CrossRef]
- Flavell, S.J.; Hou, T.Z.; Lax, S.; Filer, A.D.; Salmon, M.; Buckley, C.D. Fibroblasts as novel therapeutic targets in chronic inflammation. Br. J. Pharmacol. 2007, 153, S241–S246. [Google Scholar] [CrossRef]
- Just, S.A.; Lindegaard, H.M.; Hejbøl, E.K.; Davidsen, J.R.; Bjerring, N.; Hansen, S.; Schrøder, H.D.; Hansen, I.M.J.; Barington, T.; Nielsen, C. Fibrocyte measurement in peripheral blood correlates with number of cultured mature fibrocytes in vitro and is a potential biomarker for interstitial lung disease in Rheumatoid Arthritis. Respir. Res. 2017, 18, 141. [Google Scholar] [CrossRef]
- Caplan, A. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Kassem, M. Circulating osteogenic cells: Implications for injury, repair, and regeneration. J. Bone Miner. Res. 2011, 26, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W.Y.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J. Orthop. Transl. 2019, 19, 18–28. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.-J.; Wu, J.-C.; Hu, M.S.; Sanyal, M.; Hu, M.; Longaker, M.T.; Lorenz, H. Peripheral Blood-Derived Mesenchymal Stem Cells: Candidate Cells Responsible for Healing Critical-Sized Calvarial Bone Defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Q.; Lin, J.; Lai, X.; Cao, W.; Du, K.; Wang, Y.; Wu, K.; Hu, Y.; Zhang, L.; et al. Hypoxia-Inducible Factor-1α Is Essential for Hypoxia-Induced Mesenchymal Stem Cell Mobilization into the Peripheral Blood. Stem Cells Dev. 2011, 20, 1961–1971. [Google Scholar] [CrossRef]
- He, Q.; Wan, C.; Li, G. Concise Review: Multipotent Mesenchymal Stromal Cells in Blood. Stem Cells 2006, 25, 69–77. [Google Scholar] [CrossRef]
- Cornelissen, A.S.; Maijenburg, M.W.; Nolte, M.; Voermans, C. Organ-specific migration of mesenchymal stromal cells: Who, when, where and why? Immunol. Lett. 2015, 168, 159–169. [Google Scholar] [CrossRef]
- Roufosse, C.; Direkze, N.; Otto, W.; Wright, N.A. Circulating mesenchymal stem cells. Int. J. Biochem. Cell Boil. 2004, 36, 585–597. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Wang, Y.; Wu, G.; Qiu, S.; Liu, C.; Tan, Z.; Guo, J.; Zhu, L. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect. Tissue Res. 2019, 60, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Qadan, M.A.; Piuzzi, N.S.; Boehm, C.; Bova, W.; Moos, M.; Midura, R.J.; Hascall, V.C.; Malcuit, C.; Muschler, G. Variation in primary and culture-expanded cells derived from connective tissue progenitors in human bone marrow space, bone trabecular surface and adipose tissue. Cytotherapy 2018, 20, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006, 37, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Simon, V.; Herrera, G.; Cao, C.; Del Favero, H.; Minguell, J.J. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997, 20, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.P.; Waldron, J.A.; Upuda, K.B.; Lipschitz, D.A. Morphological characterization of stromal cell types in hematopoietically active long-term murine bone marrow cultures. J. Histochem. Cytochem. 1995, 43, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Farrington-Rock, C.; Crofts, N.; Doherty, M.; Ashton, B.; Griffin-Jones, C.; Canfield, A. Chondrogenic and Adipogenic Potential of Microvascular Pericytes. Circulation 2004, 110, 2226–2232. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P. Circulating Skeletal Stem Cells. J. Cell Boil. 2001, 153, 1133–1140. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Precursor Cells of Mechanocytes. Adv. Clin. Chem. 1976, 47, 327–359. [Google Scholar]
- Darby, I.; Hewitson, T. Fibroblast Differentiation in Wound Healing and Fibrosis. Adv. Clin. Chem. 2007, 257, 143–179. [Google Scholar]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, M.; Heaysman, J.E.; Pegrum, S.M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp. Cell Res. 1971, 67, 359–367. [Google Scholar] [CrossRef]
- Shamis, Y.; Hewitt, K.J.; Carlson, M.W.; Margvelashvili-Malament, M.; Dong, S.; Kuo, C.K.; Dahéron, L.; Egles, C.; Garlick, J.A. Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents. Stem Cell Res. Ther. 2011, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Hematti, P. Mesenchymal stromal cells and fibroblasts: A case of mistaken identity? Cytotherapy 2012, 14, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Hematti, P. Role of Extracellular Matrix in Cardiac Cellular Therapies. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Cham, Switzerland, 2018; Volume 1098, pp. 173–188. [Google Scholar]
- Raz, Y.; Cohen, N.; Shani, O.; Bell, R.E.; Novitskiy, S.V.; Abramovitz, L.; Levy, C.; Milyavsky, M.; Leider-Trejo, L.; Moses, H.L.; et al. Bone marrow–derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J. Exp. Med. 2018, 215, 3075–3093. [Google Scholar] [CrossRef]
- Feehan, J.; Nurgali, K.; Apostolopoulos, V.; Al Saedi, A.; Duque, G. Circulating osteogenic precursor cells: Building bone from blood. EBioMedicine 2019, 39, 603–611. [Google Scholar] [CrossRef]
- Cieslik, K.A.; Trial, J.; Carlson, S.; Taffet, G.E.; Entman, M.L. Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: Role of elevated circulating insulin levels. FASEB J. 2013, 27, 1761–1771. [Google Scholar] [CrossRef]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000, 2, 477–488. [Google Scholar] [CrossRef]
- Birk, D.E.; Trelstad, R.L. Fibroblasts Create Compartments in the Extracellular Space Where Collagen Polymerizes into Fibrils and Fibrils Associate into Bundles. Ann. N. Y. Acad. Sci. 1985, 460, 258–266. [Google Scholar] [CrossRef]
- Wiegner, R.; Rudhart, N.-E.; Barth, E.; Gebhard, F.; Lampl, L.; Huber-Lang, M.; Brenner, R.E. Mesenchymal stem cells in peripheral blood of severely injured patients. Eur. J. Trauma Emerg. Surg. 2017, 44, 627–636. [Google Scholar] [CrossRef]
- Forbes, S.J.; Russo, F.P.; Rey, V.; Burra, P.; Rugge, M.; Wright, N.A.; Alison, M. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 2004, 126, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Burdick, M.D.; Wandersee, N.J.; Shahir, K.S.; Zhang, L.; Simpson, P.M.; Strieter, R.M.; Field, J.J. Circulating fibrocytes as biomarkers of impaired lung function in adults with sickle cell disease. Blood Adv. 2017, 1, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Shipe, R.; Burdick, M.D.; Strieter, B.A.; Liu, L.; Shim, Y.M.; Sung, S.-S.; Teague, W.G.; Mehrad, B.; Strieter, R.M.; Rose, C.E. Number, activation, and differentiation of circulating fibrocytes correlate with asthma severity. J. Allergy Clin. Immunol. 2015, 137, 750–757.e3. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Su, Z.-Z.; Liang, S.-M.; Chen, Y.-Y.; Shu, X.-R.; Nie, R.-Q.; Wang, J.-F.; Xie, S.-L. Role of Circulating Fibrocytes in Cardiac Fibrosis. Chin. Med. J. 2016, 129, 326–331. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Mangold, A.; Scherz, T.; Seidl, V.; Panzenböck, A.; Ondracek, A.S.; Müller, J.; Schneider, M.; Binder, T.; Hell, L.; et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic Res. Cardiol. 2019, 114, 33. [Google Scholar] [CrossRef]
- Roife, D.; Fleming, J.B.; Gomer, R.H. Fibrocytes in the Tumor Microenvironment; Springer Science and Business Media LLC: Cham, Switzerland, 2020; Volume 1224, pp. 79–85. [Google Scholar]
- Chong, S.G.; Sato, S.; Kolb, M.; Gauldie, J. Fibrocytes and fibroblasts-Where are we now. Int. J. Biochem. Cell Boil. 2019, 116, 105595. [Google Scholar] [CrossRef]
- Reilkoff, R.A.; Bucala, R.; Herzog, E.L. Fibrocytes: Emerging effector cells in chronic inflammation. Nat. Rev. Immunol. 2011, 11, 427–435. [Google Scholar] [CrossRef]
- Cao, T.; Rajasingh, S.; Rajasingh, J. Circulating fibrocytes serve as a marker for clinical diagnosis. Ann. Transl. Med. 2016, 4, S38. [Google Scholar] [CrossRef]
- Ling, C.; Nishimoto, K.; Rolfs, Z.; Smith, L.M.; Frey, B.L.; Welham, N.V. Differentiated fibrocytes assume a functional mesenchymal phenotype with regenerative potential. Sci. Adv. 2019, 5, eaav7384. [Google Scholar] [CrossRef]
- Direkze, N.C. Bone Marrow Contribution to Tumor-Associated Myofibroblasts and Fibroblasts. Cancer Res. 2004, 64, 8492–8495. [Google Scholar] [CrossRef]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; DiPrete, B.; Betz, K.S.; et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Schellerer, V.; Langheinrich, M.; Hohenberger, W.; Croner, R.S.; Merkel, S.; Rau, T.; Sturzl, M.; Naschberger, E. Tumor-associated fibroblasts isolated from colorectal cancer tissues exhibit increased ICAM-1 expression and affinity for monocytes. Oncol. Rep. 2013, 31, 255–261. [Google Scholar] [CrossRef]
- Kimura, T.; Monslow, J.; Klampatsa, A.; Leibowitz, M.; Sun, J.; Liousia, M.; Woodruff, P.; Moon, E.; Todd, L.; Pure, E.; et al. Loss of cells expressing fibroblast activation protein has variable effects in models of TGF-β and chronic bleomycin-induced fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L271–L282. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xia, H.; Kang, L.; Sun, Q.; Su, Z.; Hao, C.; Xue, Y. Effects of Intermittent Parathyroid Hormone 1–34 Administration on Circulating Mesenchymal Stem Cells in Postmenopausal Osteoporotic Women. Med. Sci. Monit. 2019, 25, 259–268. [Google Scholar] [CrossRef]
- Platzbecker, U.; Prange-Krex, G.; Bornhauser, M.; Koch, R.; Soucek, S.; Aikele, P.; Haack, A.; Haag, C.; Schuler, U.; Berndt, A.; et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion 2001, 41, 184–189. [Google Scholar] [CrossRef]
- Langrzyk, A.; Nowak, W.N.; Stępniewski, J.; Jazwa, A.; Florczyk-Soluch, U.; Jozkowicz, A.; Dulak, J. Critical View on Mesenchymal Stromal Cells in Regenerative Medicine. Antioxidants Redox Signal. 2018, 29, 169–190. [Google Scholar] [CrossRef]
- Al Saedi, A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 2019, 14, 389–398. [Google Scholar] [CrossRef]
- Gunawardene, P.; Al Saedi, A.; Singh, L.; Bermeo, S.; Vogrin, S.; Phu, S.; Suriyaarachchi, P.; Pignolo, R.J.; Duque, G. Age, gender, and percentage of circulating osteoprogenitor (COP) cells: The COP Study. Exp. Gerontol. 2017, 96, 68–72. [Google Scholar] [CrossRef]
- Kucia, M.; Reca, R.; Campbell, F.R.; Zuba-Surma, E.; Majka, M.; Ratajczak, J.; Ratajczak, M.Z. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006, 20, 857–869. [Google Scholar] [CrossRef]
- Miyanishi, M.; Mori, Y.; Seita, J.; Chen, J.Y.; Karten, S.; Chan, C.K.; Nakauchi, H.; Weissman, I.L. Do Pluripotent Stem Cells Exist in Adult Mice as Very Small Embryonic Stem Cells? Stem Cell Rep. 2013, 1, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Gounari, E.; Daniilidis, A.; Tsagias, N.; Michopoulou, A.; Kouzi, K.; Koliakos, G. Isolation of a novel embryonic stem cell cord blood–derived population with in vitro hematopoietic capacity in the presence of Wharton’s jelly–derived mesenchymal stromal cells. Cytotherapy 2019, 21, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, W.; Kucia, M.; Liu, R.; Zuba-Surma, E.; Jadczyk, T.; Bachowski, R.; Nabiałek, E.; Kazmierski, M.; Ratajczak, M.Z.; Tendera, M. Circulating Very Small Embryonic-Like Stem Cells in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2010, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zuba-Surma, E.; Kucia, M.; Wu, W.; Klich, I.; Lillard, J.W.; Ratajczak, J.; Ratajczak, M.Z. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytom. Part A 2008, 73, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Wysoczynski, M.; Wu, W.; Ratajczak, J.; Ratajczak, M.Z.; Zuba-Surma, E. Evidence That Very Small Embryonic-Like Stem Cells Are Mobilized into Peripheral Blood. Stem Cells 2008, 26, 2083–2092. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J.; Kucia, M. Very Small Embryonic-Like Stem Cells (VSELs). Circ. Res. 2019, 124, 208–210. [Google Scholar] [CrossRef]
- Bhartiya, D.; Shaikh, A.; Nagvenkar, P.; Kasiviswanathan, S.; Pethe, P.; Pawani, H.; Mohanty, S.; Rao, S.A.; Zaveri, K.; Hinduja, I. Very Small Embryonic-Like Stem Cells with Maximum Regenerative Potential Get Discarded During Cord Blood Banking and Bone Marrow Processing for Autologous Stem Cell Therapy. Stem Cells Dev. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Kuruca, S.E.; Çelik, D.D.; Özerkan, D.; Erdemir, G. Characterization and Isolation of Very Small Embryonic-like (VSEL) Stem Cells Obtained from Various Human Hematopoietic Cell Sources. Stem Cell Rev. Rep. 2019, 15, 730–742. [Google Scholar] [CrossRef]
- Eljaszewicz, A.; Kleina, K.; Grubczak, K.; Radzikowska, U.; Zembko, P.; Kaczmarczyk, P.; Tynecka, M.; Dworzanczyk, K.; Naumnik, B.; Moniuszko, M. Elevated Numbers of Circulating Very Small Embryonic-Like Stem Cells (VSELs) and Intermediate CD14++CD16+ Monocytes in IgA Nephropathy. Stem Cell Rev. Rep. 2018, 14, 686–693. [Google Scholar] [CrossRef]
- Wojakowski, W.; Tendera, M.; Michałowska, A.; Majka, M.; Kucia, M.; Maślankiewicz, K.; Wyderka, R.; Ochała, A.; Ratajczak, M.Z. Mobilization of CD34/CXCR4 +, CD34/CD117 +, c-met + Stem Cells, and Mononuclear Cells Expressing Early Cardiac, Muscle, and Endothelial Markers Into Peripheral Blood in Patients With Acute Myocardial Infarction. Circulation 2004, 110, 3213–3220. [Google Scholar] [CrossRef]
- Kucia, M.; Dawn, B.; Hunt, G.; Guo, Y.; Wysoczynski, M.; Majka, M.; Ratajczak, J.; Rezzoug, F.; Ildstad, S.T.; Bolli, R.; et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 2004, 95, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, W.; Tendera, M.; Kucia, M.; Zuba-Surma, E.; Paczkowska, E.; Ciosek, J.; Hałasa, M.; Krol, M.; Kaźmierski, M.; Buszman, P.; et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sovalat, H.; Scrofani, M.; Eidenschenk, A.; Pasquet, S.; Rimelen, V.; Hénon, P. Identification and isolation from either adult human bone marrow or G-CSF−mobilized peripheral blood of CD34+/CD133+/CXCR4+/ Lin−CD45− cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp. Hematol. 2011, 39, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, E.; Kucia, M.; Koziarska, D.; Halasa, M.; Safranow, K.; Masiuk, M.; Karbicka, A.; Nowik, M.; Nowacki, P.; Ratajczak, M.Z.; et al. Clinical Evidence That Very Small Embryonic-Like Stem Cells Are Mobilized Into Peripheral Blood in Patients After Stroke. Stroke 2009, 40, 1237–1244. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Depinho, R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Boil. 2007, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Dawn, B.; Tiwari, S.; Kucia, M.; Zuba-Surma, E.; Guo, Y.; Sanganalmath, S.K.; Abdel-Latif, A.; Hunt, G.; Vincent, R.J.; Taher, H.; et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells 2008, 26, 1646–1655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kucia, M.; Halasa, M.; Wysoczynski, M.; Baskiewicz-Masiuk, M.; Moldenhawer, S.; Zuba-Surma, E.; Czajka, R.; Wojakowski, W.; Machalinski, B.; Ratajczak, M.Z. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood—Preliminary report. Leukemia 2006, 21, 297–303. [Google Scholar] [CrossRef]
- Tang, X.-L.; Rokosh, D.G.; Guo, Y.; Bolli, R. Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circ. J. 2010, 74, 390–404. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.F.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Müller, P.; et al. From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742. [Google Scholar] [CrossRef]
- Gomperts, B.N.; Belperio, J.A.; Rao, P.N.; Randell, S.H.; Fishbein, M.C.; Burdick, M.D.; Strieter, R.M. Circulating Progenitor Epithelial Cells Traffic via CXCR4/CXCL12 in Response to Airway Injury. J. Immunol. 2006, 176, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.; Eaves, C.J.; Zandieh, I.; Emerman, J.T. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 2001, 67, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Koopman, C.D.; Werb, Z. Circulating Tumor Cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Resetkova, E.; Hoda, S.A.; Clarke, J.L.; Rosen, P.P. Benign heterotopic epithelial inclusions in axillary lymph nodes. Histological and immunohistochemical patterns. Arch. Pathol. Lab. Med. 2003, 127, 25. [Google Scholar]
- Rao, R.S.; Taylor, J.; Palmer, J.; Jennings, W.C. Breast Cancer Pseudometastasis in a Sentinel Lymph Node with Cytokeratin-Positive Debris. Breast J. 2005, 11, 134–137. [Google Scholar] [CrossRef]
- Dunphy, C.H. Pitfalls of Frozen Section to Intraoperative Consultations of Evaluating Lymph Nodes for Involvement by Metastatic Malignancies: Benign Processes Mimicking Metastatic Carcinoma. In Frozen Section Library: Pancreas; Springer Science and Business Media LLC: New York, NY, USA, 2011; Volume 10, pp. 95–119. [Google Scholar]
- Lustberg, M.; Balasubramanian, P.; Miller, B.; Garcia-Villa, A.; Deighan, C.; Wu, Y.; Carothers, S.; Berger, M.; Ramaswamy, B.; Macrae, E.R.; et al. Heterogeneous atypical cell populations are present in blood of metastatic breast cancer patients. Breast Cancer Res. 2014, 16, R23. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Cauley, C.E.; Kulemann, B.; Liss, A.; Castillo, C.F.-D.; Warshaw, A.L.; Lillemoe, K.D.; Thayer, S.P.; Pitman, M.B. Cytologic characteristics of circulating epithelioid cells in pancreatic disease. Cancer Cytopathol. 2017, 125, 332–340. [Google Scholar] [CrossRef]
- Davis, J.W.; Nakanishi, H.; Kumar, V.S.; Bhadkamkar, V.A.; McCormack, R.; Fritsche, H.A.; Handy, B.; Gornet, T.; Babaian, R.J. Circulating Tumor Cells in Peripheral Blood Samples From Patients With Increased Serum Prostate Specific Antigen: Initial Results in Early Prostate Cancer. J. Urol. 2008, 179, 2187–2191. [Google Scholar] [CrossRef]
- Hardingham, J.E.; Hewett, P.J.; Sage, R.E.; Finch, J.L.; Nuttall, J.D.; Kotasek, D.; Dobrovic, A. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int. J. Cancer 2000, 89, 8–13. [Google Scholar] [CrossRef]
- Murray, N.P.; Reyes, E.; Badínez, L.; Orellana, N.; Fuentealba, C.; Olivares, R.; Porcell, J.; Dueñas, R. Circulating Prostate Cells Found in Men with Benign Prostate Disease Are P504S Negative: Clinical Implications. J. Oncol. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Hao, Q.; Shah, A.; Thiemann, F.; Smogorzewska, E.; Crooks, G. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood 1995, 86, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Copelan, E.A. Hematopoietic Stem-Cell Transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Ciraci, E.; Della Bella, S.; Salvucci, O.; Rofani, C.; Segarra, M.; Bason, C.; Molinari, A.; Maric, D.; Tosato, G.; Berardi, A.C. Adult human circulating CD34−Lin−CD45−CD133− cells can differentiate into hematopoietic and endothelial cells. Blood 2011, 118, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Varner, J. Circulating endothelial progenitor cells. Br. J. Cancer 2005, 93, 855–858. [Google Scholar] [CrossRef]
- Lancrin, C.; Sroczynska, P.; Serrano, A.G.; Gandillet, A.; Ferreras, C.; Kouskoff, V.; Lacaud, G. Blood cell generation from the hemangioblast. J. Mol. Med. 2009, 88, 167–172. [Google Scholar] [CrossRef]
- Sicco, C.L.; Tasso, R.; Reverberi, D.; Cilli, M.; Pfeffer, U.; Cancedda, R. Identification of a New Cell Population Constitutively Circulating in Healthy Conditions and Endowed with a Homing Ability Toward Injured Sites. Sci. Rep. 2015, 5, 16574. [Google Scholar] [CrossRef]
- Sicco, C.L.; Reverberi, D.; Villa, F.; Pfeffer, U.; Quarto, R.; Cancedda, R.; Tasso, R. Circulating healing (CH) cells expressing BST2 are functionally activated by the injury-regulated systemic factor HGFA. Stem Cell Res. Ther. 2018, 9, 300. [Google Scholar] [CrossRef]
- Hatt, L.; Brinch, M.; Singh, R.; Møller, K.; Lauridsen, R.H.; Uldbjerg, N.; Huppertz, B.; Christensen, B.; Kølvraa, S. Characterization of Fetal Cells from the Maternal Circulation by Microarray Gene Expression Analysis - Could the Extravillous Trophoblasts Be a Target for Future Cell-Based Non-Invasive Prenatal Diagnosis? Fetal Diagn. Ther. 2013, 35, 218–227. [Google Scholar] [CrossRef]
- Qiao, X.; Loudovaris, M.; Unverzagt, K.; Walker, D.E.; Smith, S.L.; Martinson, J.; Schilling, M.; Lee, W.; Williams, S.F.; Van Epps, D.E.; et al. Immunocytochemistry and flow cytometry evaluation of human megakaryocytes in fresh samples and cultures of CD34+ cells. Cytometry 1996, 23, 250–259. [Google Scholar] [CrossRef]
- Woywodt, A.; Blann, A.D.; Kirsch, T.; Erdbruegger, U.; Banzet, N.; Haubitz, M.; Dignat-George, F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: Proposal of a definition and a consensus protocol. J. Thromb. Haemost. 2006, 4, 671–677. [Google Scholar] [CrossRef]
- Sielatycka, K.; Poniewierska-Baran, A.; Nurek, K.; Torbe, A.; Ratajczak, M.Z. Novel View on Umbilical Cord Blood and Maternal Peripheral Blood-an Evidence for an Increase in the Number of Circulating Stem Cells on Both Sides of the Fetal-Maternal Circulation Barrier. Stem Cell Rev. Rep. 2017, 13, 774–780. [Google Scholar] [CrossRef]
- Pantel, K.; DeNeve, E.; Nocca, D.; Coffy, A.; Vendrell, J.-P.; Maudelonde, T.; Riethdorf, S.; Alix-Panabières, C. Circulating Epithelial Cells in Patients with Benign Colon Diseases. Clin. Chem. 2012, 58, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.-I.; Morgan, B.; Trautwein, J.; et al. Inertial Focusing for Tumor Antigen-Dependent and -Independent Sorting of Rare Circulating Tumor Cells. Sci. Transl. Med. 2013, 5, 179ra47. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-S.; Chen, J.-S.; Shao, H.-J.; Wu, J.-C.; Lai, J.-M.; Lu, S.-H.; Hung, T.-F.; Chiu, Y.-C.; You, J.-F.; Hsieh, P.-S.; et al. Circulating Tumor Cell Count Correlates with Colorectal Neoplasm Progression and Is a Prognostic Marker for Distant Metastasis in Non-Metastatic Patients. Sci. Rep. 2016, 6, 24517. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Thege, F.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Ginsberg, G.G.; Lieb, J.G.; et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2013, 146, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, H.E.; Baech, J.; Nikolajsen, K. Validation of the Nordic Flow Cytometry Standard for CD34+ Cell Enumeration in Blood and Autografts: Report from the Third Workshop. J. Hematotherapy 1999, 8, 15–28. [Google Scholar] [CrossRef]
- Sutherland, D.R.; Keating, A. The CD34 Antigen: Structure, Biology, and Potential Clinical Applications. J. Hematotherapy 1992, 1, 115–129. [Google Scholar] [CrossRef]
- Andersson-Sjöland, A.; De Alba, C.G.; Nihlberg, K.; Becerril, C.; Ramírez, R.; Pardo, A.; Westergren-Thorsson, G.; Selman, M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Boil. 2008, 40, 2129–2140. [Google Scholar] [CrossRef]
- Simkens, L.H.J.; Tol, J.; Terstappen, L.W.M.M.; Teerenstra, S.; Punt, C.J.A.; Nagtegaal, I.D. The predictive and prognostic value of circulating endothelial cells in advanced colorectal cancer patients receiving first-line chemotherapy and bevacizumab. Ann. Oncol. 2010, 21, 2447–2448. [Google Scholar] [CrossRef]
- Byeon, Y.; Ki, C.-S.; Han, K.-H. Isolation of nucleated red blood cells in maternal blood for Non-invasive prenatal diagnosis. Biomed. Microdevices 2015, 17, 118. [Google Scholar] [CrossRef]


| Cellular Marker Characteristic | Quality | Indication/Application |
|---|---|---|
| MEP | Present and cultivatable | Platelet therapy |
| Cytoplasmic MKC (cMKC) | Elevation | Myeloproliferative neoplasms |
| Proliferative cMKC | Elevation | Essential thrombocythaemia |
| Pro-apoptotic impaired cMKC | Elevation | Myelofibrosis (MF) |
| Naked MKC | Elevation | mCRPC good prognosis |
| Polyploidy | High ploidy >8N | Prediction of metastasis |
| Thrombospondin-1 | Marker expression | Lowered tumor progression |
| Lysyl oxidase positive cMKC | Elevation | Fibrosis myeloproliferative disorders |
| CRC Type | Description | Cell Concentration per mL | Clinical Usefulness (cbLB) | References |
|---|---|---|---|---|
| Megakaryocyte-Erythrocyte Progenitors | Bone marrow dwelling megakaryocyte progenitor | N.A. | Therapy: thrombocytopenia | [46,48,49] |
| Naked Megakaryocyte | Large bare lobulated nuclei with high density DNA | <25 | (I) solid tissue cancer prognosis | [11] |
| Cytoplasmic Megakaryocyte | Largest circulating round cell being original megakaryocyte containing platelet load | <0.5 | (I) Therapy: thrombocytopenia (ii) Diagnostic biomarker and therapy intervention target in myeloproliferative disorders (iii) prediction of bone metastasis | [17,20,21,46,48,49] |
| Mature Endothelial phalanx cell | Cobblestone-like quiescent cells | <100 cells (0.5 to 3 on average) * | (I) Prognosis, Predictive biomarker Solid tissue cancer by marker elevation | [34,68,76,94] |
| Mature Endothelial tip cell | Larger activated cell status | |||
| Mature Endothelial sprout cell | Larger, activated cell status | |||
| Endothelial progenitor cell | Bone marrow-derived | 140–360 (early EPC) <1 (late EPC) ** | (I) therapy: coronaryartery disease, neo or re vascularization (ii) prediction biomarker myocardial infarction, pulmonary hypertension and diabetes II, atherosclerotic disease progression etc. | [68,74,94,100] |
| Erythroblasts -Normoblast | Small late matured erythroblast | <50 | (I) Predictive biomarker for leukemia (ii) Prediction of death in critical ill patients | [2,118,122] |
| Erythroblast - Baso-Eb, Poly-Eb | Larger cells with lower N/C ratio | <0.5 | [10] | |
| Fibroblast like cells/Fibrocytes (CD45+) | Rare elongated spindle-shaped leukocyte | <5000 | (I) prediction of pulmonary fibrosis | [138,150] |
| Fibroblast like cells/Fibrocytes (CD45−) | Rare elongated spindle-shaped fibrocyte subpopulation (activated?) | 250 *** | unknown | [134] |
| Fibroblast-like cells/myofibroblast (hematopoietic lineage) | Activated fibrocyte differentiated into tissue resident contractile cell type | unknown | (I) predictive biomarker myocardial infarction | [186] |
| Fibroblast-like cells/mesenchymal stem cells | Bone marrow derived cell | <10 | (I) Prognosis and predictive biomarker in many pathologies e.g., cancer, polytrauma (II) fetal cell marker | [181,183] |
| Fibroblast-like cells/myofibroblast (mesenchymal lineage) | Activated MSC or fibroblast differentiated into tissue resident contractile cell type | unknown | (I) predictive biomarker for solid tissue cancer (ii) therapeutic target in cancer (iii) stem cell therapy for bone and cartilage repair | [194,198] |
| Hematopoietic stem cell | Uncommitted quiescent small blastoid cells type | <1000 | To be researched | [212,224] |
| Very small embryonic stem cells | Smallest rare cell subset with high density nucleus | <350 | (I) Predictive marker diaease non-specific (ii) age/lifestyle marker (iii) stem cell therapy | [203] |
| Mature Epithelial cells type 1 | Large squamous, columnar, cuboidal shaped cells with very low N/C-ratio | Unknown | Not investigated | |
| Mature epithelial cell type 2 | Round or oval blastoid like cells with low N/C ratio= “CellSearch CTC” | 0.42 cells (average of reports) | (i) prognosis, prediction in solid tissue cancer | [1] |
| Circulating epithelial progenitor cells | Round blastoid cells | Unknown | Pulmonary disorder | [146] |
| Hemangioblast | Small blastoid round cells (similar to VSELS) | Unknown | Not specified | [237] |
| CH-Cells | Small blastoid cells | Unknown | Not specified | [241] |
| extravillous trophoblasts | Large irregular shaped blastoid cells | <0.5 cells | Fetal origin | [242] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreier, S.; Triampo, W. The Blood Circulating Rare Cell Population. What Is It and What Is It Good for? Cells 2020, 9, 790. https://doi.org/10.3390/cells9040790
Schreier S, Triampo W. The Blood Circulating Rare Cell Population. What Is It and What Is It Good for? Cells. 2020; 9(4):790. https://doi.org/10.3390/cells9040790
Chicago/Turabian StyleSchreier, Stefan, and Wannapong Triampo. 2020. "The Blood Circulating Rare Cell Population. What Is It and What Is It Good for?" Cells 9, no. 4: 790. https://doi.org/10.3390/cells9040790
APA StyleSchreier, S., & Triampo, W. (2020). The Blood Circulating Rare Cell Population. What Is It and What Is It Good for? Cells, 9(4), 790. https://doi.org/10.3390/cells9040790




