Agonist Effects of Propranolol on Non-Tumor Human Breast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs, Reagents and Antibodies
2.2. Cell Lines, Culture and Transfection
2.3. Cell Proliferation
2.4. Resistance to Trypsinization
2.5. Fluorescence Staining
2.6. Western Blot
2.7. cAMP Quantification
2.8. Data and Statistical Analysis
3. Results
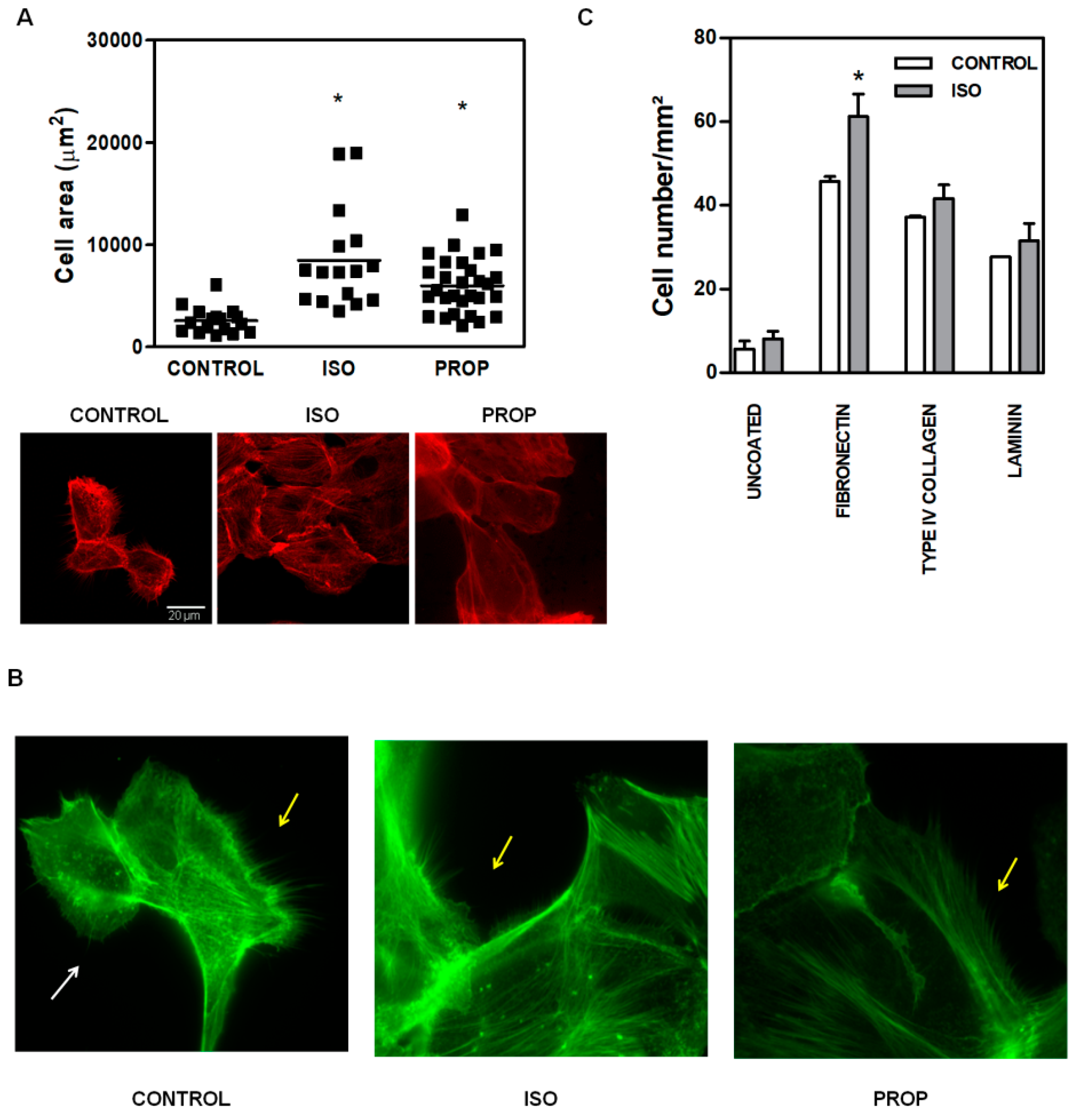
3.1. Comparison of ISO and PROP Effect on Cell Proliferation and Cell Adhesion
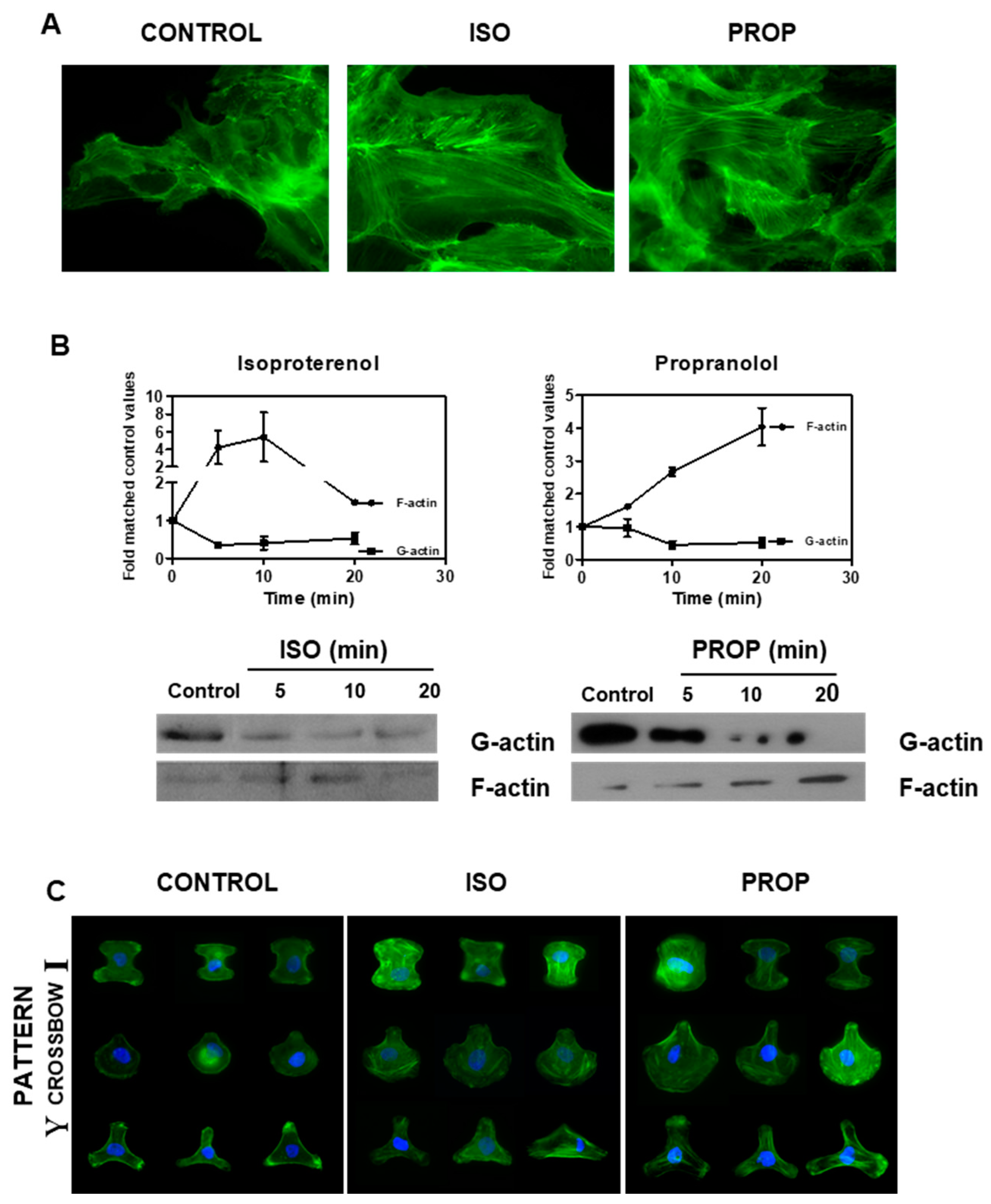
3.2. Actin Reorganization Induced by ISO and PROP
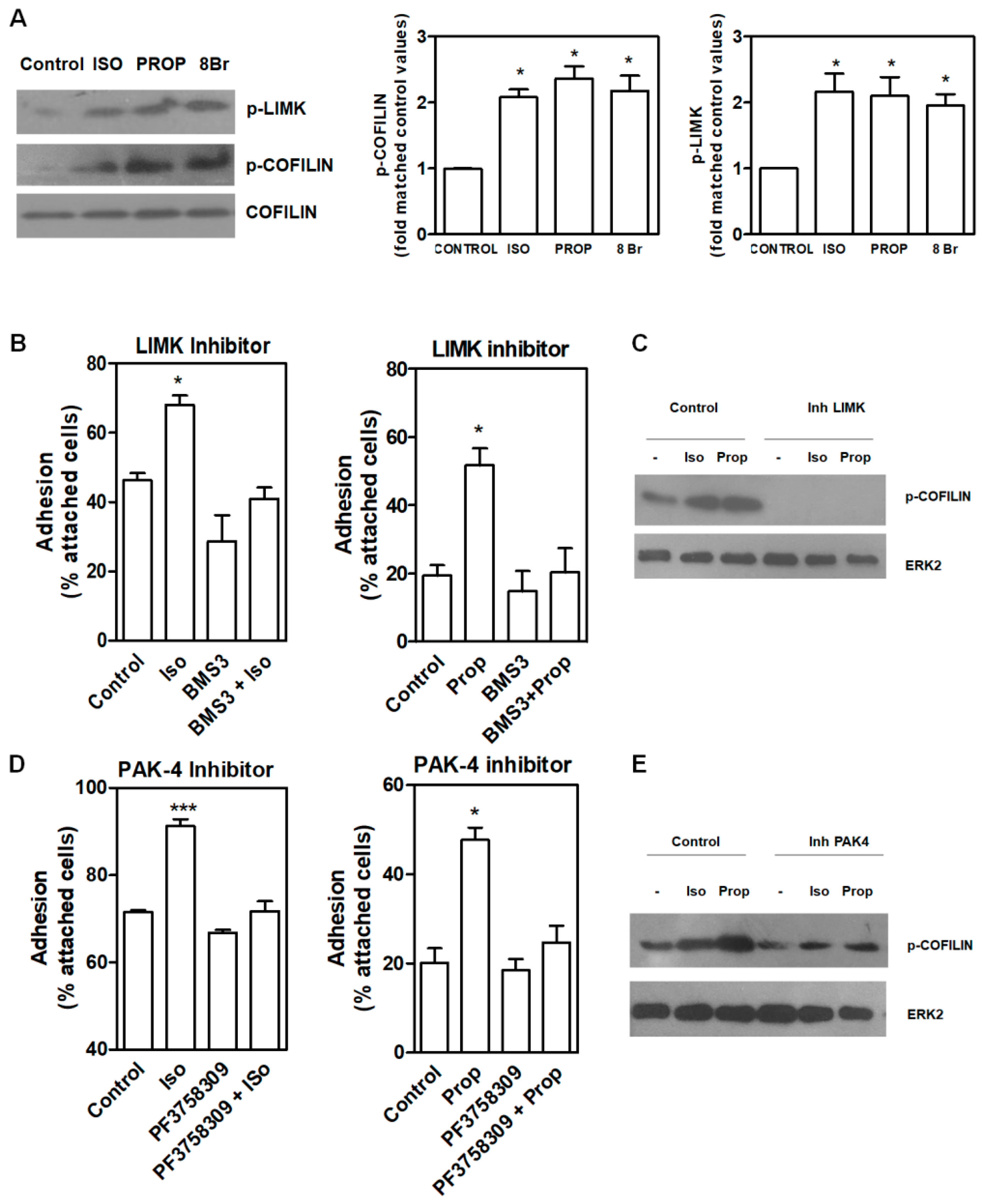
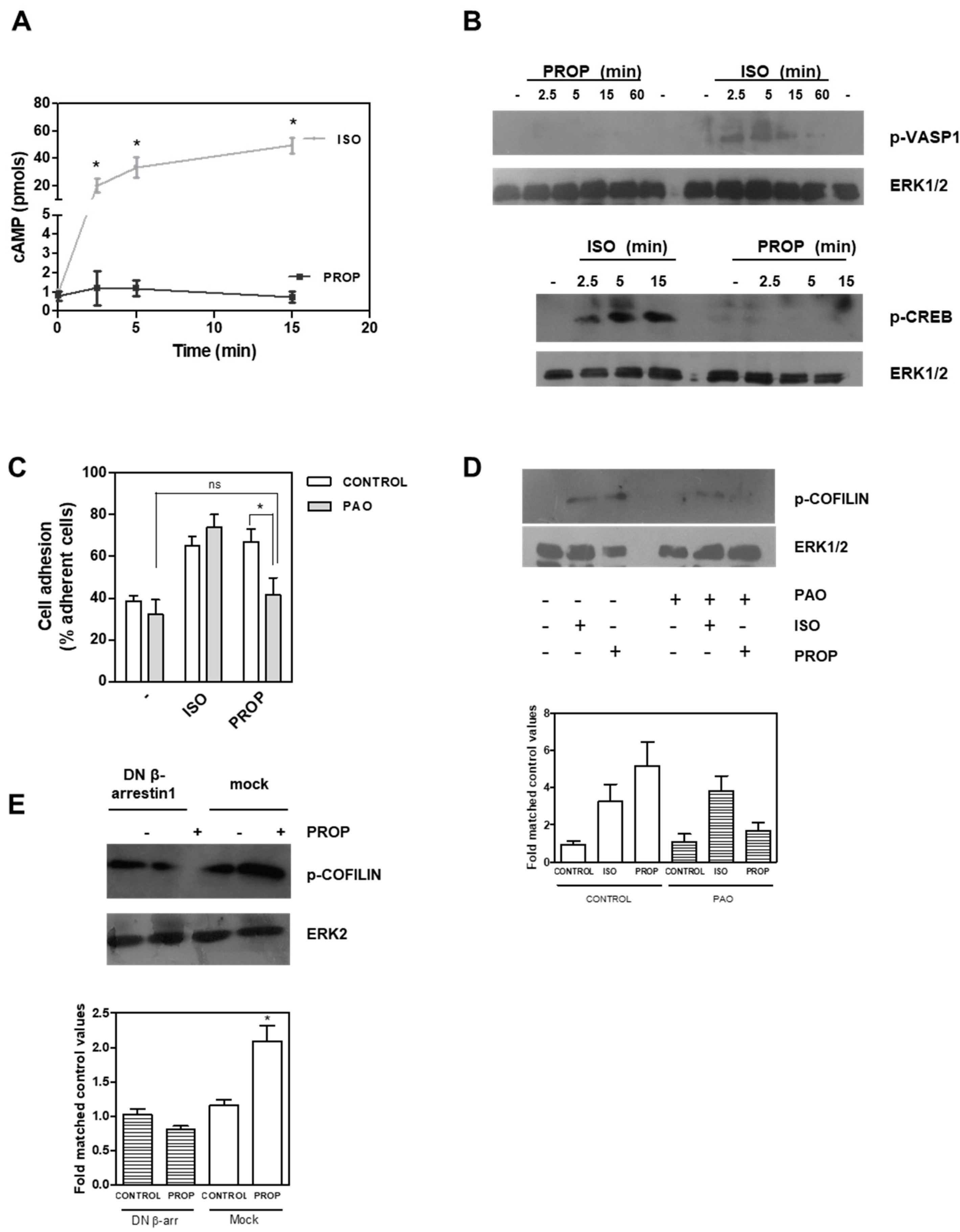
3.3. Signal Pathway Regulating Cell Adhesion Induced by ISO or PROP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Choi, M.; Staus, D.P.; Wingler, L.M.; Ahn, S.; Pani, B.; Capel, W.D.; Lefkowitz, R.J. G protein-coupled receptor kinases (GRKs) orchestrate biased agonism at the beta2-adrenergic receptor. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Lu, L.; Cho, C.H. beta-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin. Cancer Biol. 2013, 23, 533–542. [Google Scholar] [CrossRef]
- Draoui, A.; Vandewalle, B.; Hornez, L.; Revillion, F.; Lefebvre, J. Beta-adrenergic receptors in human breast cancer: Identification, characterization and correlation with progesterone and estradiol receptors. Anticancer Res. 1991, 11, 677–680. [Google Scholar] [PubMed]
- Slotkin, T.A.; Zhang, J.; Dancel, R.; Garcia, S.J.; Willis, C.; Seidler, F.J. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000, 60, 153–166. [Google Scholar] [CrossRef]
- Vandewalle, B.; Revillion, F.; Lefebvre, J. Functional beta-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 1990, 116, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Carie, A.E.; Sebti, S.M. A chemical biology approach identifies a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathway. Oncogene 2007, 26, 3777–3788. [Google Scholar] [CrossRef]
- Rivero, E.M.; Pinero, C.P.; Gargiulo, L.; Entschladen, F.; Zanker, K.; Bruzzone, A.; Luthy, I.A. The beta 2-Adrenergic Agonist Salbutamol Inhibits Migration, Invasion and Metastasis of the Human Breast Cancer MDA-MB- 231 Cell Line. Curr. Cancer Drug Targets 2017, 17, 756–766. [Google Scholar] [CrossRef]
- Gargiulo, L.; Copsel, S.; Rivero, E.M.; Gales, C.; Senard, J.M.; Luthy, I.A.; Davio, C.; Bruzzone, A. Differential beta(2)-adrenergic receptor expression defines the phenotype of non-tumorigenic and malignant human breast cell lines. Oncotarget 2014, 5, 10058–10069. [Google Scholar] [CrossRef]
- Perez, P.C.; Bruzzone, A.; Sarappa, M.; Castillo, L.; Luthy, I. Involvement of alpha2- and beta2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef]
- Gargiulo, L.; May, M.; Rivero, E.M.; Copsel, S.; Lamb, C.; Lydon, J.; Davio, C.; Lanari, C.; Luthy, I.A.; Bruzzone, A. A Novel Effect of beta-Adrenergic Receptor on Mammary Branching Morphogenesis and its Possible Implications in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2017, 22, 43–57. [Google Scholar] [CrossRef]
- Entschladen, F.; Thyssen, D.A.; Drell, D.W. Re-Use of Established Drugs for Anti-Metastatic Indications. Cells 2016, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Entschladen, F. Targeted therapies: Using beta-blockers to inhibit breast cancer progression. Nat. Rev. Clin. Oncol. 2011, 8, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Voss, M.J.; Zanker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Barron, T.I.; Sharp, L.; Visvanathan, K. Beta-adrenergic blocking drugs in breast cancer: A perspective review. Ther. Adv. Med. Oncol. 2012, 4, 113–125. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Coleman, H.G.; Murray, L.J.; Entschladen, F.; Powe, D.G. Beta-blocker usage and breast cancer survival: A nested case-control study within a UK clinical practice research datalink cohort. Int. J. Epidemiol. 2013, 42, 1852–1861. [Google Scholar] [CrossRef]
- Childers, W.K.; Hollenbeak, C.S.; Cheriyath, P. beta-Blockers Reduce Breast Cancer Recurrence and Breast Cancer Death: A Meta-Analysis. Clin. Breast Cancer 2015, 15, 426–431. [Google Scholar] [CrossRef]
- Numbere, B.; Fleming, K.M.; Walker, A.; Card, T.R. Adrenergic blockers and the risk for common solid cancers: A case-control study. Eur. J. Cancer Prev. 2017, 26, 86–93. [Google Scholar] [CrossRef]
- Raimondi, S.; Botteri, E.; Munzone, E.; Cipolla, C.; Rotmensz, N.; DeCensi, A.; Gandini, S. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: Systematic review and meta-analysis. Int. J. Cancer 2016, 139, 212–219. [Google Scholar] [CrossRef]
- Kenakin, T. Principles: Receptor theory in pharmacology. Trends Pharmacol. Sci. 2004, 25, 186–192. [Google Scholar] [CrossRef]
- van der Westhuizen, E.T.; Breton, B.; Christopoulos, A.; Bouvier, M. Quantification of ligand bias for clinically relevant beta2-adrenergic receptor ligands: Implications for drug taxonomy. Mol. Pharmacol. 2014, 85, 492–509. [Google Scholar] [CrossRef]
- Krupnick, J.G.; Santini, F.; Gagnon, A.W.; Keen, J.H.; Benovic, J.L. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J. Biol. Chem. 1997, 272, 32507–32512. [Google Scholar] [CrossRef]
- Bruzzone, A.; Sauliere, A.; Finana, F.; Senard, J.M.; Luthy, I.; Gales, C. Dosage-dependent regulation of cell proliferation and adhesion through dual beta2-adrenergic receptor/cAMP signals. FASEB J. 2014, 28, 1342–1354. [Google Scholar] [CrossRef]
- Romarowski, A.; Battistone, M.A.; La Spina, F.A.; Puga Molina Ldel, C.; Luque, G.M.; Vitale, A.M.; Cuasnicu, P.S.; Visconti, P.E.; Krapf, D.; Buffone, M.G. PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Dev. Biol. 2015, 405, 237–249. [Google Scholar] [CrossRef]
- Davio, C.A.; Cricco, G.P.; Bergoc, R.M.; Rivera, E.S. H1 and H2 histamine receptors in N-nitroso-N-methylurea (NMU)-induced carcinomas with atypical coupling to signal transducers. Biochem. Pharmacol. 1995, 50, 91–96. [Google Scholar] [CrossRef]
- Semenova, G.; Chernoff, J. Targeting PAK1. Biochem. Soc. Trans. 2017, 45, 79–88. [Google Scholar] [CrossRef]
- Soosairajah, J.; Maiti, S.; Wiggan, O.; Sarmiere, P.; Moussi, N.; Sarcevic, B.; Sampath, R.; Bamburg, J.R.; Bernard, O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005, 24, 473–486. [Google Scholar] [CrossRef]
- Srinivasan, A.V. Propranolol: A 50-Year Historical Perspective. Ann. Indian Acad. Neurol. 2019, 22, 21–26. [Google Scholar] [CrossRef]
- Holmes, S.; Griffith, E.J.; Musto, G.; Minuk, G.Y. Antihypertensive medications and survival in patients with cancer: A population-based retrospective cohort study. Cancer Epidemiol. 2013, 37, 881–885. [Google Scholar] [CrossRef]
- Kenakin, T.; Christopoulos, A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013, 12, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kahsai, A.W.; Xiao, K.; Rajagopal, S.; Ahn, S.; Shukla, A.K.; Sun, J.; Oas, T.G.; Lefkowitz, R.J. Multiple ligand-specific conformations of the beta2-adrenergic receptor. Nat. Chem. Biol. 2011, 7, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Wisler, J.W.; DeWire, S.M.; Whalen, E.J.; Violin, J.D.; Drake, M.T.; Ahn, S.; Shenoy, S.K.; Lefkowitz, R.J. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 16657–16662. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.E.; Gul, R.; Blessing, C.P.; Nguyen, J.; Liu, T.; Pulakat, L.; Bastepe, M.; Jackson, E.K.; Andresen, B.T. The beta-blocker Nebivolol Is a GRK/beta-arrestin biased agonist. PLoS ONE 2013, 8, e71980. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, L.; Li, H.H.; Huang, B.; Li, Y.X.; Tao, Y.Z. beta-Arrestin-biased signaling mediates memory reconsolidation. Proc. Natl. Acad. Sci. USA 2015, 112, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Tzingounis, A.V.; von Zastrow, M.; Yudowski, G.A. {Beta}-blocker drugs mediate calcium signaling in native central nervous system neurons by {beta}-arrestin-biased agonism. Proc. Natl. Acad. Sci. USA 2010, 107, 21028–21033. [Google Scholar] [CrossRef]
- Martin, L.J.; Piltonen, M.H.; Gauthier, J.; Convertino, M.; Acland, E.L.; Dokholyan, N.V.; Mogil, J.S.; Diatchenko, L.; Maixner, W. Differences in the Antinociceptive Effects and Binding Properties of Propranolol and Bupranolol Enantiomers. J. Pain 2015, 16, 1321–1333. [Google Scholar] [CrossRef]
- Cakir, Y.; Plummer, H.K., III.; Tithof, P.K.; Schuller, H.M. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 2002, 21, 153–157. [Google Scholar] [CrossRef]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.P.; Serdjebi, C.; Kavallaris, M.; Andre, N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2, 797–809. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, R.H.; Zhang, J.; He, Y.J.; Wan, Z.; Zhou, C.F.; Tang, Y.J.; Li, Z.; McLeod, H.L.; Liu, J. betablockers inhibit the viability of breast cancer cells by regulating the ERK/COX2 signaling pathway and the drug response is affected by ADRB2 singlenucleotide polymorphisms. Oncol. Rep. 2019, 41, 341–350. [Google Scholar]
- Langslet, A. Membrane stabilization and cardiac effects of d,1-propranolol, d-propranolol and chlorpromazine. Eur. J. Pharmacol. 1970, 13, 6–14. [Google Scholar] [CrossRef]
- Sozzani, S.; Agwu, D.E.; McCall, C.E.; O’Flaherty, J.T.; Schmitt, J.D.; Kent, J.D.; McPhail, L.C. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J. Biol. Chem. 1992, 267, 20481–20488. [Google Scholar] [PubMed]
- Shand, D.G.; Nuckolls, E.M.; Oates, J.A. Plasma propranolol levels in adults with observations in four children. Clin. Pharmacol. Ther. 1970, 11, 112–120. [Google Scholar] [CrossRef]
- Vrydag, W.; Michel, M.C. Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 374, 385–398. [Google Scholar] [CrossRef]
- Baker, J.G.; Hall, I.P.; Hill, S.J. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 2003, 64, 1357–1369. [Google Scholar] [CrossRef]
- Wu, B.; Yang, S.; Sun, H.; Sun, T.; Ji, F.; Wang, Y.; Xu, L.; Zhou, D. Keap1 Inhibits Metastatic Properties of NSCLC Cells by Stabilizing Architectures of F-Actin and Focal Adhesions. Mol. Cancer Res. 2018, 16, 508–516. [Google Scholar] [CrossRef]
- Komalavilas, P.; Penn, R.B.; Flynn, C.R.; Thresher, J.; Lopes, L.B.; Furnish, E.J.; Guo, M.; Pallero, M.A.; Murphy-Ullrich, J.E.; Brophy, C.M. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L69–L78. [Google Scholar] [CrossRef]
- Zoudilova, M.; Min, J.; Richards, H.L.; Carter, D.; Huang, T.; DeFea, K.A. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J. Biol. Chem. 2010, 285, 14318–14329. [Google Scholar] [CrossRef]
- Harbeck, B.; Huttelmaier, S.; Schluter, K.; Jockusch, B.M.; Illenberger, S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 2000, 275, 30817–30825. [Google Scholar] [CrossRef]
- Goncharova, E.A.; Goncharov, D.A.; Zhao, H.; Penn, R.B.; Krymskaya, V.P.; Panettieri, R.A., Jr. beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am. J. Respir. Cell Mol. Biol. 2012, 46, 48–54. [Google Scholar] [CrossRef]
- Sartoretto, J.L.; Jin, B.Y.; Bauer, M.; Gertler, F.B.; Liao, R.; Michel, T. Regulation of VASP phosphorylation in cardiac myocytes: Differential regulation by cyclic nucleotides and modulation of protein expression in diabetic and hypertrophic heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1697–H1710. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargiulo, L.; Rivero, E.M.; di Siervi, N.; Buzzi, E.D.; Buffone, M.G.; Davio, C.A.; Lüthy, I.A.; Bruzzone, A. Agonist Effects of Propranolol on Non-Tumor Human Breast Cells. Cells 2020, 9, 1036. https://doi.org/10.3390/cells9041036
Gargiulo L, Rivero EM, di Siervi N, Buzzi ED, Buffone MG, Davio CA, Lüthy IA, Bruzzone A. Agonist Effects of Propranolol on Non-Tumor Human Breast Cells. Cells. 2020; 9(4):1036. https://doi.org/10.3390/cells9041036
Chicago/Turabian StyleGargiulo, Lucía, Ezequiel Mariano Rivero, Nicolás di Siervi, Edgardo David Buzzi, Mariano Gabriel Buffone, Carlos Alberto Davio, Isabel Alicia Lüthy, and Ariana Bruzzone. 2020. "Agonist Effects of Propranolol on Non-Tumor Human Breast Cells" Cells 9, no. 4: 1036. https://doi.org/10.3390/cells9041036
APA StyleGargiulo, L., Rivero, E. M., di Siervi, N., Buzzi, E. D., Buffone, M. G., Davio, C. A., Lüthy, I. A., & Bruzzone, A. (2020). Agonist Effects of Propranolol on Non-Tumor Human Breast Cells. Cells, 9(4), 1036. https://doi.org/10.3390/cells9041036





