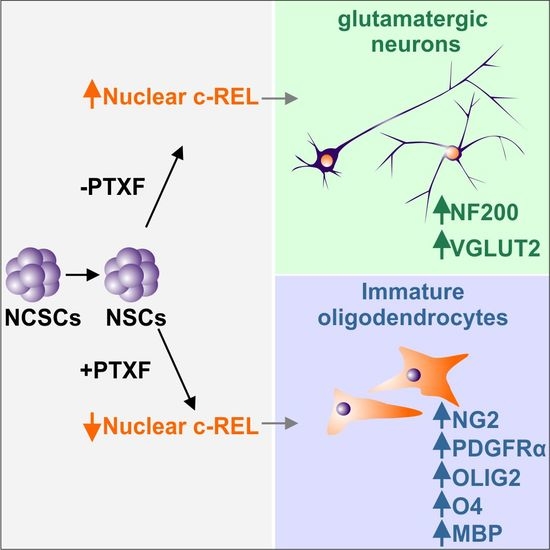
A Matter of Choice: Inhibition of c-Rel Shifts Neuronal to Oligodendroglial Fate in Human Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Human NCSCs
2.2. Neuronal Differentiation of Human NCSCs to Glutamatergic Neurons
2.3. Pentoxifylline Treatment
2.4. Cerebellar Slice Culture, Demyelination and Cell Transplantation
2.5. Immunocytochemistry
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Image Analyses and Quantification
2.8. Statistical Analyses
3. Results
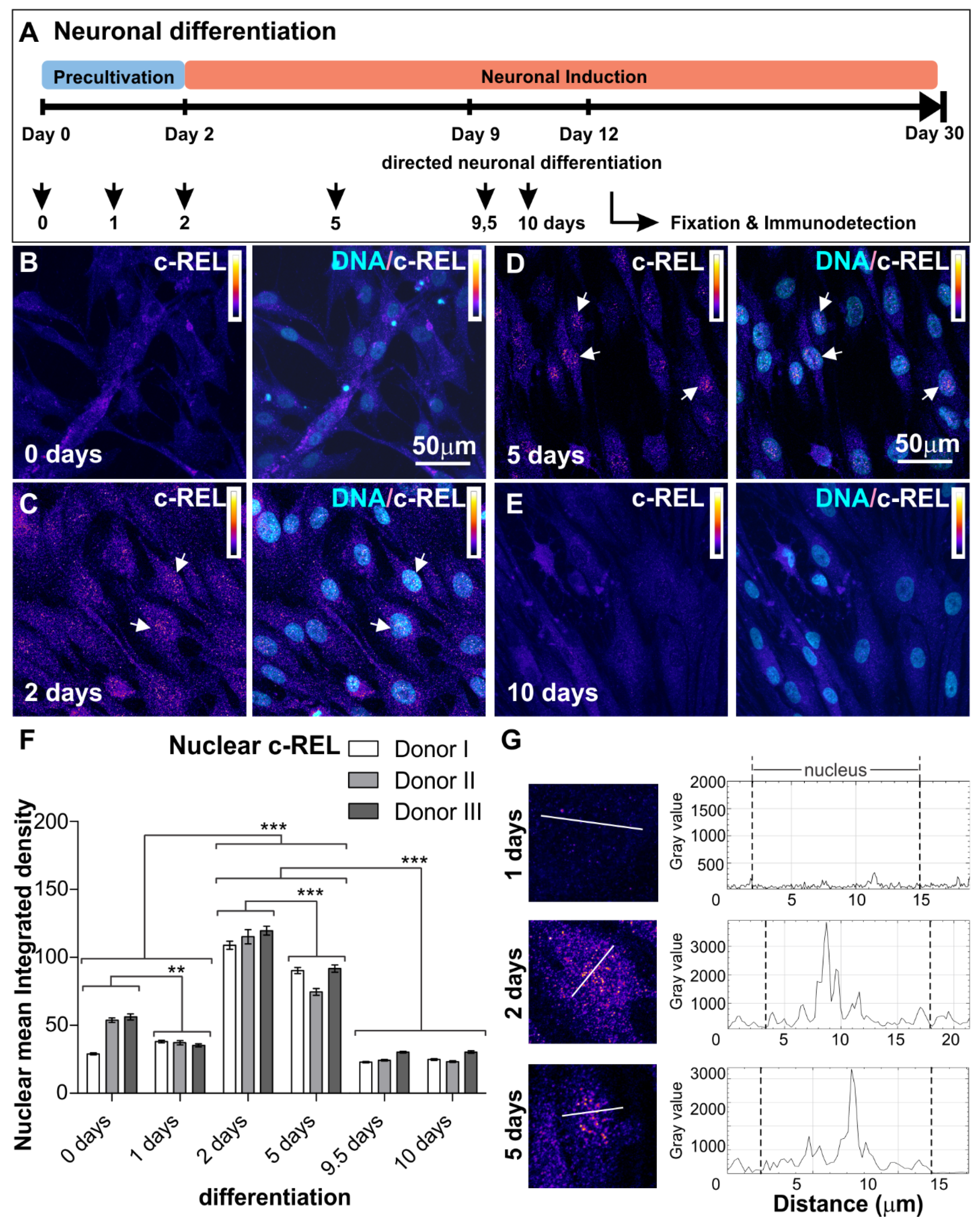
3.1. NF-κB-c-REL during Early Stages of Glutamatergic Differentiation in NCSC-Derived NSCs
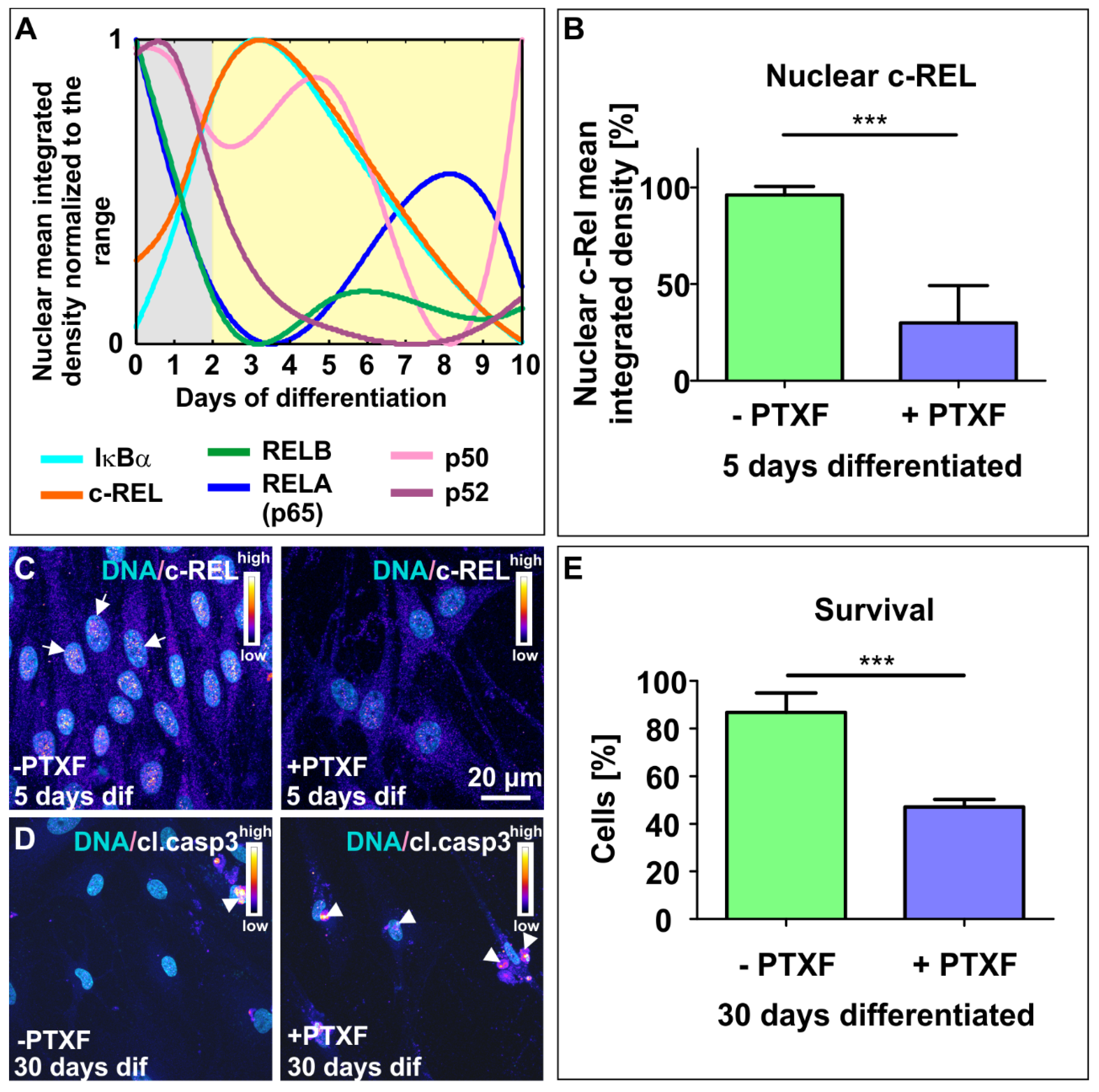
3.2. NF-κB-c-REL is Crucial for Cell Survival during Glutamatergic Differentiation of Adult Human Stem Cells
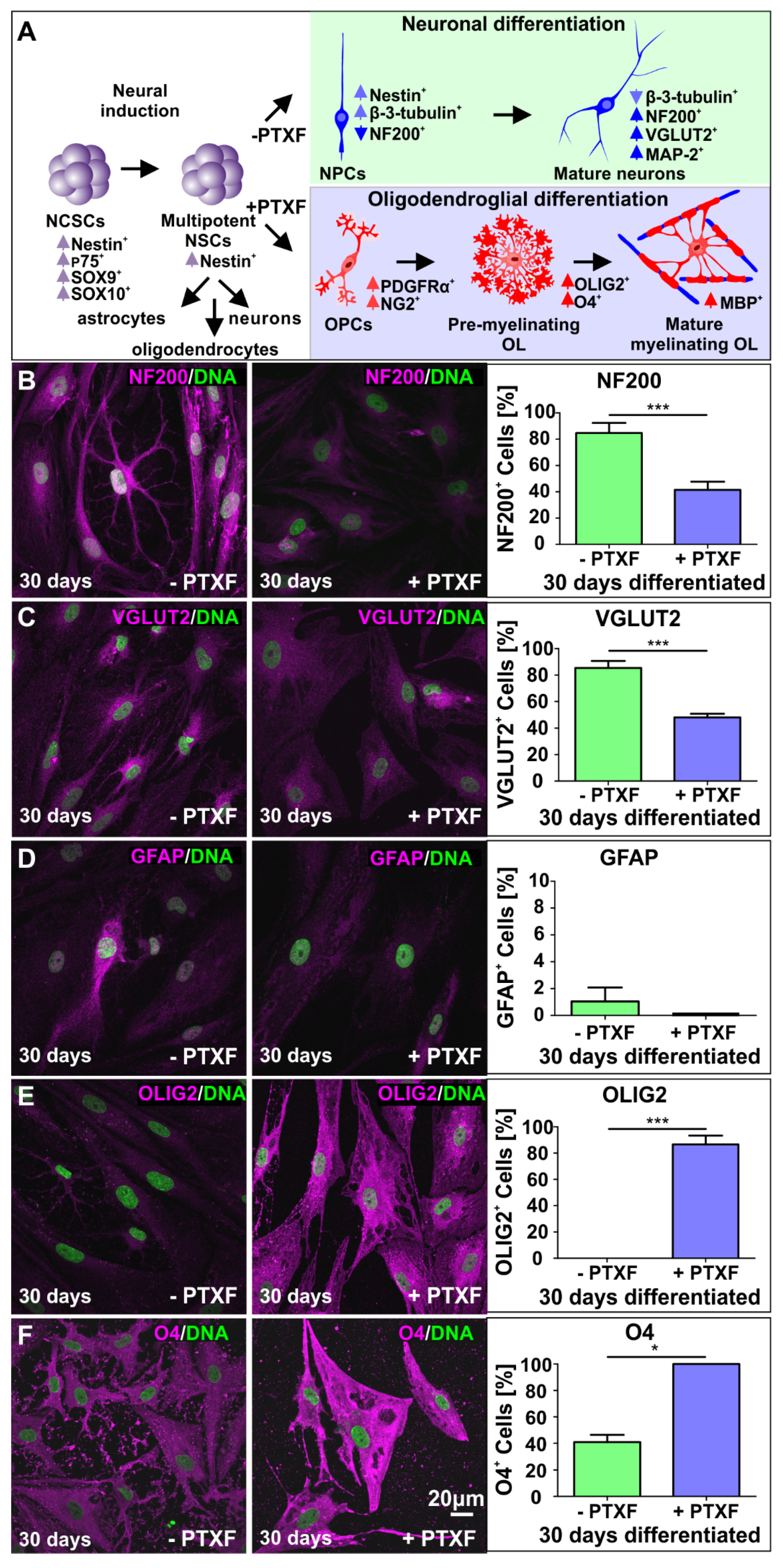
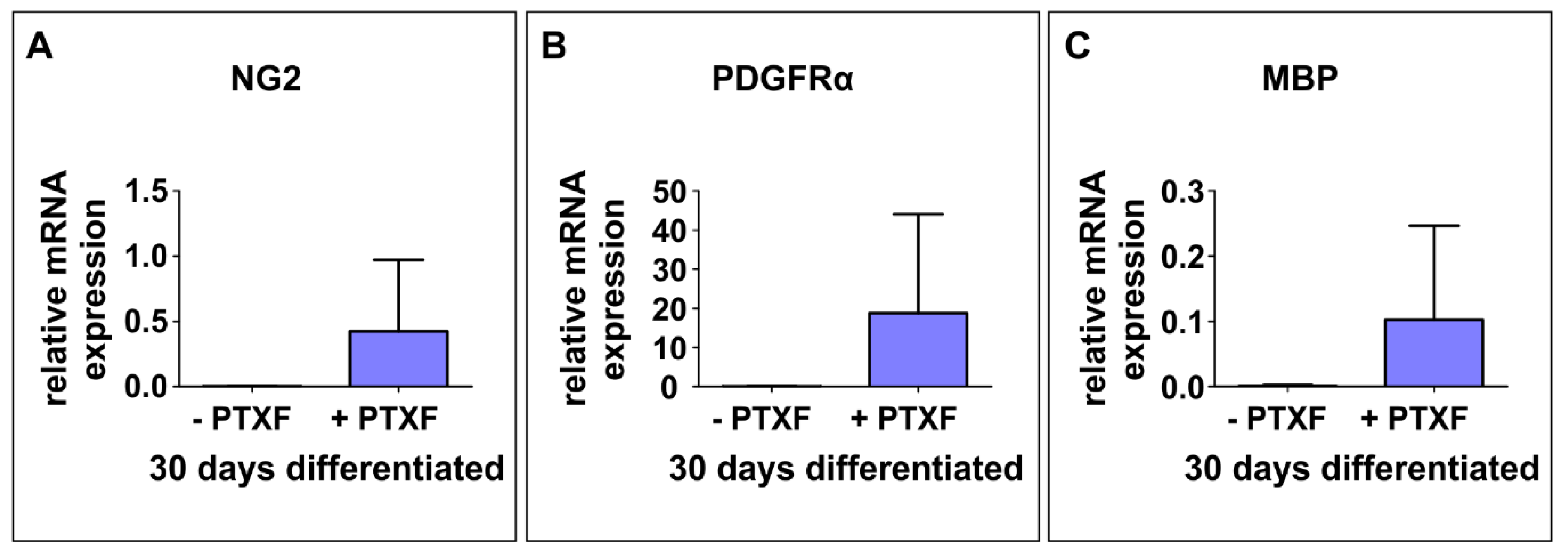
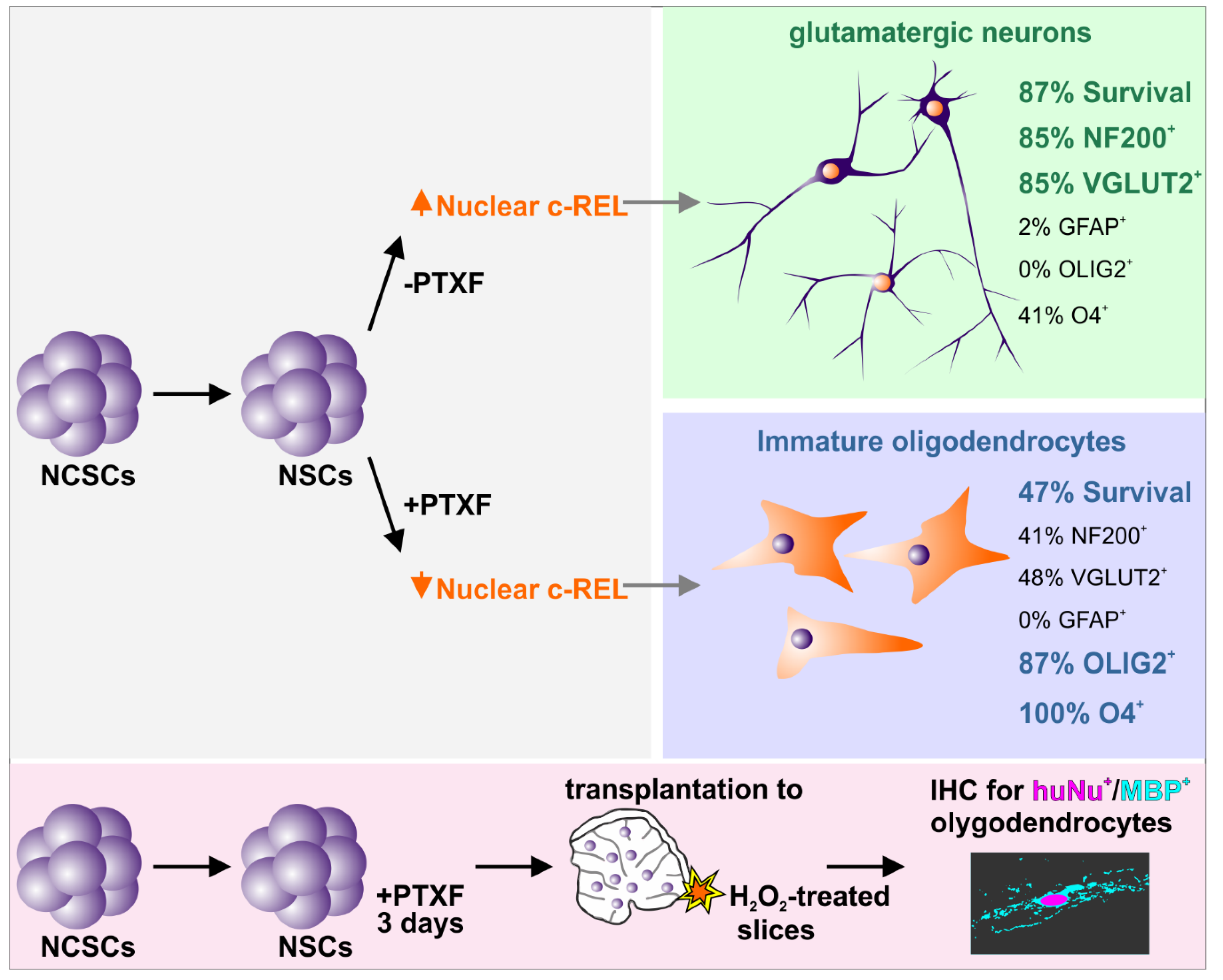
3.3. c-REL Inhibition by Pentoxifylline Induces a Shift into the Oligodendrocyte Fate
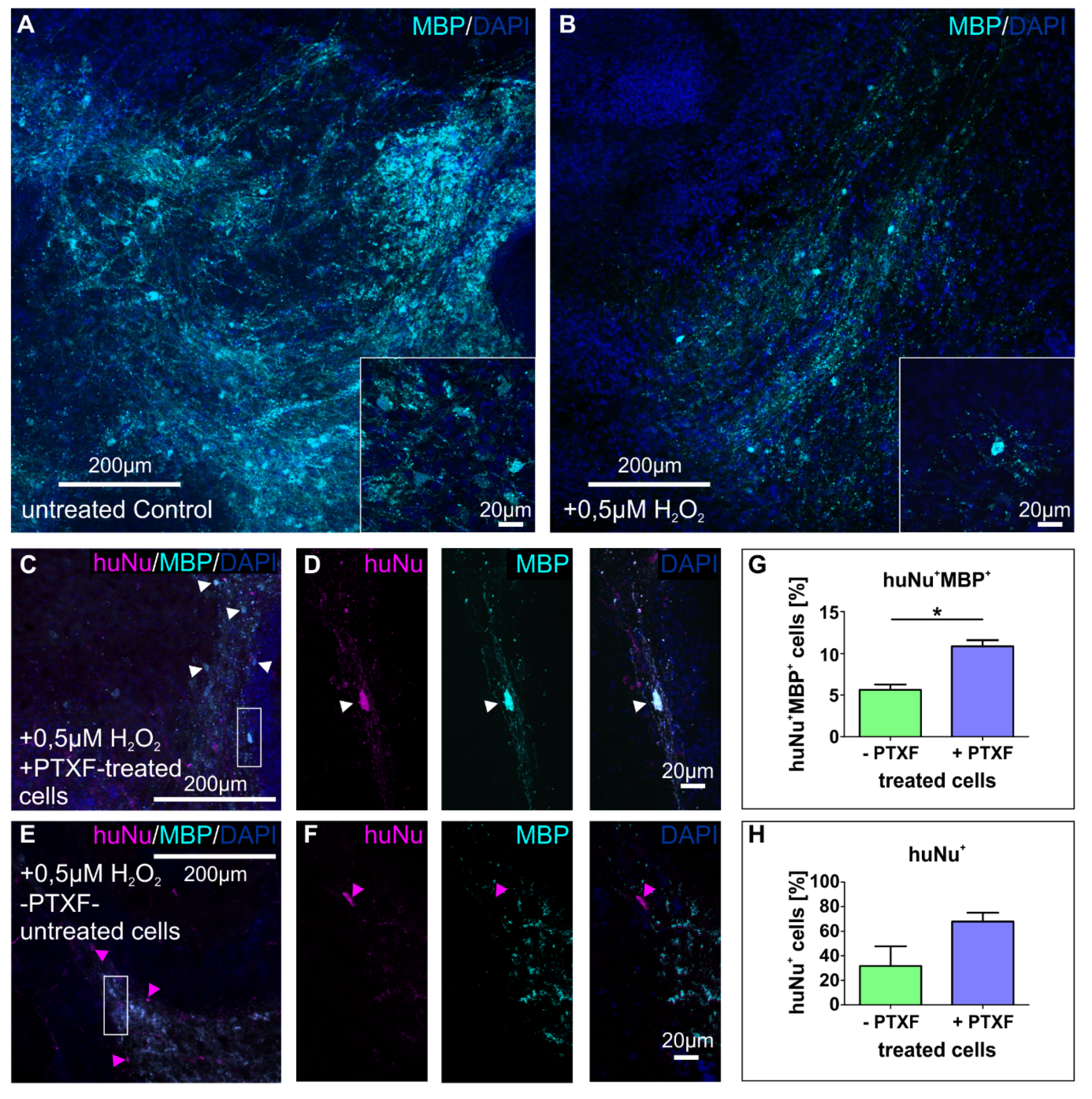
3.4. Transplanted PTXF-Treated Predifferentiated NSCs into Demyelinated Mouse Organotypic Cerebellar Slices Integrate and Differentiate into Myelinating Oligodendrocytes
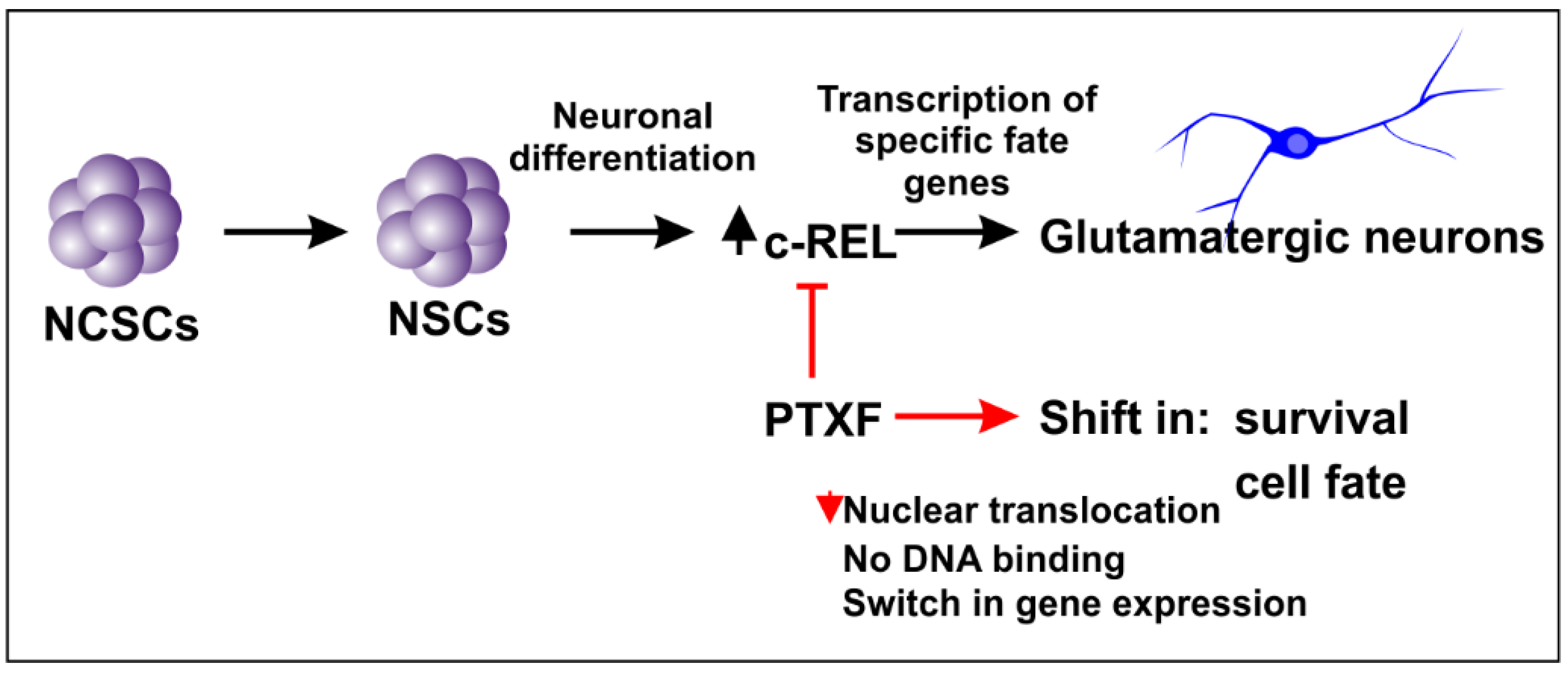
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Ribeiro, M.F.; Genebra, T.; Rego, A.C.; Rodrigues, C.M.P.; Sola, S. Amyloid beta Peptide Compromises Neural Stem Cell Fate by Irreversibly Disturbing Mitochondrial Oxidative State and Blocking Mitochondrial Biogenesis and Dynamics. Mol. Neurobiol. 2019, 56, 3922–3936. [Google Scholar] [CrossRef]
- Miron, V.E.; Kuhlmann, T.; Antel, J.P. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim. Biophys. Acta 2011, 1812, 184–193. [Google Scholar] [CrossRef]
- Kaltschmidt, C.; Kaltschmidt, B.; Baeuerle, P.A. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech. Dev. 1993, 43, 135–147. [Google Scholar] [CrossRef]
- Kaltschmidt, C.; Kaltschmidt, B.; Baeuerle, P.A. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 9618–9622. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Uherek, M.; Volk, B.; Baeuerle, P.A.; Kaltschmidt, C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2642–2647. [Google Scholar] [CrossRef]
- Imielski, Y.; Schwamborn, J.C.; Lüningschrör, P.; Heimann, P.; Holzberg, M.; Werner, H.; Leske, O.; Puschel, A.W.; Memet, S.; Heumann, R.; et al. Regrowing the adult brain: NF-kappaB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS ONE 2012, 7, e30838. [Google Scholar] [CrossRef]
- Sanchez-Ponce, D.; Tapia, M.; Munoz, A.; Garrido, J.J. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol. Cell Neurosci. 2008, 37, 832–844. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yao, S.; Li, F.; Xin, L.; Lai, M.; Bracchi-Ricard, V.; Xu, H.; Yen, W.; Meng, W.; et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells 2012, 30, 510–524. [Google Scholar] [CrossRef]
- Ruiz-Perera, L.M.; Schneider, L.; Windmoller, B.A.; Müller, J.; Greiner, J.F.W.; Kaltschmidt, C.; Kaltschmidt, B. NF-kappaB p65 directs sex-specific neuroprotection in human neurons. Sci. Rep. 2018, 8, 16012. [Google Scholar] [CrossRef]
- Hauser, S.; Widera, D.; Qunneis, F.; Müller, J.; Zander, C.; Greiner, J.; Strauss, C.; Lüningschrör, P.; Heimann, P.; Schwarze, H.; et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef]
- Greiner, J.F.; Hauser, S.; Widera, D.; Müller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cell Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef]
- Müller, J.; Ossig, C.; Greiner, J.F.; Hauser, S.; Fauser, M.; Widera, D.; Kaltschmidt, C.; Storch, A.; Kaltschmidt, B. Intrastriatal transplantation of adult human neural crest-derived stem cells improves functional outcome in parkinsonian rats. Stem Cells Transl. Med. 2015, 4, 31–43. [Google Scholar] [CrossRef]
- Weber, M.; Apostolova, G.; Widera, D.; Mittelbronn, M.; Dechant, G.; Kaltschmidt, B.; Rohrer, H. Alternative generation of CNS neural stem cells and PNS derivatives from neural crest-derived peripheral stem cells. Stem Cells 2015, 33, 574–588. [Google Scholar] [CrossRef]
- Rieckmann, P.; Weber, F.; Gunther, A.; Martin, S.; Bitsch, A.; Broocks, A.; Kitze, B.; Weber, T.; Borner, T.; Poser, S. Pentoxifylline, a phosphodiesterase inhibitor, induces immune deviation in patients with multiple sclerosis. J. Neuroimmunol. 1996, 64, 193–200. [Google Scholar] [CrossRef]
- Wang, W.; Tam, W.F.; Hughes, C.C.; Rath, S.; Sen, R. c-Rel is a target of pentoxifylline-mediated inhibition of T lymphocyte activation. Immunity 1997, 6, 165–174. [Google Scholar] [CrossRef]
- Grinberg-Bleyer, Y.; Oh, H.; Desrichard, A.; Bhatt, D.M.; Caron, R.; Chan, T.A.; Schmid, R.M.; Klein, U.; Hayden, M.S.; Ghosh, S. NF-kappaB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell 2017, 170, 1096–1108.e13. [Google Scholar] [CrossRef]
- Windmeier, C.; Gressner, A.M. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen. Pharmacol. 1997, 29, 181–196. [Google Scholar] [CrossRef]
- Carter, B.D.; Kaltschmidt, C.; Kaltschmidt, B.; Offenhauser, N.; Bohm-Matthaei, R.; Baeuerle, P.A.; Barde, Y.A. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science 1996, 272, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, U.; Lachance, C.; Simoneau, S.J.; Bhakar, A.; Barker, P.A.; Antel, J.P. p75 neurotrophin receptor expression on adult human oligodendrocytes: Signaling without cell death in response to NGF. J. Neurosci. 1998, 18, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Schubart, A.; Mir, A.K.; Dev, K.K. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, N.; Mikuni, T.; Hashimoto, K.; Hirai, H.; Sakimura, K.; Kano, M. Organotypic coculture preparation for the study of developmental synapse elimination in mammalian brain. J. Neurosci. 2012, 32, 11657–11670. [Google Scholar] [CrossRef]
- Birgbauer, E.; Rao, T.S.; Webb, M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J. Neurosci. Res. 2004, 78, 157–166. [Google Scholar] [CrossRef]
- O’Sullivan, S.A.; Velasco-Estevez, M.; Dev, K.K. Demyelination induced by oxidative stress is regulated by sphingosine 1-phosphate receptors. Glia 2017, 65, 1119–1136. [Google Scholar] [CrossRef]
- Armstrong, L.; Hughes, O.; Yung, S.; Hyslop, L.; Stewart, R.; Wappler, I.; Peters, H.; Walter, T.; Stojkovic, P.; Evans, J.; et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006, 15, 1894–1913. [Google Scholar] [CrossRef]
- Takase, O.; Yoshikawa, M.; Idei, M.; Hirahashi, J.; Fujita, T.; Takato, T.; Isagawa, T.; Nagae, G.; Suemori, H.; Aburatani, H.; et al. The role of NF-kappaB signaling in the maintenance of pluripotency of human induced pluripotent stem cells. PLoS ONE 2013, 8, e56399. [Google Scholar] [CrossRef]
- FitzPatrick, L.M.; Hawkins, K.E.; Delhove, J.; Fernandez, E.; Soldati, C.; Bullen, L.F.; Nohturfft, A.; Waddington, S.N.; Medina, D.L.; Bolanos, J.P.; et al. NF-kappaB Activity Initiates Human ESC-Derived Neural Progenitor Cell Differentiation by Inducing a Metabolic Maturation Program. Stem Cell Rep. 2018, 10, 1766–1781. [Google Scholar] [CrossRef]
- Deng, P.; Zhou, C.; Alvarez, R.; Hong, C.; Wang, C.Y. Inhibition of IKK/NF-kappaB Signaling Enhances Differentiation of Mesenchymal Stromal Cells from Human Embryonic Stem Cells. Stem Cell Rep. 2016, 6, 456–465. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kang, H.B.; Park, J.A.; Nam, K.H.; Kwon, H.J.; Lee, Y. Upregulation of NF-kappaB upon differentiation of mouse embryonic stem cells. BMB Rep. 2008, 41, 705–709. [Google Scholar] [CrossRef]
- Lüningschrör, P.; Stocker, B.; Kaltschmidt, B.; Kaltschmidt, C. miR-290 cluster modulates pluripotency by repressing canonical NF-kappaB signaling. Stem Cells 2012, 30, 655–664. [Google Scholar] [CrossRef]
- Pizzi, M.; Goffi, F.; Boroni, F.; Benarese, M.; Perkins, S.E.; Liou, H.C.; Spano, P. Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J. Biol. Chem. 2002, 277, 20717–20723. [Google Scholar] [CrossRef] [PubMed]
- Widera, D.; Mikenberg, I.; Elvers, M.; Kaltschmidt, C.; Kaltschmidt, B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Flores, G.; Ortiz-Lazareno, P.C.; Lerma-Diaz, J.M.; Dominguez-Rodriguez, J.R.; Jave-Suarez, L.F.; Aguilar-Lemarroy Adel, C.; de Celis-Carrillo, R.; del Toro-Arreola, S.; Castellanos-Esparza, Y.C.; Bravo-Cuellar, A. Pentoxifylline sensitizes human cervical tumor cells to cisplatin-induced apoptosis by suppressing NF-kappa B and decreased cell senescence. BMC Cancer 2011, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef]
- Tomita, K.; Moriyoshi, K.; Nakanishi, S.; Guillemot, F.; Kageyama, R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000, 19, 5460–5472. [Google Scholar] [CrossRef]
- Lauriola, L.; Coli, A.; Cocchia, D.; Tallini, G.; Michetti, F. Comparative study by S-100 and GFAP immunohistochemistry of glial cell populations in the early stages of human spinal cord development. Brain Res. 1987, 465, 251–255. [Google Scholar] [CrossRef]
- Valerio-Gomes, B.; Guimaraes, D.M.; Szczupak, D.; Lent, R. The Absolute Number of Oligodendrocytes in the Adult Mouse Brain. Front. Neuroanat. 2018, 12, 90. [Google Scholar] [CrossRef]
- Correa, J.O.; Aarestrup, B.J.; Aarestrup, F.M. Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2010, 226, 15–23. [Google Scholar] [CrossRef]
- Myers, L.W.; Ellison, G.W.; Merrill, J.E.; El Hajjar, A.; St Pierre, B.; Hijazin, M.; Leake, B.D.; Bentson, J.R.; Nuwer, M.R.; Tourtellotte, W.W.; et al. Pentoxifylline is not a promising treatment for multiple sclerosis in progression phase. Neurology 1998, 51, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Polak, T.; Gunther, A.; Kubuschok, B.; Janovskaja, J.; Bitsch, A.; Poser, S.; Rieckmann, P. Synergistic immunomodulatory effects of interferon-beta1b and the phosphodiesterase inhibitor pentoxifylline in patients with relapsing-remitting multiple sclerosis. Ann. Neurol. 1998, 44, 27–34. [Google Scholar] [CrossRef]
- Hof, P.R.; Haroutunian, V.; Friedrich, V.L., Jr.; Byne, W.; Buitron, C.; Perl, D.P.; Davis, K.L. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiatry 2003, 53, 1075–1085. [Google Scholar] [CrossRef]
- Petersen, M.A.; Ryu, J.K.; Chang, K.J.; Etxeberria, A.; Bardehle, S.; Mendiola, A.S.; Kamau-Devers, W.; Fancy, S.P.J.; Thor, A.; Bushong, E.A.; et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron 2017, 96, 1003–1012.e7. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Ehrenreich, H. Time to revisit oligodendrocytes in multiple sclerosis. Nat. Med. 2019, 25, 364–366. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Perera, L.M.; Greiner, J.F.W.; Kaltschmidt, C.; Kaltschmidt, B. A Matter of Choice: Inhibition of c-Rel Shifts Neuronal to Oligodendroglial Fate in Human Stem Cells. Cells 2020, 9, 1037. https://doi.org/10.3390/cells9041037
Ruiz-Perera LM, Greiner JFW, Kaltschmidt C, Kaltschmidt B. A Matter of Choice: Inhibition of c-Rel Shifts Neuronal to Oligodendroglial Fate in Human Stem Cells. Cells. 2020; 9(4):1037. https://doi.org/10.3390/cells9041037
Chicago/Turabian StyleRuiz-Perera, Lucia Mercedes, Johannes Friedrich Wilhelm Greiner, Christian Kaltschmidt, and Barbara Kaltschmidt. 2020. "A Matter of Choice: Inhibition of c-Rel Shifts Neuronal to Oligodendroglial Fate in Human Stem Cells" Cells 9, no. 4: 1037. https://doi.org/10.3390/cells9041037
APA StyleRuiz-Perera, L. M., Greiner, J. F. W., Kaltschmidt, C., & Kaltschmidt, B. (2020). A Matter of Choice: Inhibition of c-Rel Shifts Neuronal to Oligodendroglial Fate in Human Stem Cells. Cells, 9(4), 1037. https://doi.org/10.3390/cells9041037