Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer
Abstract
1. Introduction
2. Cytotoxin-Associated Gene A
2.1. CagA and EMT
2.2. CagA and Cancer Stem Cell Properties
2.3. CagA and Yes-Associated Protein Pathway
3. Tumor Necrosis Factor α-Inducing Protein of H. pylori in EMT
Antigenic Lpp20 Protein
4. Afadin Protein Downregulation
5. Penicillin-Binding Protein 1A
6. Upregulation of MicroRNA-29a-3p
7. Downregulation of Programmed Cell Death Protein 4
8. Upregulation of lysosomal-Associated Protein Transmembrane 4β
9. Cancer-Associated Fibroblasts
10. Heparin-Binding Epidermal Growth Factor and Matrix Metalloproteinase-7
11. Stem Cells
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbrevations
| α-SMA | α-smooth muscle actin |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein 1 |
| AREG | amphiregulin |
| Bcl-2 | B-cell lymphoma 2 |
| CAFs | cancer associated fibroblasts |
| CagA | cytotoxin-associated gene A |
| cagPAI | cag pathogenicity island |
| Ccl2 | chemokine (C-C motif) ligand 2 |
| Ccl20 | chemokine (C-C motif) ligand 20 |
| Ccl7 | chemokine (C-C motif) ligand 7 |
| CK7 | cytokeratin 7 |
| COX-2 | cyclooxygenase-2 |
| CSC | cancer stem-cell |
| CTGF | connective tissue growth factor |
| Cxc11 | C-X-C motif chemokine 11 |
| Cxcl1 | chemokine (C-X-C motif) ligand 1 |
| Cxcl10 | C-X-C motif chemokine 10 |
| Cxcl12 | stromal cell-derived factor 1 |
| Cxcl2 | chemokine (C-X-C motif) ligand 2 |
| Cxcl5 | C-X-C motif chemokine 5 |
| Cxcl9 | chemokine (C-X-C motif) ligand 9 |
| CYR61 | cysteine-rich angiogenic inducer 61 |
| DupA | duodenal ulcer promoting gene A |
| EGF | epidermal growth protein |
| EGFR | epidermal growth factor receptor |
| eIF4A | eukaryotic initiation factor-4A |
| eIF4G | eukaryotic translation initiation factor 4 G |
| EMT | epithelial–mesenchymal transition |
| EphA2 | erythropoietin-producing hepatocellular A2 receptor |
| ERK-1/2 | extracellular regulated kinases ½ |
| FAP | fibroblast activation protein |
| FGF9 | fibroblast growth factor 9 |
| FGF | fibroblast growth factor |
| FSP | fibroblast surface protein |
| FSP1 | fibroblast-specific protein 1 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GOLPH3 | Golgi phosphoprotein 3 |
| GSK-3 | glycogen synthase kinase 3 |
| HB-EGF | heparin-binding epidermal growth factor |
| HGF | hepatocyte growth factor |
| HIF1α | hypoxia inducible factor 1α |
| hucMSCs | human umbilical cord MSCs |
| Il-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| JNK | c-Jun N-terminal kinase |
| LAPTM4B | lysosomal-associated protein transmembrane 4β |
| LATS2 | large tumor suppressor 2 |
| MCP-1 | monocyte chemoattractant protein 1 |
| miRNA | microRNA |
| MMP-7 | matrix metalloproteinase-7 |
| MNNG | N-nitrosoguanidine |
| MSCs | mesenchymal stem cells |
| NANOGP8 | Nanog Homebox Retrogene P8 |
| NAP | neutrophil activating protein A |
| NF-κB | nuclear factor κB |
| OipA | outer inflammatory protein A |
| PBP | penicillin–binding protein |
| PDCD4 | programmed cell death protein 4 |
| PDGF-B | platelet-derived growth factor subunit B |
| PTEN | phosphatase and tensin homolog |
| SabA | sialic acid-binding adhesin |
| SDF-1 | stromal cell-derived factor 1 |
| SHP-2 | protein tyrosine phosphatase 2 |
| SSP-1 | osteopontin |
| T4SS | type 4 secretion system |
| TGF-α | transforming growth factor-α |
| TGF-β | transforming growth factor β |
| Tipα protein | tumor necrosis factor- α-inducing protein |
| TNF-α | tumor necrosis factor-α |
| TNC | tenascin-C |
| TRAIL1R | Trail receptor 1, death receptor 4 |
| VacA | vacuolating cytotoxin |
| VEGF | vascular endothelial factor |
| WT | wild-type |
| YAP | Yes-Associated-Protein |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| ZO-1 | zonula occludens-1 |
References
- Correia, M.; Machado, J.C.; Ristimäki, A. Basic Aspects of Gastric Cancer. Helicobacter 2009, 14, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-Analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Isomoto, H.; Moriyama, M.; Fujioka, T.; Machado, J.C.; Yamaoka, Y. Helicobacterand Gastric Malignancies. Helicobacter 2008, 13, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri Assadi, M.; Chamanrokh, P.; Whitehouse, C.A.; Huq, A. Methods for Detecting the Environmental Coccoid Form of Helicobacter pylori. Front. Public Health 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef]
- Morais, S.; Costa, A.R.; Ferro, A.; Lunet, N.; Peleteiro, B. Contemporary migration patterns in the prevalence of Helicobacter pylori infection: A systematic review. Helicobacter 2017, 22, 1–11. [Google Scholar] [CrossRef]
- Chojnacki, C.; Popławski, T.; Błońska, A.; Błasiak, J.; Romanowski, M.; Chojnacki, J. Expression of tryptophan hydroxylase in gastric mucosa in symptomatic and asymptomatic Helicobacter pylori infection. Arch. Med. Sci. 2019, 15, 416–423. [Google Scholar] [CrossRef]
- Seta, T.; Takahashi, Y.; Noguchi, Y.; Shikata, S.; Sakai, T.; Sakai, K.; Yamashita, Y.; Nakayama, T. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: A systematic review and meta-analysis comparing risk ratio with risk difference. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Figueiredo, C.; Costa, S.; Karameris, A.; Machado, J.C. Pathogenesis of Gastric Cancer. Helicobacter 2015, 20, 30–35. [Google Scholar] [CrossRef]
- Resende, C.; Regalo, G.; Durães, C.; Pinto, M.T.; Wen, X.; Figueiredo, C.; Carneiro, F.; Machado, J.C. Interleukin-1B signalling leads to increased survival of gastric carcinoma cells through a CREB-C/EBPβ-Associated mechanism. Gastric Cancer 2015, 19, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.; Leite, M.; Seruca, R.; Figueiredo, C. Adherens junctions as targets of microorganisms: A focus onHelicobacter pylori. FEBS Lett. 2012, 587, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Figueiredo, C.; Touati, E. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2009, 1, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Graham, D.Y. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014, 10, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Kabamba, E.T.; Yamaoka, Y. Helicobacter pylori and Related Virulence Factors for Gastrointestinal Diseases. In Gastric Cancer; Shiotani, A., Ed.; Springer: Singapore, 2018; pp. 31–50. [Google Scholar]
- Pormohammad, A.; Ghotaslou, R.; Leylabadlo, H.E.; Nasiri, M.J.; Dabiri, H.; Hashemi, A. Risk of gastric cancer in association with Helicobacter pylori different virulence factors: A systematic review and meta-Analysis. Microb. Pathog. 2018, 118, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sgouras, D.; Tegtmeyer, N.; Wessler, S. Activity and Functional Importance of Helicobacter pylori Virulence Factors. In Advances in Experimental Medicine and Biology; Kluwer Academic Publishers: Boston, MA, USA, 2019. [Google Scholar]
- Olivera-Severo, D.; Uberti, A.F.; Marques, M.S.; Pinto, M.T.; Gomez-Lazaro, M.; Figueiredo, C.; Leite, M.; Carlini, C.R. A New Role for Helicobacter pylori Urease: Contributions to Angiogenesis. Front. Microbiol. 2017, 8, 1883. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Machado, J.C.; Figueiredo, C. Clinical relevance of Helicobacter pylori vacA and cagA genotypes in gastric carcinoma. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1003–1015. [Google Scholar] [CrossRef]
- Guimarães, N.M.; Azevedo, N.F.; Vieira, M.J.; Figueiredo, C. Water-Induced modulation of Helicobacter pylori virulence properties. Memórias Inst. Oswaldo Cruz. 2014, 109, 414–419. [Google Scholar] [CrossRef][Green Version]
- Ferreira, R.M.; Figueiredo, C.; Bonet, C.; Pardo, M.L.; Liso, J.M.R.; Alonso, P.; Sala, N.; Capella, G.; Sanz-Anquela, J.M.; Gonzalez, C.A. Helicobacter pylori vacA Intermediate Region Genotyping and Progression of Gastric Preneoplastic Lesions. Am. J. Gastroenterol. 2012, 107, 145–146. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Costa, A.M.; Costa, A.C.; Machado, J.C.; Seruca, R.; Mareel, M.; Figueiredo, C. 26 E-Cadherin and CagA Associate in a Tetrameric Complex That Suppresses Helicobacter pylori-Mediated Cell Invasion. Gastroenterology 2008, 134, A-3. [Google Scholar] [CrossRef]
- Peleteiro, B.; Lunet, N.; Figueiredo, C.; Carneiro, F.; David, L.; Barros, H. Smoking, Helicobacter pylori Virulence, and Type of Intestinal Metaplasia in Portuguese Males. Cancer Epidemiol. Biomark. Prev. 2007, 16, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.D.; Desler, C.; Bøggild, S.; Strickertsson, J.A.; Friis-Hansen, L.; Figueiredo, C.; Seruca, R.; Rasmussen, L.J. Helicobacter pylori infection affects mitochondrial function and DNA repair, thus, mediating genetic instability in gastric cells. Mech. Ageing Dev. 2013, 134, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Marques, M.S.; Melo, J.; Pinto, M.T.; Cavadas, B.; Aroso, M.; Gomez-Lazaro, M.; Seruca, R.; Figueiredo, C. Helicobacter Pylori Targets the EPHA2 Receptor Tyrosine Kinase in Gastric Cells Modulating Key Cellular Functions. Cells 2020, 9, 513. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, J.; Maciejewski, R.; Polkowski, W. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Pinto-Ribeiro, I.; Ferreira, R.; Batalha, S.; Hlaing, T.; Wong, S.; Carneiro, F.; Figueiredo, C. Helicobacter pylori vacA Genotypes in Chronic Gastritis and Gastric Carcinoma Patients from Macau, China. Toxins 2016, 8, 142. [Google Scholar] [CrossRef]
- Yanovich, O.; Doroshko, M.; Titov, L. Helicobacter pylori genotypes among Belarus patients with gastroduodenal disorders and their association with clinical outcome. Acta Microbiol. Immunol. Hung. 2019, 66, 399–411. [Google Scholar] [CrossRef]
- Hnatyszyn, A.; Szalata, M.; Skrzypczak-Zielinska, M.; Wielgus, K.; Stanczyk, J.; Dziuba, I.; Mikstacki, A.; Dobrowolska, A.; Waszak, M.; Hnatyszyn, P.T.; et al. DNA Variants in Helicobacter Pylori Infected Patients with Chronic Gastritis, Dysplasia and Gastric Cancer. Adv. Med. Sci. 2019, 64, 79–84. [Google Scholar] [CrossRef]
- Kakelar, H.M.; Barzegari, A.; Dehghani, J.; Hanifian, S.; Saeedi, N.; Barar, J.; Omidi, Y. Pathogenicity of Helicobacter pylori in cancer development and impacts of vaccination. Gastric Cancer 2018, 22, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Machado, J.C.; Pharoah, P.; Seruca, R.; Sousa, S.; Carvalho, R.; Capelinha, A.F.; Quint, W.; Caldas, C.; van Doorn, L.J.; et al. Helicobacter pylori and interleukin 1 genotyping: An opportunity to identify high-Risk individuals for gastric carcinoma. JNCI J. Natl. Cacncer Inst. 2002, 94, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, S.Z.; Latifi-Navid, S.; Mohammadi, S.; Zahri, S.; Bakhti, F.S.; Feizi, F.; Yazdanbod, A.; Siavoshi, F. Relevance ofHelicobacter pylori vacA 3’-End Region Polymorphism to Gastric Cancer. Helicobacter 2015, 21, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pinto, R.; Dinis-Ribeiro, M.J.; Carneiro, F.; Machado, J.C.; Figueiredo, C.; Wen, X.; Reis, C.A.; Ferreira, R.M.; Lopes, C.; Ferreira, J.; et al. Mo1556 High-Risk Host Genotype and H. pylori Strains in First Degree Relatives of Patients with Early-Onset Gastric Cancer. Gastroenterology 2012, 142, S-627–S-628. [Google Scholar] [CrossRef]
- González, C.A.; Figueiredo, C.; Lic, B.C.; Ferreira, R.M.; Pardo, M.L.; Liso, R.J.M.; Alonso, P.; Sala, N.; Capella, G.; Sanz-Anquela, J.M. Helicobacter pylori cagA and vacA Genotypes as Predictors of Progression of Gastric Preneoplastic Lesions: A Long-Term Follow-Up in a High-Risk Area in Spain. Am. J. Gastroenterol. 2011, 106, 867–874. [Google Scholar] [CrossRef]
- Machado, A.M.D.; Figueiredo, C.; Seruca, R.; Rasmussen, L.J. Helicobacter pylori infection generates genetic instability in gastric cells. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 58–65. [Google Scholar] [CrossRef]
- Figueiredo, C.; Quint, W.; Nouhan, N.; Munckhof, H.V.D.; Herbrink, P.; Scherpenisse, J.; de Boer, W.; Schneeberger, P.; Perez-Perez, G.; Blaser, M.J.; et al. Assessment of Helicobacter pylori vacA and cagA Genotypes and Host Serological Response. J. Clin. Microbiol. 2001, 39, 1339–1344. [Google Scholar] [CrossRef][Green Version]
- Nogueira, C.; Figueiredo, C.; Carneiro, F.; Gomes, A.T.; Barreira, R.; Figueira, P.; Salgado, C.; Belo, L.; Peixoto, A.; Bravo, J.C.; et al. Helicobacter pylori Genotypes May Determine Gastric Histopathology. Am. J. Pathol. 2001, 158, 647–654. [Google Scholar] [CrossRef]
- Watari, J. Helicobacter pyloriassociated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Thieblemont, C.; Zucca, E. Clinical aspects and therapy of gastrointestinal MALT lymphoma. Best Pract. Res. Clin. Haematol. 2017, 30, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, G.S.; Axon, A.T.R. Helicobacter pylori and Nonmalignant Diseases. Helicobacter 2015, 20, 26–29. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2017, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Puculek, M.; Machlowska, J.; Wierzbicki, R.; Baj, J.; Maciejewski, R.; Sitarz, R. Helicobacter pylori associated factors in the development of gastric cancer with special reference to the early-Onset subtype. Oncotarget 2018, 9, 31146–31162. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Gattani, S.G.; Farhat, S.A. Helicobacter pylori and cardiovascular complications: A mechanism based review on role of Helicobacter pylori in cardiovascular diseases. Integr. Med. Res. 2016, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bazmamoun, H.; Rafeey, M.; Nikpouri, M.; Ghergherehchi, R. Helicobacter Pylori Infection in Children with Type 1 Diabetes Mellitus: A Case-Control Study. J. Res. Health Sci. 2016, 16, 68–71. [Google Scholar]
- Mortazavi, E.; Eslami, B.; Aghahosseini, P.; Ahron, F.; Amininejad, A.; Mahmoodi, S.; Satarpour, H.; Radmanesh, N.; Rassi, H. Association of Mannose-Binding Lectin rs1800450 and Tumor Necrotic Factor-α rs1800620 Polymorphism with Helicobacter pylori in Type II Diabetes Mellitus. Monoclon. Antibodies Immunodiagn. Immunother. 2017, 36, 236–241. [Google Scholar] [CrossRef]
- Doulberis, M.; Kotronis, G.; Thomann, R.; Polyzos, S.A.; Boziki, M.; Gialamprinou, D.; Deretzi, G.; Katsinelos, P.; Kountouras, J. Review: Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter 2018, 23, 1–18. [Google Scholar] [CrossRef]
- Ribatti, D. Epithelial-Mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp. Cell Res. 2017, 353, 1–5. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-Mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial–Mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K. Contribution of epithelial-Mesenchymal transitions to organogenesis and cancer metastasis. Curr. Opin. Cell Biol. 2018, 55, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial-Mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell. Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.-H.; Leong, C.O.; Ngai, S.C. E-Cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Muhammad, N.; Bhattacharya, S.; Steele, R.; Phillips, N.; Ray, R.B. Involvement of c-Fos in the Promotion of Cancer Stem-Like Cell Properties in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 23, 3120–3128. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene 2016, 36, 667–677. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Lu, Z.; Zhang, H.; Zhuang, N.; Wang, B.; Song, Z.; Chen, G.; Huang, C.; Xu, D.; et al. miR-127 promotes EMT and stem-Like traits in lung cancer through a feed-Forward regulatory loop. Oncogene 2016, 36, 1631–1643. [Google Scholar] [CrossRef]
- Xiao, H. MiR-7-5p suppresses tumor metastasis of non-Small cell lung cancer by targeting NOVA2. Cell. Mol. Biol. Lett. 2019, 24, 1–13. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-To-Mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.; Chang, H.; Lee, H.S.; Park, S.M.; Park, J.H.; Shin, C.M.; Kim, J.M.; Kim, J.S.; Lee, D.H.; et al. Helicobacter pylori-induced epithelial-Mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015, 36, 553–563. [Google Scholar] [CrossRef]
- Teeuwssen, F. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Liang, S.; Lang, G.; Liu, G.; Liu, P.; Deng, X. Long non-Coding RNA VIM-AS1 promotes prostate cancer growth and invasion by regulating epithelial-Mesenchymal transition. JBUON 2019, 24, 2090–2098. [Google Scholar] [PubMed]
- Scimeca, M.; Bonfiglio, R.; Menichini, E.; Albonici, L.; Urbano, N.; Caro, M.T.D.; Mauriello, A.; Schillaci, O.; Gambacurta, A.; Bonanno, E. Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 5633. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Antonacci, C.; Colombo, D.; Bonfiglio, R.; Buonomo, O.C.; Bonanno, E. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumor Biol. 2015, 37, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.; Fernandes, M.S.; Figueiredo, J.; Caldeira, J.; Carvalho, J.; Pinheiro, H.; Leite, M.; Melo, S.; Oliveira, P.; Simoes-Correia, J.; et al. E-Cadherin dysfunction in gastric cancer-Cellular consequences, clinical applications and open questions. FEBS Lett. 2012, 586, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Figueiredo, J.; Albergaria, A.; Oliveira, P.; Carvalho, J.; Ribeiro, A.S.; Caldeira, J.; Costa, A.M.; Simoes-Correia, J.; Oliveira, M.J.; et al. Epithelial E- and P-Cadherins: Role and clinical significance in cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 297–311. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the Epithelial-to-Mesenchymal Transition of Human Colorectal Cancer by Human TNF-β (Lymphotoxin) and its Reversal by Resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Feng, J.; Deng, L.-L.; Liu, Y.; Li, B.; Zhu, M.; Lu, C.; Zhou, L. MT2-MMP induces proteolysis and leads to EMT in carcinomas. Oncotarget 2016, 7, 48193. [Google Scholar] [CrossRef]
- Kim, W.K.; Kwon, Y.; Jang, M.; Park, M.; Kim, J.; Cho, S.; Jang, D.G.; Lee, W.B.; Jung, S.H.; Choi, H.J.; et al. β-Catenin activation down-regulates cell-Cell junction-Related genes and induces epithelial-To-Mesenchymal transition in colorectal cancers. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, X.; Xiao, W.; Liu, X.; Li, C.; Guo, Y.; Xiong, W.; Li, Y. N3ICD with the transmembrane domain can effectively inhibit EMT by correcting the position of tight/adherens junctions. Cell Adhes. Migr. 2019, 13, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Pan, J.; Zhao, T.; Ji, J.; Zhang, C.; Che, Y.; Yang, J.; Shi, H.; Li, J.; Zhou, H.; et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-To-Mesenchymal transition through β-Catenin/Erk/Akt signaling in gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 208. [Google Scholar] [CrossRef]
- Yu, W.; Li, L.; Zheng, F.; Yang, W.; Zhao, S.; Tian, C.; Yin, W.; Chen, Y.; Guo, W.; Zou, L.; et al. β-Catenin Cooperates with CREB Binding Protein to Promote the Growth of Tumor Cells. Cell. Physiol. Biochem. 2017, 44, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.-W. The regulation of β-Catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Y.-X.; Yu, J.-H.; Chen, X.; Luo, X.-J.; Yin, Y.-R. Research of the Relationship Between β-Catenin and C-Myc-Mediated Wnt Pathway and Laterally Spreading Tumors Occurrence. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 252–257. [Google Scholar]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Liu, X.; Fan, D. The Epithelial-Mesenchymal Transition and Cancer Stem Cells: Functional and Mechanistic Links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef]
- Giancotti, F.G. Mechanisms Governing Metastatic Dormancy and Reactivation. Cell 2013, 155, 750–764. [Google Scholar] [CrossRef]
- Begicevic, R.-R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Liu, X.-Z.; Liang, C.-M. Inflammatory microenvironment contributes to epithelial-Mesenchymal transition in gastric cancer. World J. Gastroenterol. 2016, 22, 6619. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Brzozowska, K.; Forma, A.; Maani, A.; Sitarz, E.; Portincasa, P. Immunological Aspects of the Tumor Microenvironemnt and Epithelial-Mesenchymal Transition in Gastric Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 2544. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chakrabarti, R. Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells. J. Clin. Med. 2019, 8, 642. [Google Scholar] [CrossRef]
- Soundararajan, R.; Fradette, J.; Konen, J.; Moulder, S.; Zhang, X.; Gibbons, D.; Varadarajan, N.; Wistuba, I.I.; Tripathy, D.; Bernatchez, C.; et al. Targeting the Interplay between Epithelial-to-Mesenchymal-Transition and the Immune System for Effective Immunotherapy. Cancers 2019, 11, 714. [Google Scholar] [CrossRef]
- Zakharchenko, O.; Cojoc, M.; Dubrovska, A.; Souchelnytskyi, S. A Role of TGFß1 Dependent 14-3-3σ Phosphorylation at Ser69 and Ser74 in the Regulation of Gene Transcription, Stemness and Radioresistance. PLoS ONE 2013, 8, e65163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Shao, M.; Zang, X.; Zhang, J.; Mao, F.; Qian, H.; Xu, W. SALL4 activates TGF-β/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag. Res. 2018, 10, 4459–4470. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef]
- Katoh, M. Dysregulation of stem cell signaling network due to germline mutation, SNP, helicobacter pylori infection, epigenetic change, and genetic alteration in gastric cancer. Cancer Biol. Ther. 2007, 6, 832–839. [Google Scholar] [CrossRef]
- Magalhães, A.; Marcos, N.T.; Carvalho, A.S.; David, L.; Figueiredo, C.; Bastos, J.; David, G.; Reis, C.A. Helicobacter pylori cagpathogenicity island-Positive strains induce syndecan-4 expression in gastric epithelial cells. FEMS Immunol. Med. Microbiol. 2009, 56, 223–232. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Costa, A.C.; Costa, A.M.; Henriques, L.; Suriano, G.; Atherton, J.C.; Machado, J.C.; Carneiro, F.; Seruca, R.; Mareel, M.; et al. Helicobacter pyloriInduces Gastric Epithelial Cell Invasion in a c-Met and Type IV Secretion System-dependent Manner. J. Biol. Chem. 2006, 281, 34888–34896. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Machado, J.C.; Yamaoka, Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter 2005, 10, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Neddermann, M.; Maubach, G.; Naumann, M. Pathogenesis of Helicobacter pylori infection. Helicobacter 2016, 21, 19–25. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Machado, J.C.; Leite, M.; Carneiro, F.; Figueiredo, C. The number of Helicobacter pylori CagA EPIYA C tyrosine phosphorylation motifs influences the pattern of gastritis and the development of gastric carcinoma. Histopathology 2012, 60, 992–998. [Google Scholar] [CrossRef]
- Hu, B.; Khara, P.; Song, L.; Lin, A.S.; Frick-Cheng, A.E.; Harvey, M.L.; Cover, T.L.; Christie, P.J. In situ molecular architecture of the helicobacter pylori cag type IV secretion system. mBio 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Wessler, S.; Necchi, V.; Rohde, M.; Harrer, A.; Rau, T.T.; Asche, C.I.; Boehm, M.; Loessner, H.; Figueiredo, C.; et al. Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery. Cell Host Microbe 2017, 22, 552–560. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, B.; Wen, C.; Zheng, S.; Chen, H.; She, F. YWHAE is a novel interaction partner of Helicobacter pylori CagA. FEMS Microbiol. Lett. 2018, 365, 1–7. [Google Scholar] [CrossRef]
- Zhu, S.; Soutto, M.; Chen, Z.; Peng, D.F.; Romero-Gallo, J.; Krishna, U.S.; Belkhiri, A.; Washington, M.K.; Peek, R.; El-Rifai, W. Helicobacter pylori-Induced cell death is counteracted by NF-κB-Mediated transcription of DARPP-32. Gut 2016, 66, 802–812. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, J.Y.; Cho, Y.; Woo, H.J.; Kwon, H.J.; Kim, D.H.; Park, M.; Moon, C.; Yeon, M.J.; Kim, H.W.; et al. Inhibitory effects of menadione on Helicobacter pylori growth and helicobacter pylori-Induced inflammation via NF-κB inhibition. Int. J. Mol. Sci. 2019, 20, 1169. [Google Scholar] [CrossRef]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharm. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Bessède, E.; Staedel, C.; Acuña Amador, L.A.; Nguyen, P.H.; Chambonnier, L.; Hatakeyama, M.; Belleannee, G.; Megraud, F.; Varon, C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-Mesenchymal transition-Like changes. Oncogene 2014, 33, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Senda, M.; Suzuki, N.; Nishikawa, H.; Ben, C.; Tang, C.; Nagase, L.; Inoue, K.; Senda, T.; Hatakeyama, M. Differential Mechanisms for SHP2 Binding and Activation Are Exploited by Geographically Distinct Helicobacter pylori CagA Oncoproteins. Cell Rep. 2017, 20, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Nagase, L.; Hayashi, T.; Senda, T.; Hatakeyama, M. Dramatic increase in SHP2 binding activity of Helicobacter pylori Western CagA by EPIYA-C duplication: Its implications in gastric carcinogenesis. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, H.S.; Lee, Y.S.; Kim, S.; Cha, S.Y.; Ota, I.; Kim, N.H.; Cha, Y.H.; Yand, D.H.; Lee, Y.; et al. Helicobacter pylori CagA promotes Snail-Mediated epithelial-Mesenchymal transition by reducing GSK-3 activity. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sougleri, I.S.; Papadakos, K.S.; Zadik, M.P.; Mavri-Vavagianni, M.; Mentis, A.F.; Sgouras, D.N. Helicobacter pylori CagA protein induces factors involved in the epithelial to mesenchymal transition (EMT) in infected gastric epithelial cells in an EPIYA- Phosphorylation-Dependent manner. FEBS J. 2016, 283, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Hashi, K.; Imai, C.; Yahara, K.; Tahmina, K.; Hayashi, T.; Azuma, T.; Miyabe-Nishiwaki, T.; Sato, H.; Matsuoka, M.; Niimi, S.; et al. Evaluating the origin and virulence of a Helicobacter pylori cagA-Positive strain isolated from a non-human primate. Sci. Rep. 2018, 8, 15981. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Machado, J.C.; Carneiro, F.; Figueiredo, C. T1645 the Number of Helicobacter pylori cagA EPIYA C Tyrosine Phosphorylation Motifs Is Associated with Histopathological Features of Chronic Gastritis. Gastroenterology 2009, 136, A-549. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Machado, J.C.; Carneiro, F.; Figueiredo, C. T1801 Helicobacter pylori CagA EPIYA C Tyrosine Phosphorylation Motif Influences the Risk for Gastric Carcinoma Development. Gastroenterology 2008, 134, A-566. [Google Scholar] [CrossRef]
- Naumann, M.; Sokolova, O.; Tegtmeyer, N.; Backert, S. Helicobacter pylori: A Paradigm Pathogen for Subverting Host Cell Signal Transmission. Trends Microbiol. 2017, 25, 316–328. [Google Scholar] [CrossRef]
- Noto, J.M.; Zackular, J.P.; Varga, M.G.; Delgado, A.; Romero-gallo, J.; Scholz, M.B. Crossm Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic. mBio 2019, 10, e00955-19. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Costa, A.M.; Costa, A.C.; Ferreira, R.M.; Sampaio, P.; Machado, J.C. CagA Associates with c-Met, E-Cadherin, and p120-Catenin in a Multiproteic Complex that Suppresses Helicobacter pylori–Induced Cell-Invasive Phenotype. J. Infect. Dis. 2009, 200, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Nell, S.; Estibariz, I.; Krebes, J.; Bunk, B.; Graham, D.Y.; Overmann, J.; Song, Y.; Sproer, C.; Yang, I.; Wex, T.; et al. Genome and Methylome Variation in Helicobacter pylori With a cag Pathogenicity Island During Early Stages of Human Infection. Gastroenterology 2018, 154, 612–623.e7. [Google Scholar] [CrossRef]
- Li, H.; He, C.; Wang, X.; Wang, H.; Nan, G.; Fang, L. MicroRNA-183 affects the development of gastric cancer by regulating autophagy via MALAT1-miR-183-SIRT1 axis and PI3K/AKT/mTOR signals. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3163–3171. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Guan, J.; Ji, T.; Wang, X. Serum microRNA-381: A Potential Marker for Early Diagnosis of Gastric Cancer. Yonsei Med. J. 2019, 60, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Oncogenic Metabolism Acts as a Prerequisite Step for Induction of Cancer Metastasis and Cancer Stem Cell Phenotype. Oxidative Med. Cell. Longev. 2018, 2018, 1–28. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–Mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef]
- Park, A.H.; Shin, J.E.; Park, H.W. The role of Hippo Pathway in Cancer Stem Cell Biology. Mol. Cells 2018, 41, 83–92. [Google Scholar]
- Zhou, P.; Li, B.; Liu, F.; Zhang, M.; Wang, Q.; Liu, Y.; Yao, Y.; Li, D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer 2017, 16, 52. [Google Scholar] [CrossRef]
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94. [Google Scholar] [CrossRef]
- Pradella, D.; Naro, C.; Sette, C.; Ghigna, C. EMT and stemness: Flexible processes tuned by alternative splicing in development and cancer progression. Mol. Cancer 2017, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J. Gastric Cancer Originating from Bone Marrow-Derived Cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.-F.A.; Boziki, M.; Grigoriadis, N.; Papavassiliou, A.G. Helicobacter pylori infection and gastric cancer biology: Tempering a double-Edged sword. Cell. Mol. Life Sci. 2019, 76, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Toh, T.B.; Lim, J.J.; Chow, E.K.H. Epigenetics in cancer stem cells. Mol. Cancer 2017, 16, 1–20. [Google Scholar] [CrossRef]
- Santos, J.C.; Carrasco-Garcia, E.; Garcia-Puga, M.; Aldaz, P.; Montes, M.; Fernandez-Reyes, M.; de Oliveira, C.C.; Lawrie, C.H.; Arauzo-Bravo, M.J.; Ribeiro, M.L.; et al. SOX9 Elevation Acts with Canonical WNT Signaling to Drive Gastric Cancer Progression. Cancer Res. 2016, 76, 6735–6746. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Choi, E.; Petersen, C.; Delgado, A.G.; Piazuelo, M.B.; Romero-Gallo, J.; Lantz, T.L.; Zavros, Y.; Coffey, R.J.; Goldenring, J.R.; et al. Targeted mobilization of Lrig1 gastric epithelial stem cell populations by a carcinogenic Helicobacter pylori type IV secretion system. Proc. Natl. Acad. Sci. USA 2019, 116, 19652–19658. [Google Scholar] [CrossRef]
- Zavros, Y. Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Morath, I.; Hartmann, T.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. In Methods in Molecular Biology Cancer Stem Cells; Humana Press: New York, NY, USA, 2018; pp. 31–42. [Google Scholar]
- Wu, K.; Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K. The role of CD44 in epithelial–Mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783. [Google Scholar] [CrossRef]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.W.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kato, C.; Mori, H.; Matsuzaki, J.; Kameyama, K.; Saya, H.; Hatakeyama, M.; Suematsu, M.; Suzuki, H. Cancer Stem-Cell Marker CD44v9-Positive Cells Arise from Helicobacter pylori–Infected CAPZA1-Overexpressing Cells. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Bertaux-Skeirik, N.; Feng, R.; Schumacher, M.A.; Li, J.; Mahe, M.M.; Engevik, A.C.; Javier, J.E.; Peek, R.M.; Ottemann, K.; Orian-Rousseau, V.; et al. CD44 Plays a Functional Role in Helicobacter pylori-Induced Epithelial Cell Proliferation. PLoS Pathog. 2015, 11, e1004663. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wei, H.; Yi, J.; Xie, B.; Chen, J.; Zhou, C.; Wang, L.; Yang, Y. Chronic CagA-Positive Helicobacter pylori infection with MNNG stimulation synergistically induces mesenchymal and cancer stem cell-Like properties in gastric mucosal epithelial cells. J. Cell. Biochem. 2019, 120, 17635–17649. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Suzuki, H.; Saya, H.; Hatakeyama, M.; Hirayama, T.; Hirata, K.; Nagano, O.; Matsuzaki, J.; Hibi, T. Reactive Oxygen Species-Induced Autophagic Degradation of Helicobacter pylori CagA Is Specifically Suppressed in Cancer Stem-Like Cells. Cell Host Microbe 2012, 12, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Tang, B.; Xiao, Y.-F.; Xie, R.; Qin, Y.; Luo, G.; Hu, C.J.; Dong, H.; Yang, S.M. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-Catenin signaling pathway to promote cancer stem cell-Like properties in human gastric cancer. Cancer Lett. 2016, 374, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Sigal, M.; Rothenberg, M.E.; Logan, C.Y.; Lee, J.Y.; Honaker, R.W.; Cooper, R.L.; Passarelli, B.; Camorlinga, M.; Bouley, D.M.; Alvarez, G.; et al. Helicobacter pylori Activates and Expands Lgr5 Stem Cells through Direct Colonization of the Gastric Glands. Gastroenterology 2015, 148, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Yoon, C.; Park, M.R.; Lee, D.; Kook, M.C.; Lin, J.X.; Kang, J.H.; Ashktorab, H.; Smoot, D.T.; Yoon, S.S.; et al. CDX1 Expression Induced by CagA-Expressing Helicobacter pylori Promotes Gastric Tumorigenesis. Mol. Cancer Res. 2019, 17, 2169–2183. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K.-L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lu, C.-Y.; Cheng, T.-Y.; Pan, S.-H.; Chen, H.-F.; Chang, N.-S. WW Domain-Containing Proteins YAP and TAZ in the Hippo Pathway as Key Regulators in Stemness Maintenance, Tissue Homeostasis, and Tumorigenesis. Front. Oncol. 2019, 9, 60. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Sardo, F.L.; Strano, S.; Blandino, G. YAP and TAZ in Lung Cancer: Oncogenic Role and Clinical Targeting. Cancers 2018, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhu, Y.; Zhang, W.; Liao, Q.; Chen, Y.; Zhao, X.; Guo, Q.; Shen, P.; Zhen, B.; Qian, X.; et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol. Cell 2017, 68, 591–604. [Google Scholar] [CrossRef]
- Santucci, M.; Vignudelli, T.; Ferrari, S.; Mor, M.; Scalvini, L.; Bolognesi, M.L.; Uliassi, E.; Costi, M.P. The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment. J. Med. Chem. 2015, 58, 4857–4873. [Google Scholar] [CrossRef]
- Lei, Q.-Y.; Zhang, H.; Zhao, B.; Zha, Z.-Y.; Bai, F.; Pei, X.-H.; Zhao, S.; Xiong, Y.; Guan, K.-L. TAZ Promotes Cell Proliferation and Epithelial-Mesenchymal Transition and Is Inhibited by the Hippo Pathway. Mol. Cell. Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef]
- Li, N.; Feng, Y.; Hu, Y.; He, C.; Xie, C.; Ouyang, Y.; Artim, S.C.; Huang, D.; Zhu, Y.; Luo, Z.; et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef]
- Mo, J.S.; Meng, Z.; Kim, Y.C.; Park, H.W.; Hansen, C.G.; Kim, S.; Lim, D.S.; Guan, K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 2015, 17, 500–510. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, P.; Wang, Z.; Zhang, Y.; Xie, P.; Geng, D.; Jiang, Y.; Yu, R.; Zhou, X. β-Catenin-Mediated YAP signaling promotes human glioma growth. J. Exp. Clin. Cancer Res. 2017, 36, 1–11. [Google Scholar] [CrossRef]
- Molina-Castro, S.E.; Tiffon, C.; Giraud, J.; Boeuf, H.; Sifre, E.; Giese, A.; Belleannee, G.; Lehours, P.; Bessede, E.; Megraud, F.; et al. The Hippo Kinase LATS2 Controls Helicobacter pylori-Induced Epithelial-Mesenchymal Transition and Intestinal Metaplasia in Gastric Mucosa. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 257–276. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Li, X.; Wu, C.; Jiang, J. Yes-Associated Protein 1 as a Novel Prognostic Biomarker for Gastrointestinal Cancer: A Meta-Analysis. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Wang, K.; Zhang, X.; Yu, Y.; Lv, Y.; Zhang, S.; Zhang, L.; Guo, Y.; Li, Y.; et al. A novel YAP1/SLC35B4 regulatory axis contributes to proliferation and progression of gastric carcinoma. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, L.; Liang, X.; Li, S.; Ma, L.; Zheng, L.; Li, T.; Yu, H.; Chan, H.; Chen, C.; et al. Helicobacter pylori-Induced YAP1 nuclear translocation promotes gastric carcinogenesis by enhancing IL-1β expression. Cancer Med. 2019, 8, 3965–3980. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Takahashi, A.; Suzuki, K.; Kurusu-Kanno, M.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Epithelial-Mesenchymal transition in human gastric cancer cell lines induced by TNF-α-Inducing protein of Helicobacter pylori. Int. J. Cancer 2014, 134, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, E.; Saberi, S.; Esmaeili, M.; Karimi, Z.; Najafi, S.; Tashakoripoor, M.; Abdirad, A.; Hosseini, M.E.; Mohagheghi, M.A.; Khalaj, V.; et al. Multiplex serology of Helicobacter pylori antigens in detection of current infection and atrophic gastritis-A simple and cost-Efficient method. Microb. Pathog. 2018, 119, 137–144. [Google Scholar] [CrossRef]
- Ashrafi, H.; Siraji, M.I.; Showva, N.N.; Hossain, M.M.; Hossan, T.; Hasan, M.A.; Shohael, A.M.; Shawan, M.M.A.K. Structure to function analysis with antigenic characterization of a hypothetical protein, HPAG1_0576 from Helicobacter pylori HPAG1. Bioinformation 2019, 15, 456–466. [Google Scholar] [CrossRef]
- Suganuma, M.; Yamaguchi, K.; Ono, Y.; Matsumoto, H.; Hayashi, T.; Ogawa, T.; Imai, K.; Kuzuhara, T.; Nishizono, A.; Fujiki, H. TNF-α-Inducing protein, a carcinogenic factor secreted fromH. pylori, enters gastric cancer cells. Int. J. Cancer 2008, 123, 117–122. [Google Scholar] [CrossRef]
- Suganuma, M.; Kuzuhara, T.; Yamaguchi, K.; Fujiki, H. Carcinogenic Role of Tumor Necrosis Factor-α Inducing Protein of Helicobacter pylori in Human Stomach. BMB Rep. 2006, 39, 1–8. [Google Scholar] [CrossRef]
- Tsuge, H.; Tsurumura, T.; Utsunomiya, H.; Kise, D.; Kuzuhara, T.; Watanabe, T.; Fujiki, H.; Suganuma, M. Structural basis for the Helicobacter pylori-Carcinogenic TNF-α-Inducing protein. Biochem. Biophys. Res. Commun. 2009, 388, 193–198. [Google Scholar] [CrossRef]
- Tang, C.; Hao, B.; Zhang, G.; Shi, R.; Cheng, W. Helicobacter pylori tumor necrosis factor-α inducing protein promotes cytokine expression via nuclear factor-κB. World J. Gastroenterol. 2013, 19, 399–403. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Suganuma, M.; Oka, K.; Fujiki, H. DNA-Binding activity of TNF-α inducing protein from Helicobacter pylori. Biochem. Biophys. Res. Commun. 2007, 362, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Beug, H.; Wirth, T. Epithelial-Mesenchymal Transition: NF-kappaB takes center stage. Cell Cycle 2004, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Bo, F.; Holzenburg, J.; Godek, F.; Kauffmann, P.; Moser, N.; Schliephake, H. Influence of tumour necrosis factor alpha on epithelial–Mesenchymal transition of oral cancer cells in co-Culture with mesenchymal stromal cells. Int. J. Maxillofac. Surg. 2020, 49, 157–165. [Google Scholar]
- Watanabe, T.; Tsuge, H.; Imagawa, T.; Kise, D.; Hirano, K.; Beppu, M.; Takahashi, A.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Nucleolin as cell surface receptor for tumor necrosis factor-α inducing protein: A carcinogenic factor of Helicobacter pylori. J. Cancer Res. Clin. Oncol. 2010, 136, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wang, F.; Jiang, A.; Xia, A.; Kong, S.; Gong, C.; Zhu, M.; Zhou, X.; Zhu, J.; Zhu, W.; et al. MicroRNA-3178 ameliorates inflammation and gastric carcinogenesis promoted byHelicobacter pylorinew toxin, Tip-α, by targeting TRAF3. Helicobacter 2016, 22, e12348. [Google Scholar] [CrossRef]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Nishizono, A.; Murakami, K.; Fujioka, T.; Fujiki, H. New tumor necrosis factor-alpha Inducing protein released from Helicobacter pylori for gastric cancer progression. J. Cancer Res. Clin. Oncol. 2004, 131, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Furuta, Y.; Yahara, K.; Tsuru, T.; Oshima, K.; Handa, N.; Takahashi, N.; Yoshida, M.; Azuma, T.; Hattori, M.; et al. Evolution in an oncogenic bacterial species with extreme genome plasticity: Helicobacter pylori East Asian genomes. BMC Microbiol. 2011, 11, 104. [Google Scholar] [CrossRef]
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 Paracrine Network Links Cancer Chemoresistance and Metastasis. Cell 2012, 150, 165–178. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Suganuma, M.; Kurusu, M.; Fujiki, H. Helicobacter pylori-Secreting protein Tipα is a potent inducer of chemokine gene expressions in stomach cancer cells. J. Cancer Res. Clin. Oncol. 2006, 133, 287–296. [Google Scholar] [CrossRef]
- Jang, J.Y.; Yoon, H.J.; Yoon, J.Y.; Kim, H.S.; Lee, S.J.; Kim, K.H.; Kim, D.J.; Jang, S.; Han, B.G.; Lee, B.I. Crystal structure of the TNF-Alpha inducing protein (Tip alpha) from Helicobacter pylori: Insights into Its DNA-Binding activity. J. Mol. Biol. 2009, 392, 191–197. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Marcos-Pinto, R.; Nairn, A.; dela Rosa, M.; Ferreira, R.M.; Junqueira-Neto, S.; Freitas, D.; Gomes, J.; Oliveira, P.; Santos, M.R.; et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta 2015, 1852, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, M.; Liu, J.; Yuan, Y. Host genetic factors respond to pathogenic step-Specific virulence factors of Helicobacter pylori in gastric carcinogenesis. Mutat. Res. Rev. Mutat. Res. 2014, 759, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Carneiro, F.; Letley, D.; Atherton, J.C.; Figueiredo, C. T1639 Helicobacter pylori vacA Intermediate Region i1 Strains Are Associated with More Severe Histological Features of Chronic Gastritis and Increased Gastric Carcinoma Risk in Portugal. Gastroenterology 2009, 136, A-548. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. The concept of the okadaic acid class of tumor promoters is revived in endogenous protein inhibitors of protein phosphatase 2A, SET and CIP2A, in human cancers. J. Cancer Res. Clin. Oncol. 2018, 144, 2339–2349. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Suganuma, M. Tumor promoters: From chemicals to inflammatory proteins. J. Cancer Res. Clin. Oncol. 2013, 139, 1603–1614. [Google Scholar] [CrossRef]
- Devanand, P.; Oya, Y.; Sundaramoorthy, S.; Song, K.Y.; Watanabe, T.; Kobayashi, Y.; Shimizu, Y.; Hong, S.A.; Suganuma, M.; Lim, I.K. Inhibition of TNFα-interacting protein α (Tipα)-Associated gastric carcinogenesis by BTG2/TIS21 via downregulating cytoplasmic nucleolin expression. Exp. Mol. Med. 2018, 50, e449. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Suganuma, M. Cell-Surface nucleolin acts as a central mediator for carcinogenic, anti-Carcinogenic, and disease-related ligands. J. Cancer Res. Clin. Oncol. 2014, 140, 689–699. [Google Scholar] [CrossRef]
- Suganuma, M.; Watanabe, T.; Yamaguchi, K.; Takahashi, A.; Fujiki, H. Human gastric cancer development with TNF-α-Inducing protein secreted from Helicobacter pylori. Cancer Lett. 2012, 322, 133–138. [Google Scholar] [CrossRef]
- Watanabe, T.; Hirano, K.; Takahashi, A.; Yamaguchi, K.; Beppu, M.; Fujiki, H.; Suganuma, M. Nucleolin on the Cell Surface as a New Molecular Target for Gastric Cancer Treatment. Biol. Pharm. Bull. 2010, 33, 796–803. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Tumor Promoters-Microcystin-LR, Nodularin and TNF-α and Human Cancer Development. Anti Cancer Agents Med. Chem. 2011, 11, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tang, N.; Wang, C.; Xiao, L.; Yu, M.; Zhao, L.; Cai, H.; Han, L.; Xie, C.; Zhang, Y. TNF-α-Inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shiota, S.; Yamada, K.; Gotoh, K.; Suganuma, M.; Fujioka, T.; Ahmed, K.; Iha, H.; Nishizono, A. Evaluation of a New Tumor Necrosis Factor-α-Inducing Membrane Protein of Helicobacter pylori as a Prophylactic Vaccine Antigen. Helicobacter 2009, 14, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.; Allardyce, R.; Roake, J.; Oliaro, J.; Domigan, N.; Potter, H.; Aitken, G.; Allardyce, R.; Roake, J. Immune response to an 18-Kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect. Immun. 2000, 68, 3337–3343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Y.; Marcus, E.A.; Matrubutham, U.; Gleeson, M.A.; Scott, D.R.; Sachs, G. Acid-Adaptive Genes of Helicobacter pylori. Society 2003, 71, 5921–5939. [Google Scholar]
- Ernst, F.D.; Bereswill, S.; Waidner, B.; Stoof, J.; Mäder, U.; Kusters, J.G.; Kuipers, E.J.; Kist, M.; van Vliet, A.H.M.; Homuth, G. Transcriptional profiling of Helicobacter pylori Fur- and iron-Regulated gene expression. Microbiology 2005, 151, 533–546. [Google Scholar] [CrossRef]
- Vallese, F.; Mishra, N.M.; Pagliari, M.; Berto, P.; Codolo, G.; de Bernard, M.; Zanotti, G. Helicobacter pylori antigenic Lpp20 is a structural homologue of Tipα and promotes epithelial-Mesenchymal transition. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3263–3271. [Google Scholar] [CrossRef]
- Ikeda, W.; Nakanishi, H.; Miyoshi, J.; Mandai, K.; Ishizaki, H.; Tanaka, M.; Togawa, A.; Takahashi, K.; Nishioka, H.; Yoshida, H.; et al. Afadin: A key molecule essential for structural organization of cell-Cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 1999, 146, 1117–1132. [Google Scholar] [CrossRef]
- Tabariès, S.; Mcnulty, A.; Ouellet, V.; Annis, M.G.; Dessureault, M.; Vinette, M.; Hachem, Y.; Lavoie, B.; Omeroglu, A.; Simon, H.G.; et al. Afadin cooperates with Claudin-2 to promote breast cancer metastasis. Genes Dev. 2019, 33, 180–193. [Google Scholar] [CrossRef]
- Maruo, T.; Sakakibara, S.; Miyata, M.; Itoh, Y.; Kurita, S. Molecular and Cellular Neuroscience Involvement of l-Afadin, but not s-Afadin, in the formation of puncta adherentia junctions of hippocampal synapses. Mol. Cell. Neurosci. 2018, 92, 40–49. [Google Scholar] [CrossRef]
- Peng, Y.F.; Mandai, K.; Nakanishi, H.; Ikeda, W.; Asada, M.; Momose, Y.; Shibamoto, S.; Yanagihara, K.; Shiozaki, K.; Monden, M.; et al. Restoration of E-Cadherin-based cell–Cell adhesion by overexpression of nectin in HSC-39 cells, a human signet ring cell gastric cancer cell line. Oncogene 2002, 21, 4108–4119. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.S.; Melo, J.; Cavadas, B.; Mendes, N.; Pereira, L.; Carneiro, F.; Figueiredo, C.; Leite, M. Afadin downregulation by helicobacter pyloriInduces epithelial to mesenchymal transition in gastric cells. Front. Microbiol. 2018, 9, 2712. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, J.G. Substitutions in Penicillin-Binding Protein 1 in Amoxicillin-Resistant Helicobacter pylori Strains Isolated from Korean Patients. Gut Liver 2013, 7, 655. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Davies, T.A.; Jacobs, M.R.; Appelbaum, P.C. Effects of Amino Acid Alterations in and 2× on PBP Affinities of Penicillin, Clinical Isolates of Penicillin-Susceptible, Effects of Amino Acid Alterations in Penicillin-Binding Proteins (PBPs) 1a, 2b, and 2× on PBP Affinities of Penicillin, Ampici. Antimicrob. Agents Chemother. 2002, 46, 1273–1280. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.Y.; Pan, D.D. Penicillin-Binding protein 1A mutation-Positive Helicobacter pylori promotes epithelial-Mesenchymal transition in gastric cancer via the suppression of microRNA-134. Int. J. Oncol. 2019, 54, 916–928. [Google Scholar] [CrossRef]
- Qiu, Z.; He, G. MicroRNA-134 functions as a tumor suppressor gene in gastric cancer. Am. J. Transl. Res. 2016, 8, 4320–4328. [Google Scholar]
- Liu, Y.; Sun, Y.; Zhao, A. MicroRNA-134 suppresses cell proliferation in gastric cancer cells via targeting of GOLPH3. Oncol. Rep. 2017, 37, 2441–2448. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Jin, M.; Wang, J.; Li, S.; Chen, Z.H.E.; Yu, W. MicroRNA-134 reverses multidrug resistance in human lung adenocarcinoma cells by targeting FOXM1. Oncol. Lett. 2017, 13, 1451–1455. [Google Scholar] [CrossRef]
- Damasceno, J.; Rodrigues, R.; Gonçalves, R.; Kitagawa, R. Anti-Helicobacter pylori Activity of Isocoumarin Paepalantine: Morphological and Molecular Docking Analysis. Molecules 2017, 22, 786. [Google Scholar] [CrossRef]
- Marcus, E.A.; Inatomi, N.; Nagami, G.T.; Sachs, G.; Scott, D.R. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment. Pharmacol. Ther. 2012, 36, 972–979. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Eberhardt, M.; Schmitz, U.; Vera, J. Systems biology-Based investigation of cooperating microRNAs as monotherapy or adjuvant therapy in cancer. Nucleic Acids Res. 2019, 47, 7753–7766. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Dong, P.; Xiong, Y.; Yue, J.; Konno, Y.; Ihira, K.; Kobayashi, N.; Todo, Y.; Watari, H. MicroRNA-361-Mediated Inhibition of HSP90 Expression and EMT in Cervical Cancer Is Counteracted by Oncogenic lncRNA NEAT1. Cells 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, K.; Makarova, J.; Sartorius, B.; An, P.; Winkler, C.; Chuturgoon, A.; Kramvis, A. The Regulatory Role of MicroRNA in Hepatitis-B Virus-Associated Hepatocellular Carcinoma (HBV-HCC) Pathogenesis. Cells 2019, 8, 1504. [Google Scholar] [CrossRef]
- Ding, H.; Shi, Y.; Liu, X.; Qiu, A. MircoRNA-4513 promotes gastric cancer cell proliferation and epithelial–Mesenchymal transition through targeting KAT6B. Human Gene Ther. Clin. Dev. 2019, 30, 142–148. [Google Scholar] [CrossRef]
- Du, M.; Tan, P.; Wang, A. microRNA-95 knockdown inhibits epithelial–Mesenchymal transition and cancer stem cell phenotype in gastric cancer cells through MAPK pathway by upregulating DUSP5. J. Cell. Physiol. 2020, 235, 944–956. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, M.; Zhang, L.; Guo, B.; Qin, Y.; Jiang, Q.; Sun, R.; Yang, J.; Wang, L.; Liu, L.; et al. MicroRNA-4268 inhibits cell proliferation via AKT/JNK signalling pathways by targeting Rab6B in human gastric cancer. Cancer Gene Ther. 2019, 1–12. [Google Scholar] [CrossRef]
- Sun, F.; Ni, Y.; Zhu, H.; Fang, J.; Wang, H.; Xia, J.; Ding, F.; Shen, H.; Shao, S. MicroRNA-29a-3p, up-Regulated in human gastric cells and tissues with H. Pylori infection, promotes the migration of GES-1 Cells via A20-Mediated EMT Pathway. Cell. Physiol. Biochem. 2018, 51, 1250–1263. [Google Scholar] [CrossRef]
- Fukaya, M.; Brorsson, C.A.; Meyerovich, K.; Catrysse, L.; Delaroche, D.; Vanzela, E.C.; Ortis, F.; Beyaert, R.; Nielsen, L.B.; Andersen, M.L.; et al. A20 Inhibits β-Cell Apoptosis by Multiple Mechanisms and Predicts Residual β-Cell Function in Type 1 Diabetes. Mol. Endocrinol. 2016, 30, 48–61. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Manirujjaman, M.; Hamajima, H.; Ozaki, I. Control Mechanisms of the Tumor Suppressor PDCD4: Expression and Functions. Int. J. Mol. Sci. 2019, 20, 2304. [Google Scholar] [CrossRef]
- Vikhreva, P.N.; Kalinichenko, S.V.; Korobko, I.V. Programmed cell death 4 mechanism of action: The model to be updated? Cell Cycle 2017, 16, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.-S. The role of Pdcd4 in tumour suppression and protein translation. Biol. Cell 2018, 110, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Leupold, J.H.; Yang, H.; Colburn, N.H.; Asangani, I.; Post, S.; Allgayer, H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-Transcription factors. Oncogene 2007, 26, 4550–4562. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Izumi, H.; Tanimoto, A.; Takahashi, M.; Miyamoto, N.; Kashiwagi, E.; Kidani, A.; Hirano, G.; Masubuchi, D.; Fukunaka, Y.; et al. Programmed Cell Death Protein 4 Down-Regulates Y-Box Binding Protein-1 Expression via a Direct Interaction with Twist1 to Suppress Cancer Cell Growth. Cancer Res. 2009, 69, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, D.E.; Clocchiatti, A.; Dotto, G.P. PDCD4 is a CSL associated protein with a transcription repressive function in cancer associated fibroblast activation. Oncotarget 2016, 7, 58717–58727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, H.; Zeng, J.; Liang, X.; Wang, W.; Zhou, Y.; Sun, Y.; Liu, S.; Li, W.; Chen, C.; Ja, J. Helicobacter pylori Promotes Epithelial–Mesenchymal Transition in Gastric Cancer by Downregulating Programmed Cell Death Protein 4 (PDCD4). PLoS ONE 2014, 9, e105306. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Li, Y.; Lin, S.; Tomuleasa, C. MicroRNA-21 Stimulates Gastric Cancer Growth and Ivasion by Inhibiting the Tumor Suppressor Effects of Programmed Cell Deatg Protein 4 and Phosphatase and Tensin Homolog. J. BUON 2014, 19, 228–236. [Google Scholar]
- Rehman, Z.; Fahim, A.; Bhatti, A.; Sadia, H.; John, P. Co-Expression of HIF-1α, MDR1 and LAPTM4B in peripheral blood of solid tumors. PeerJ 2019, 7, e6309. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Zhang, S.; Lu, J.; Lu, Y.; Zhan, P.; Fang, Q.; Wang, F.; Zhang, X.; Xie, C.; et al. LAPTM4B facilitates tumor growth and induces autophagy in hepatocellular carcinoma. Cancer Manag. Res. 2019, 11, 2485–2497. [Google Scholar] [CrossRef]
- Li, X.; Song, C.; Wang, K.; Li, N.; Sun, S.; Li, N.; Zhao, Z.; Li, M. Prognostic significance of LAPTM4B and p27kip1 expression in triple-negative breast cancer. Cancer Biomark. 2019, 25, 19–27. [Google Scholar] [CrossRef]
- Wang, L.; Meng, Y.; Zhang, Q. LAPTM4B is a novel diagnostic and prognostic marker for lung adenocarcinoma and associated with mutant EGFR. BMC Cancer 2019, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, H.; Yuan, P.; Shi, N.; Wang, X.; Hu, J.; Liu, L. Helicobacter pylori infection promotes epithelial-to-mesenchymal transition of gastric cells by upregulating LAPTM4B. Biochem. Biophys. Res. Commun. 2019, 514, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Gao, F.; Chen, J.; Sun, Y.; Zhang, Y.; Liu, H.; Li, X.; Yang, P.; Zheng, R.; Liu, G.; et al. Overexpressed LAPTM4B-35 is a risk factor for cancer recurrence and poor prognosis in non-small-Cell lung cancer. Oncotarget 2016, 7, 56193–56199. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Zhou, J.; Lu, L.; Wang, H.; Zhang, G.; Wan, G.; Cai, S.; Du, J. Nodal Facilitates Differentiation of Fibroblasts to Cancer-Associated Fibroblasts that Support Tumor Growth in Melanoma and Colorectal Cancer. Cells 2019, 8, 538. [Google Scholar] [CrossRef] [PubMed]
- Yegodayev, K.M.; Novoplansky, O.; Golden, A.; Prasad, M.; Levin, L.; Jagadeeshan, S.; Zorea, J.; Dimitstein, O.; Joshua, B.Z.; Cohen, L.; et al. TGF-Beta-Activated Cancer-Associated Fibroblasts Limit Cetuximab Efficacy in Preclinical Models of Head and Neck Cancer. Cancers 2020, 12, 339. [Google Scholar] [CrossRef]
- Minna, E.; Brich, S.; Todoerti, K.; Pilotti, S.; Collini, P.; Bonaldi, E.; Romeo, P.; Cecco, L.D.; Dugo, M.; Perrone, F.; et al. Cancer Associated Fibroblasts and Senescent Thyroid Cells in the Invasive Front of Thyroid Carcinoma. Cancers 2020, 12, 112. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Zhang, Q.; Chai, S.; Wang, W.; Wan, C.; Zhang, F.; Li, Y.; Wang, F. Macrophages activate mesenchymal stem cells to acquire cancer-associated fibroblast-Like features resulting in gastric epithelial cell lesions and malignant transformation in vitro. Oncol. Lett. 2019, 17, 747–756. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Huang, F.; Yang, T.; Cai, J.; Zhang, X.; Zhu, W.; Qian, H.; Xu, W. H. pylori infection-induced MSC differentiation into CAFs promotes epithelial-mesenchymal transition in gastric epithelial cells. Int. J. Mol. Med. 2013, 32, 1465–1473. [Google Scholar] [CrossRef][Green Version]
- Tsukada, T.; Fushida, S.; Harada, S.; Yagi, Y.; Kinoshita, J.; Oyama, K.; Tajima, H.; Fujita, H.; Ninomiya, I.; Fujimura, T.; et al. The role of human peritoneal mesothelial cells in the fibrosis and progression of gastric cancer. Int. J. Oncol. 2012, 41, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gu, J.; Chen, J.; Zhang, P.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncol. Rep. 2017, 38, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Zhang, H.; Wang, C.; Song, X. Exosomes Released by Gastric Cancer Cells Induce Transition of Pericytes Into Cancer-Associated Fibroblasts. Med. Sci. Monit. 2018, 24, 2350–2359. [Google Scholar] [CrossRef]
- Lim, H.; Moon, A. Inflammatory fibroblasts in cancer. Arch. Pharmacal Res. 2016, 39, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Yokozaki, H.; Koma, Y.-I.; Shigeoka, M.; Nishio, M. Cancer as a tissue: The significance of cancer-Stromal interactions in the development, morphogenesis and progression of human upper digestive tract cancer. Pathol. Int. 2018, 68, 334–352. [Google Scholar] [CrossRef]
- Machlowska, J.; Maciejewski, R.; Sitarz, R. The Pattern of Signatures in Gastric Cancer Prognosis. Int. J. Mol. Sci. 2018, 19, 1658. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-Associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Itoh, G.; Chida, S.; Yanagihara, K.; Yashiro, M.; Aiba, N.; Tanaka, M. Cancer-Associated fibroblasts induce cancer cell apoptosis that regulates invasion mode of tumours. Oncogene 2017, 36, 4434–4444. [Google Scholar] [CrossRef]
- Tang, D.; Gao, J.; Wang, S.; Ye, N.; Chong, Y.; Huang, Y.; Wang, J.; Li, B.; Yin, W.; Wang, D. Cancer-Associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumor Biol. 2016, 37, 1889–1899. [Google Scholar] [CrossRef]
- Lin, N.N.; Wang, P.; Zhao, D.; Zhang, F.J.; Yang, K.; Chen, R. Significance of oral cancer-associated fibroblasts in angiogenesis, lymphangiogenesis, and tumor invasion in oral squamous cell carcinoma. J. Oral Pathol. Med. 2017, 46, 21–30. [Google Scholar] [CrossRef]
- Eiro, N.; González, L.; Martínez-Ordoñez, A.; Fernandez-Garcia, B.; González, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-Associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell. Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Krzysiek-Maczka, G.; Targosz, A.; Ptak-Belowska, A.; Korbut, E.; Szczyrk, U.; Strzalka, M.; Brzozowski, T. Molecular alterations in fibroblasts exposed to helicobacter pylori: A missing link in bacterial inflammation progressing into gastric carcinogenesis? J. Physiol. Pharmacol. 2013, 64, 77–87. [Google Scholar]
- Krzysiek-Maczka, G.; Targosz, A.; Szczyrk, U.; Strzałka, M.; Sliwowski, Z.; Brzozowski, T.; Czyz, J.; Ptak-Belowska, A. Role of Helicobacter pylori infection in cancer-Associated fibroblast-induced epithelial-Mesenchymal transition in vitro. Helicobacter 2018, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; He, X.; Jiao, F.; Wang, C.; Sun, Y.; Ren, X.; Li, Q. Fibroblast Activation Protein-α-Positive Fibroblasts Promote Gastric Cancer Progression and Resistance to Immune Checkpoint Blockade. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Koczorowska, M.M.; Tholen, S.; Bucher, F.; Lutz, L.; Kizhakkedathu, J.N.; De Wever, O.; Wellner, U.F.; Biniossek, M.L.; Stahl, A.; Lassmann, S.; et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 2016, 10, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, C.; Peng, C.; Xu, F.; Li, Y.; Yutaka, Y.; Xiong, B.; Yang, X. Stromal fibroblast activation protein alpha promotes gastric cancer progression via epithelial-Mesenchymal transition through Wnt/β-Catenin pathway. BMC Cancer 2018, 18, 1099. [Google Scholar] [CrossRef]
- Chiavarina, B.; Whitaker-Menezes, D.; Migneco, G.; Martinez-Outschoorn, U.E.; Pavlides, S.; Howell, A.; Tanowitz, H.B.; Casimiro, M.C.; Wang, C.; Pestell, R.G.; et al. HIF1-Alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle 2010, 9, 3534–3551. [Google Scholar] [CrossRef]
- Nagaharu, K.; Zhang, X.; Yoshida, T.; Katoh, D.; Hanamura, N.; Kozuka, Y.; Ogawa, T.; Shiraishi, T.; Imanaka-Yoshida, K. Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am. J. Pathol. 2011, 178, 754–763. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Tykarski, A.; Ksiazek, K. The Peritoneal “soil” for a Cancerous “Seed”: A Comprehensive Review of the Pathogenesis of Intraperitoneal Cancer Metastases. Cell. Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Krzysiek-Maczka, G.; Targosz, A.; Szczyrk, U.; Strzalka, M.; Brzozowski, T.; Ptak-Belowska, A. Involvement of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in the Mechanism of Helicobacter Pylori-Induced Fibroblasts Activation. J. Physiol. Pharmacol. 2019, 70, 727–736. [Google Scholar]
- Schoepp, M.; Ströse, A.; Haier, J. Dysregulation of miRNA Expression in Cancer Associated Fibroblasts (CAFs) and Its Consequences on the Tumor Microenvironment. Cancers 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, Y.; Yu, W.; Yan, Y.; Qiao, M.; Jiang, R.; Guan, W.; Wang, L. Downregulation of miRNA-214 in cancer-Associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J. Exp. Clin. Cancer Res. 2019, 38, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fukui, H.; Hara, K.; Zhang, X.; Kitayama, Y.; Eda, H.; Tomita, T.; Oshima, T.; Kikuchi, S.; Watari, J.; et al. FGF9 from cancer-Associated fibroblasts is a possible mediator of invasion and anti-Apoptosis of gastric cancer cells. BMC Cancer 2015, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shan, J.-X.; Chen, X.-H.; Zhang, D.; Su, L.-P.; Huang, X.-Y.; Yu, B.Q.; Zhi, G.M.; Li, C.L.; Wang, Y.Q.; et al. Epigenetic silencing of microRNA-149 in cancer-Associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res. 2015, 25, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.N.; Won, Y.J.; Shim, J.H.; Kim, H.J.; Han, S.H.; Kim, B.S.; Kim, H.S. Cancer-Associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-Independent manner. J. Cancer Res. Clin. Oncol. 2018, 144, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-Associated fibroblasts promotes epithelial-Mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741. [Google Scholar] [CrossRef]
- Chong, Y.; Tang, D.; Xiong, Q.; Jiang, X.; Xu, C.; Huang, Y.; Wang, J.; Zhou, H.; Shi, Y.; Wu, X.; et al. Galectin-1 from cancer-Associated fibroblasts induces epithelial–Mesenchymal transition through β1 integrin-Mediated upregulation of Gli1 in gastric cancer. J. Exp. Clin. Cancer Res. 2016, 35, 175. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Wang, X.; Yu, Z.; Pan, T.; Li, Z.; Chang, X.; Jin, Z.; Li, J.; Zhu, Z.; et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene 2019, 39, 1414–1428. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chou, Y.T.; Kuo, M.H.; Tsai, H.P.; Chang, J.L.; Wu, C.W. A targetable HB-EGF-CITED4 axis controls oncogenesis in lung cancer. Oncogene 2017, 36, 2946–2956. [Google Scholar] [CrossRef]
- Dickson, J.H.; Grabowska, A.; El-Zaatari, M.; Atherton, J.; Watson, S.A. Helicobacter pylori can induce heparin-Binding epidermal growth factor expression via gastrin and its receptor. Cancer Res. 2006, 66, 7524–7531. [Google Scholar] [CrossRef]
- Chung, H.W.; Kong, H.Y.; Lim, J.-B. Clinical significance and usefulness of soluble heparin binding-Epidermal growth factor in gastric cancer. World J. Gastroenterol. 2015, 21, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Naef, M.; Yokoyama, M.; Friess, H.; Büchler, M.W.; Korc, M. Co-Expression of heparin-binding EGF-Like growth factor and related peptides in human gastric carcinoma. Int. J. Cancer 1996, 66, 315–321. [Google Scholar] [CrossRef]
- Kim, J.; Kim, N.; Park, J.H.; Chang, H.; Kim, J.Y.; Lee, D.H.; Kim, J.M.; Kim, J.S.; Jung, H.C. The Effect of Helicobacter pylori on Epidermal Growth Factor Receptor-Induced Signal Transduction and the Preventive Effect of Celecoxib in Gastric Cancer Cells. Gut Liver 2013, 7, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ricci, V.; Popolo, A.D.; Sommi, P.; Blanco, C.D.V.; Bruni, C.B.; Ventura, U.; Cover, T.L.; Blaser, M.J.; Coffey, R.J.; et al. Helicobacter pylori upregulates expression of epidermal growth factor-Related peptides, but inhibits their proliferative effect in MKN 28 gastric mucosal cells. J. Clin. Investig. 1998, 101, 1604–1613. [Google Scholar] [CrossRef]
- Murayama, Y.; Miyagawa, J.-I.; Shinomura, Y.; Kanayama, S.; Isozaki, K.; Yamamori, K.; Mizuno, H.; Ishiguro, S.; Kiyohara, T.; Miyazaki, Y.; et al. Significance of the association between heparin-Binding epidermal growth factor-Like growth factor and CD9 in human gastric cancer. Int. J. Cancer 2002, 98, 505–513. [Google Scholar] [CrossRef]
- Busiello, I.; Acquaviva, R.; Di Popolo, A.; Blanchard, T.G.; Ricci, V.; Romano, M.; Zarrilli, R. Helicobacter pylori γ-Glutamyltranspeptidase upregulates COX-2 and EGF-related peptide expression in human gastric cells. Cell. Microbiol. 2004, 6, 255–267. [Google Scholar] [CrossRef]
- Lu, L.; Ma, G.-Q.; Liu, X.-D.; Sun, R.-R.; Wang, Q.; Liu, M.; Zhang, P.Y. Correlation between GDF15, MMP7 and gastric cancer and its prognosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 535–541. [Google Scholar]
- Kitoh, T.; Yanai, H.; Saitoh, Y.; Nakamura, Y.; Matsubara, Y.; Kitoh, H.; Yoshida, T.; Okita, K. Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. J. Gastroenterol. 2004, 39, 434–440. [Google Scholar] [CrossRef]
- Yang, T.F.; Guo, L.; Wang, Q. Meta-Analysis of Associations between Four Polymorphisms in the Matrix Metalloproteinases Gene and Gastric Cancer Risk. Asian Pac. J. Cancer Prev. 2014, 15, 1263–1267. [Google Scholar] [CrossRef][Green Version]
- Long, Z.-W.; Wang, J.-L.; Wang, Y.-N. Matrix metalloproteinase-7 mRNA and protein expression in gastric carcinoma: A meta-Analysis. Tumor Biol. 2014, 35, 11415–11426. [Google Scholar] [CrossRef]
- Li, G.; Su, Q.; Liu, H.; Wang, D.; Zhang, W.; Lu, Z.; Chen, Y.; Huang, X.; Li, W.; Zhang, C.; et al. Frizzled7 Promotes Epithelial-To-Mesenchymal Transition and Stemness via Activating Canonical Wnt/β-Catenin Pathway in Gastric Cancer. Int. J. Biol. Sci. 2018, 14, 280–293. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Gershtein, E.S.; Ivannikov, A.A.; Davydov, M.M.; Chang, V.L.; Ognerubov, N.A.; Stilidi, I.S. Clinical Significance of Matrix Metalloproteinases in Blood Plasma of Patients with Gastric Cancer. Bull. Exp. Biol. Med. 2019, 166, 373–376. [Google Scholar] [CrossRef]
- Naumann, M.; Wessler, S.; Bartsch, C.; Wieland, B.; Covacci, A.; Haas, R.; Meyer, T.F. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 1999, 274, 31655–31662. [Google Scholar] [CrossRef]
- Meyer-Ter-Vehn, T.; Covacci, A.; Kist, M.; Pahl, H.L. Helicobacter pylori activates mitogen-Activated protein kinase cascades and induces expression of the proto-Oncogenes c-Fos and c-Jun. J. Biol. Chem. 2000, 275, 16064–16072. [Google Scholar] [CrossRef]
- Wu, D. Isocitrate dehydrogenase 2 inhibits gastric cancer cell invasion via matrix metalloproteinase 7. Tumor Biol. 2015, 37, 5225–5230. [Google Scholar] [CrossRef]
- Gunawardhana, N.; Jang, S.; Choi, Y.H.; Hong, Y.A.; Jeon, Y.-E.; Kim, A.; Su, H.; Kim, J.H.; Yoo, Y.J.; Errell, S.; et al. Helicobacter pylori-Induced HB-EGF Upregulates Gastrin Expression via the EGF Receptor, C-Raf, Mek1, and Erk2 in the MAPK Pathway. Front. Cell. Infect. Microbiol. 2018, 7, 541. [Google Scholar] [CrossRef]
- Soleyman-Jahi, S.; Nedjat, S.; Abdirad, A.; Hoorshad, N.; Heidari, R.; Zendehdel, K. Prognostic Significance of Matrix Metalloproteinase-7 in Gastric Cancer Survival: A Meta-Analysis. PLoS ONE 2015, 10, e0122316. [Google Scholar] [CrossRef]
- Yin, F.; Grabowska, A.M.; Clarke, P.A.; Whelband, E.; Robinson, K.; Argent, R.H.; Tobias, A.; Kumari, R.; Therton, J.C.; Watson, S.A. Helicobacter pylori potentiates epithelial: Mesenchymal transition in gastric cancer: Links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut 2010, 59, 1037–1045. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, L.; Peek, R.M.; Hao, X.; Polk, D.B.; Li, H.; Yan, F. Activation of Epidermal Growth Factor Receptor in Macrophages Mediates Feedback Inhibition of M2 Polarization and Gastrointestinal Tumor Cell Growth. J. Biol. Chem. 2016, 291, 20462–20472. [Google Scholar] [CrossRef]
- Byeon, S.-J.; Lee, H.S.; Kim, M.-A.; Lee, B.L.; Kim, W.H. Expression of the ERBB Family of Ligands and Receptors in Gastric Cancer. Pathobiology 2017, 84, 210–217. [Google Scholar] [CrossRef]
- Koshikawa, N.; Mizushima, H.; Minegishi, T.; Iwamoto, R.; Mekada, E.; Seiki, M. Membrane Type 1-Matrix Metalloproteinase Cleaves Off the NH2-Terminal Portion of Heparin-Binding Epidermal Growth Factor and Converts It into a Heparin-Independent Growth Factor. Cancer Res. 2010, 70, 6093–6103. [Google Scholar] [CrossRef]
- Yotsumoto, F.; Yagi, H.; Suzuki, S.O.; Oki, E.; Tsujioka, H.; Hachisuga, T.; Sonoda, K.; Kawarabayashi, T.; Mekada, E.; Miyamoto, S. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem. Biophys. Res. Commun. 2008, 365, 555–561. [Google Scholar] [CrossRef]
- Kneissl, J.; Hartmann, A.; Pfarr, N.; Erlmeier, F.; Lorber, T.; Keller, S.; Zwingenberger, G.; Weichert, W.; Luber, B. Influence of the HER receptor ligand system on sensitivity to cetuximab and trastuzumab in gastric cancer cell lines. J. Cancer Res. Clin. Oncol. 2016, 143, 573–600. [Google Scholar] [CrossRef]
- Shimura, T.; Yoshida, M.; Fukuda, S.; Ebi, M.; Hirata, Y.; Mizoshita, T.; Tanida, S.; Kataoka, H.; Kamiya, T.; Higashiyama, S.; et al. Nuclear translocation of the cytoplasmic domain of HB-EGF induces gastric cancer invasion. BMC Cancer 2012, 12, 205. [Google Scholar] [CrossRef]
- Shimura, T.; Kataoka, H.; Ogasawara, N.; Kubota, E.; Sasaki, M.; Tanida, S.; Joh, T. Suppression of proHB-EGF Carboxy-Terminal Fragment Nuclear Translocation: A New Molecular Target Therapy for Gastric Cancer. Clin. Cancer Res. 2008, 14, 3956–3965. [Google Scholar] [CrossRef][Green Version]
- Krakowiak, M.S.; Noto, J.M.; Piazuelo, M.B.; Hardbower, D.M.; Romero-Gallo, J.; Delgado, A.; Chaturvedi, R.; Correa, P.; Wilson, K.T.; Peek, R.M. Matrix metalloproteinase 7 restrains Helicobacter pylori-Induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization. Oncogene 2014, 34, 1865–1871. [Google Scholar] [CrossRef]
- Azizi, P.; Mazhari, S.; Tokhangibli, S.; Noukabadi, F.N.; Abkenar, E.D.; Shamsafzali, E.; Baghaei, K. Paracrine Signals of Mesenchymal Stem Cells Induce Epithelial to Mesenchymal Transition in Gastric Cancer Cells. Gastroenterol. Hepatol. Bed Bench 2019, 12 (Suppl. 1), S51–S57. [Google Scholar]
- Puculek, M.; Baj, J.; Portincasa, P.; Sitarz, M.; Grochowski, C.; Radzikowska, E. The morphology and application of stem cells in digestive system surgery. Folia Morphol. 2020. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Yang, J.; Zhang, X.; Zhang, H.; Zhang, T.; Zhao, S.; Zheng, P.; Huo, J.; Wu, H. Gastric cancer-Derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J. Exp. Clin. Cancer Res. 2015, 34, 52. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Zhang, B.; Zhang, X.; Shi, H.; Liang, Z.; Wu, P.; Li, R.; You, B.; Yang, L.; et al. YAP signaling in gastric cancer-Derived mesenchymal stem cells is critical for its promoting role in cancer progression. Int. J. Oncol. 2017, 51, 1055–1066. [Google Scholar] [CrossRef]
- Ferrand, J.; Lehours, P.; Schmid-Alliana, A.; Mégraud, F.; Varon, C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-κB-Dependent pathway. PLoS ONE 2011, 6, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wu, X.; Chen, X.; Liu, Y.; Wang, X.; Wu, K.; Nie, Y.; Fan, D. Mesenchymal Stem Cells Promote Epithelial to Mesenchymal Transition and Metastasis in Gastric Cancer Though Paracrine Cues and Close Physical Contact. J. Cell. Biochem. 2015, 116, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, B.; Wang, X.; Luo, Y.; Fan, W. NANOGP8 is the key regulator of stemness, EMT, Wnt pathway, chemoresistance, and other malignant phenotypes in gastric cancer cells. PLoS ONE 2018, 13, e0192436. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, J.; Liu, J.; Wang, W.; Zhang, F.; Wang, J.; Li, Y. Helicobacter pylori-infected MSCs acquire a pro-Inflammatory phenotype and induce human gastric cancer migration by promoting EMT in gastric cancer cells. Oncol. Lett. 2016, 11, 449–457. [Google Scholar] [CrossRef][Green Version]
- Sun, L.; Wang, Q.; Chen, B.; Zhao, Y.; Shen, B.; Wang, X.; Zhu, M.; Li, Z.; Zhao, X.; Xu, C. Human Gastric Cancer Mesenchymal Stem Cell-Derived IL15 Contributes to Tumor Cell Epithelial-Mesenchymal Transition via Upregulation Tregs Ratio and PD-1 Expression in CD4 T Cell. Stem Cells Dev. 2018, 27, 1203–1214. [Google Scholar] [CrossRef]
- Jiang, Y.-X.; Yang, S.-W.; Li, P.-A.; Luo, X.; Li, Z.-Y.; Hao, Y.-X.; Yu, P.-W. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene 2016, 36, 1256–1264. [Google Scholar] [CrossRef]
- Peng, X.; Liu, G.; Peng, H.; Chen, A.; Zha, L.; Wang, Z. SOX4 contributes to TGF-β-Induced epithelial–Mesenchymal transition and stem cell characteristics of gastric cancer cells. Genes Dis. 2018, 5, 49–61. [Google Scholar] [CrossRef]
- Zhang, S.; Shang, Y.; Chen, T.; Zhou, X.; Meng, W.; Fan, C.; Lu, R.; Huang, Q.; Li, X.; Hong, X.; et al. Human circulating and tissue gastric cancer stem cells display distinct epithelial–Mesenchymal features and behaviors. J. Cancer Res. Clin. Oncol. 2017, 143, 1687–1699. [Google Scholar] [CrossRef]
- Bessède, E.; Dubus, P.; Mégraud, F.; Varon, C. Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene 2014, 34, 2547–2555. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Geng, Z.-J.; Sun, X.-Y.; Li, Y.-H.; Fu, X.-B.; Zhao, X.-Y.; Wei, B. Isoprenaline induces epithelial–Mesenchymal transition in gastric cancer cells. Mol. Cell. Biochem. 2015, 408, 1–13. [Google Scholar] [CrossRef]
- Kim, N. Chemoprevention of Gastric Cancer by Helicobacter Pylori Eradication and Its Underlying Mechanism. J. Gastroenterol. Hepatol. 2019, 34, 1287–1295. [Google Scholar]
- Shu, X.; Liu, H.; Pan, Y.; Sun, L.; Yu, L.; Sun, L.; Yang, Z.; Ran, Y. Distinct biological characterization of the CD44 and CD90 phenotypes of cancer stem cells in gastric cancer cell lines. Mol. Cell. Biochem. 2019, 459, 35–47. [Google Scholar] [CrossRef]
- Li, L.-C.; Wang, D.-L.; Wu, Y.-Z.; Nian, W.-Q.; Wu, Z.-J.; Li, Y.; Ma, H.W.; Shao, J.H. Gastric tumor-Initiating CD44 cells and epithelial-Mesenchymal transition are inhibited by γ-Secretase inhibitor DAPT. Oncol. Lett. 2015, 10, 3293–3299. [Google Scholar] [CrossRef][Green Version]
- Tanabe, S. Regulated genes in mesenchymal stem cells and gastric cancer. World J. Stem Cells 2015, 7, 208. [Google Scholar] [CrossRef]
- Yoon, C.; Till, J.; Cho, S.-J.; Chang, K.K.; Lin, J.-X.; Huang, C.-M.; Ryeom, S.; Yoon, S.S. KRAS Activation in Gastric Adenocarcinoma Stimulates Epithelial-to-Mesenchymal Transition to Cancer Stem–Like Cells and Promotes Metastasis. Mol. Cancer Res. 2019, 17, 1945–1957. [Google Scholar] [CrossRef]
- Yoon, C.; Cho, S.-J.; Chang, K.K.; Park, D.J.; Ryeom, S.W.; Yoon, S.S. Role of Rac1 Pathway in Epithelial-to-Mesenchymal Transition and Cancer Stem-Like Cell Phenotypes in Gastric Adenocarcinoma. Mol. Cancer Res. 2017, 15, 1106–1116. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, Y.; Sun, Z.; Ji, R.; Zhang, X.; Xu, W.; Yuan, X.; Zhang, B.; Yan, Y.; Yin, L.; et al. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer 2015, 15, 793. [Google Scholar] [CrossRef]
- Guo, J.; Wang, B.; Fu, Z.; Wei, J.; Lu, W. Hypoxic Microenvironment Induces EMT and Upgrades Stem-Like Properties of Gastric Cancer Cells. Technol. Cancer Res. Treat. 2015, 15, 60–68. [Google Scholar] [CrossRef]
- Hajimoradi, M.; Hassan, Z.M.; Ebrahimi, M.; Soleimani, M.; Bakhshi, M.; Firouzi, J.; Samani, F.S. STAT3 Is Overactivated in Gastric Cancer Stem-Like Cells. Cell J. 2016, 17, 617–628. [Google Scholar]
- Jung, D.H.; Bae, Y.J.; Kim, J.-H.; Shin, Y.K.; Jeung, H.-C. HER2 Regulates Cancer Stem Cell Activities via the Wnt Signaling Pathway in Gastric Cancer Cells. Oncology 2019, 97, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Ping, Y.-F.; Jiang, Y.-X.; Luo, X.; Zhang, X.; Bian, X.-W.; Yu, P.W. ATG4A promotes tumor metastasis by inducing the epithelial-Mesenchymal transition and stem-Like properties in gastric cells. Oncotarget 2016, 7, 39279–39292. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Xu, M.; Hua, R.; Tan, C.; Zhang, J.; Gong, Y.; Wu, Z.; Weng, W.; Sheng, W.; Guo, W. The polycomb group protein EZH2 induces epithelial–Mesenchymal transition and pluripotent phenotype of gastric cancer cells by binding to PTEN promoter. J. Hematol. Oncol. 2018, 11, 9. [Google Scholar] [CrossRef]
- Yang, S.-W.; Zhang, Z.-G.; Hao, Y.-X.; Zhao, Y.-L.; Qian, F.; Shi, Y.; Li, P.A.; Liu, C.Y.; Yu, P.W. HIF-1α induces the epithelial-Mesenchymal transition in gastric cancer stem cells through the Snail pathway. Oncotarget 2017, 8, 9535–9545. [Google Scholar] [PubMed]
- Yang, T.; Zhang, X.; Wang, M.; Zhang, J.; Huang, F.; Cai, J.; Zhang, Q.; Mao, F.; Zhu, W.; Qian, H.; et al. Activation of Mesenchymal Stem Cells by Macrophages Prompts Human Gastric Cancer Growth through NF-κB Pathway. PLoS ONE 2014, 9, e97569. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Park, S.; Park, S.; Lee, H.; Kim, C.; Jung, D.E.; Song, S.Y. Novel Gastric Cancer Stem Cell-Related Marker LINGO2 Is Associated with Cancer Cell Phenotype and Patient Outcome. Int. J. Mol. Sci. 2019, 20, 555. [Google Scholar] [CrossRef]
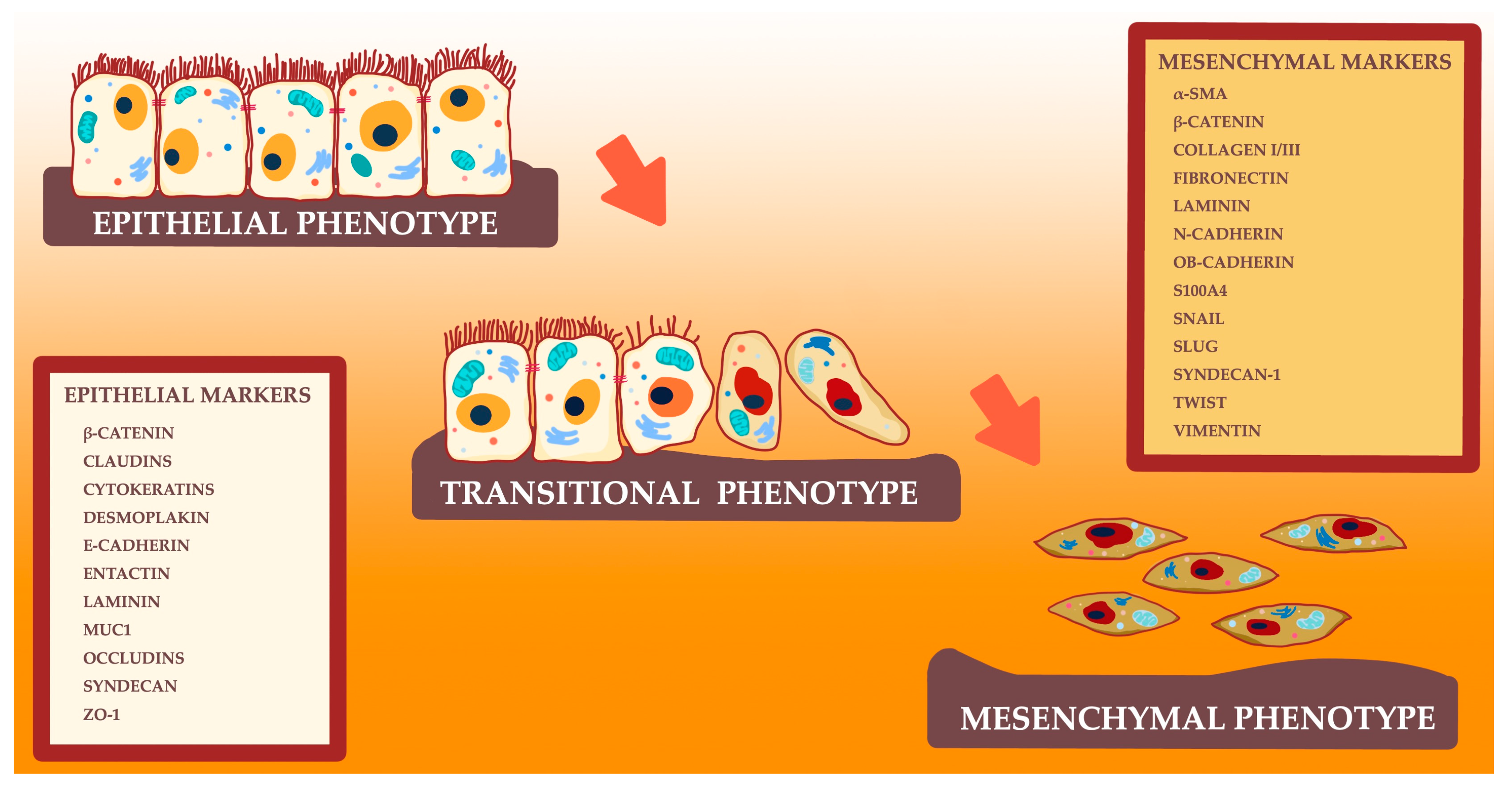

| Factors | Increase | Decrease |
|---|---|---|
| Afadin | actin stress fibers; Snail | ND |
| CAFs | α-SMA; Collagen I; Collagen III; COX-2; FAP; FGF-2; FSP1; HGF; HIF-1α; IL-6; IL-8; Integrin β-1; N-cadherin; SDF-1; Snail; TGF-β; TNC; TWIST; VEGF; vimentin | Bcl-2; Ki67 |
| CagA | CD44; Snail 1; vimentin; ZEB1 | CK7; SSP-1 |
| CagA & YAP pathway | CTGF; CYR61; MYC; N-cadherin; Slug; | E-cadherin |
| HB-EGF & MMP-7 | AP-1; NF-κB; Slug; Snail; vimentin | ND |
| LAPTM4B | β-catenin; N-cadherin; Slug; Snail; vimentin; ZEB1 | E-cadherin; ZO-1 |
| Lpp20 | ND | E-cadherin |
| miR-29a-3p | N-cadherin; Snail; vimentin | A20 gene; E-cadherin |
| MSCs | EGF; GM-CSF; IL-1β; IL-6; IL-8; MCP-1; N-cadherin; PDGF-B; TNF-α; VEGF; vimentin | E-cadherin |
| PBP1A mutation | α-SMA; FoxM1 | E-cadherin; miR-134 |
| PDCD4 | Twist1; vimentin | E-cadherin |
| Tipα | Ccl2; Ccl7; Ccl20; Cxcl1; Cxcl2; Cxcl5; Cxcl10; IL-1β; IL-6/STAT3 pathway; IL-8; N-cadherin; NF-κB; TNF-α; vimentin | E-cadherin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer. Cells 2020, 9, 1055. https://doi.org/10.3390/cells9041055
Baj J, Korona-Głowniak I, Forma A, Maani A, Sitarz E, Rahnama-Hezavah M, Radzikowska E, Portincasa P. Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer. Cells. 2020; 9(4):1055. https://doi.org/10.3390/cells9041055
Chicago/Turabian StyleBaj, Jacek, Izabela Korona-Głowniak, Alicja Forma, Amr Maani, Elżbieta Sitarz, Mansur Rahnama-Hezavah, Elżbieta Radzikowska, and Piero Portincasa. 2020. "Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer" Cells 9, no. 4: 1055. https://doi.org/10.3390/cells9041055
APA StyleBaj, J., Korona-Głowniak, I., Forma, A., Maani, A., Sitarz, E., Rahnama-Hezavah, M., Radzikowska, E., & Portincasa, P. (2020). Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter pylori-Induced Gastric Cancer. Cells, 9(4), 1055. https://doi.org/10.3390/cells9041055









