Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Human Samples
2.3. Primary Mouse Cells
2.4. S1P Modulators, Lipids, and Reagents
2.5. Cell Growth Assays
2.6. Co-Culture Studies
2.7. Cytotoxicity Assays
2.8. RT-PCR
2.9. Real Time Quantitative PCR (qPCR) Analysis
2.10. Lipidomic Analysis
2.11. Statistical Analysis
3. Results
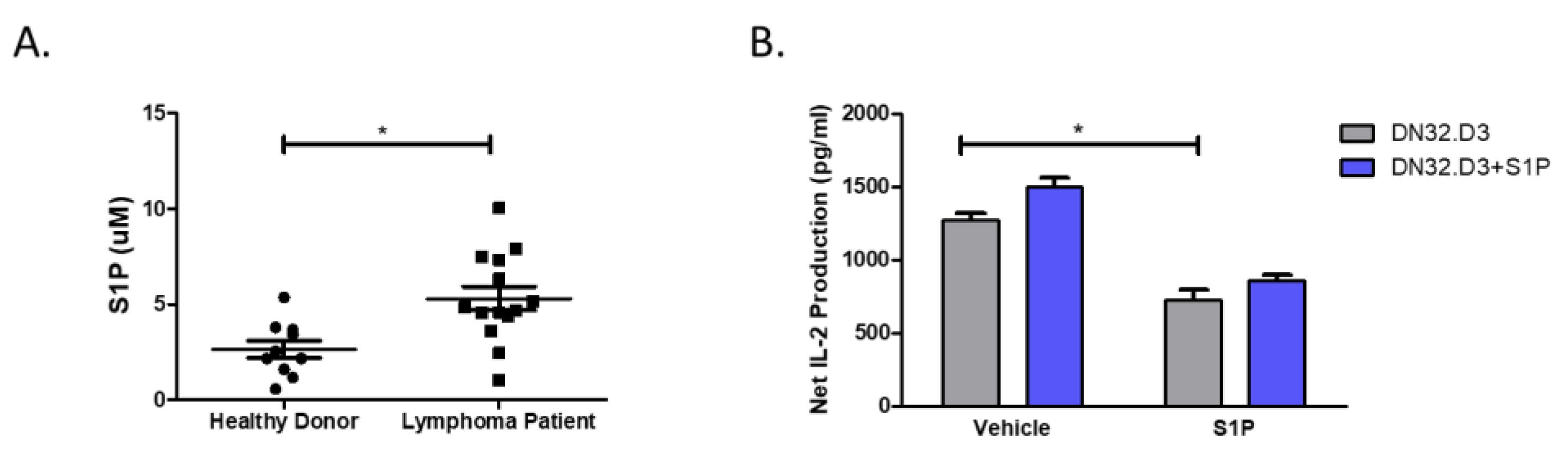
3.1. S1P Pretreatment Inhibits CD1d-Mediated NKT Cell Activation
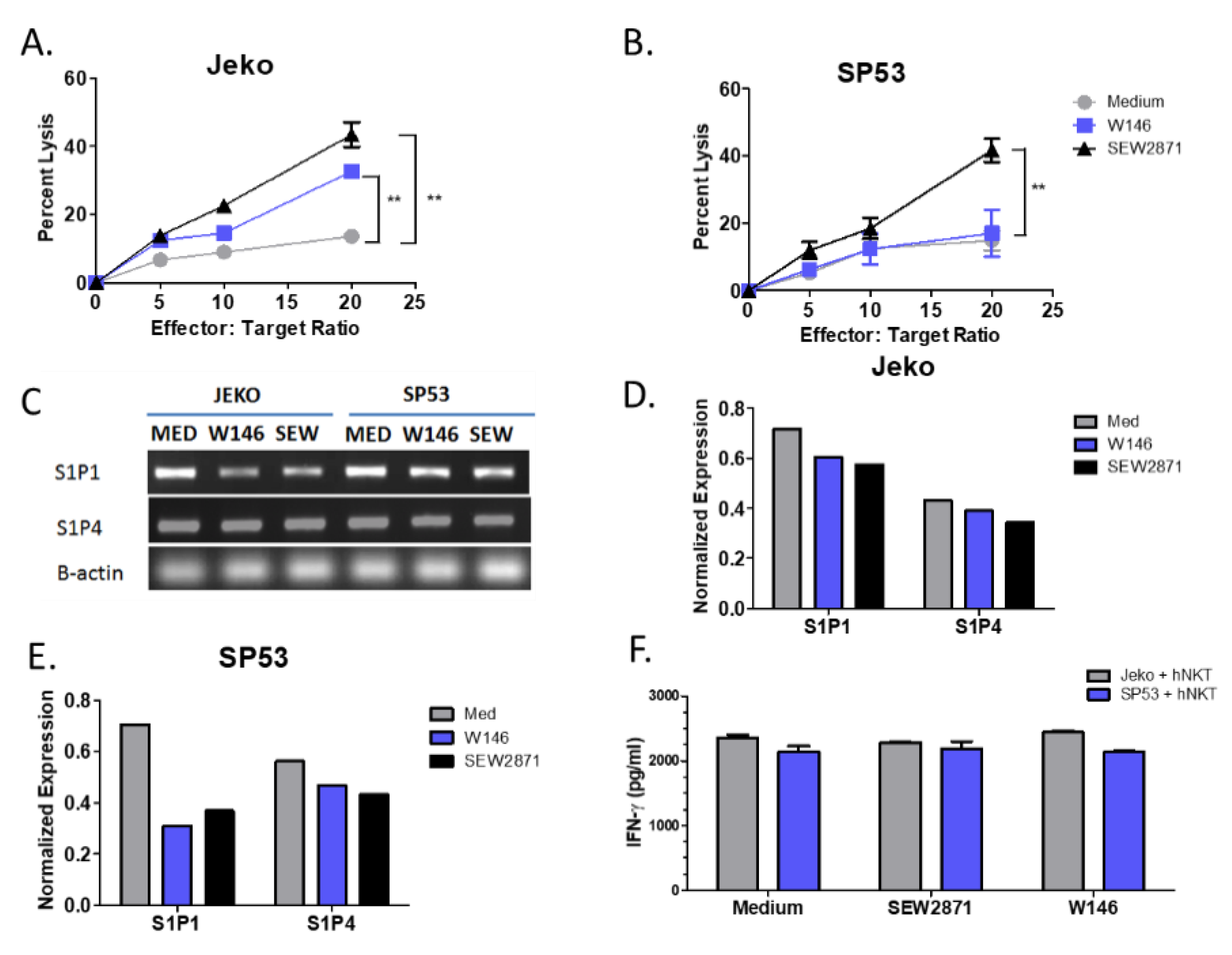
3.2. Targeting of S1P1 Signaling Enhances NKT Cell-Mediated Lysis of MCL
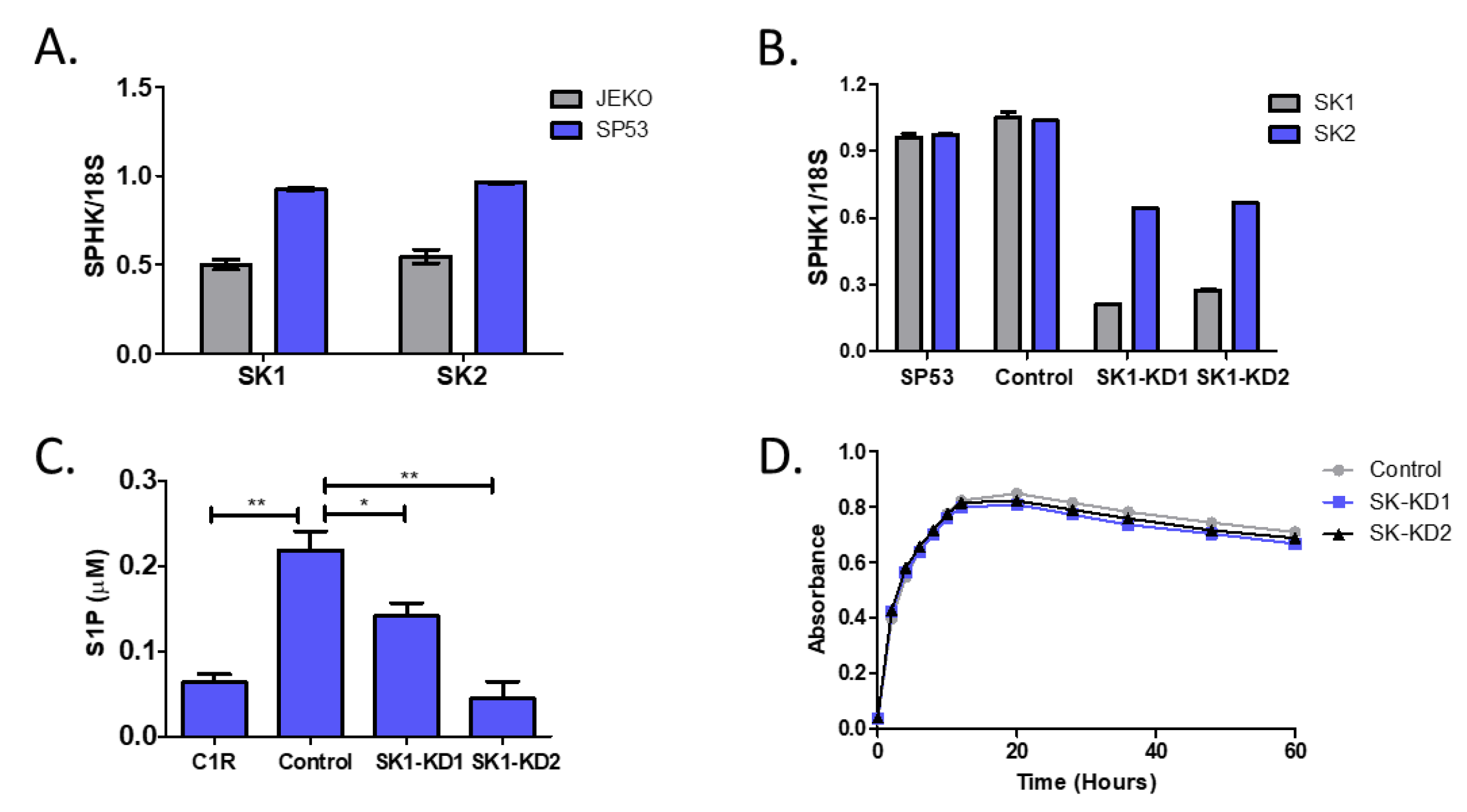
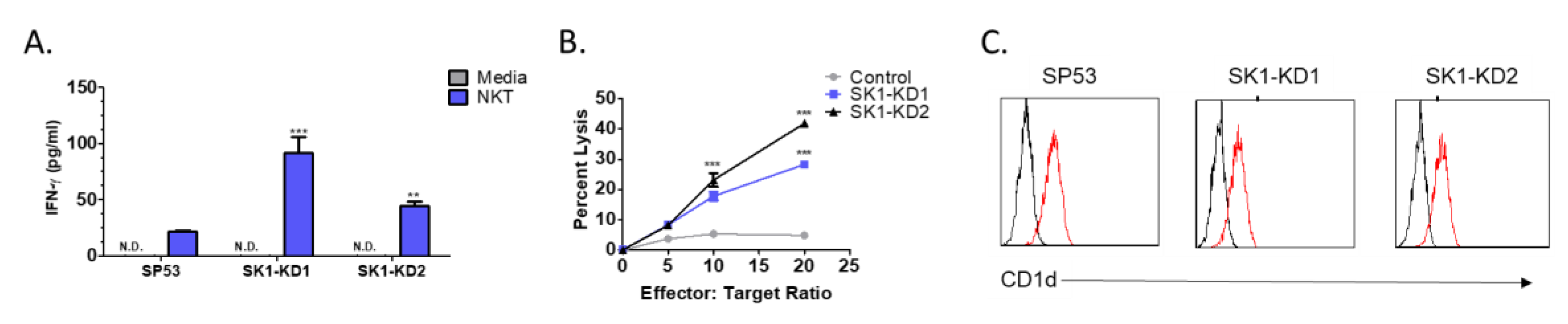
3.3. Knockdown of Sphingosine Kinase Restores NKT Cell Responses to MCL
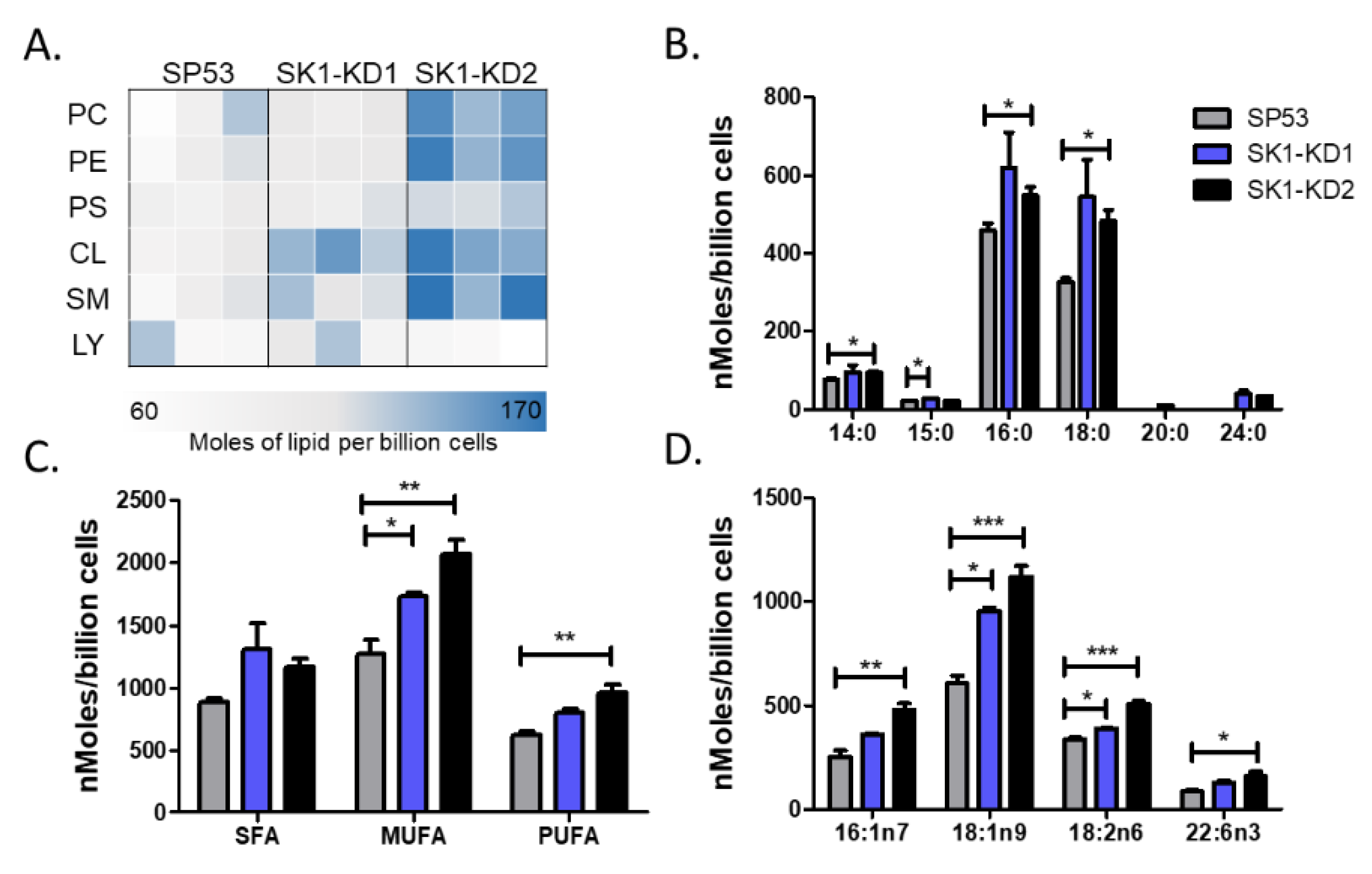
3.4. Knockdown of Sphingosine Kinase Results in Increased Cardiolipin Levels
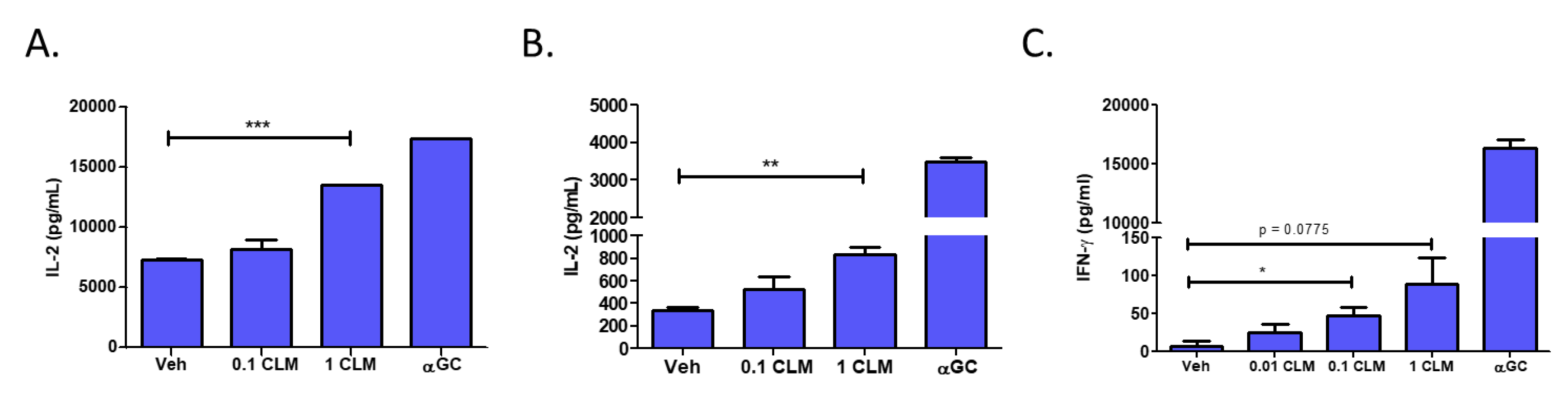
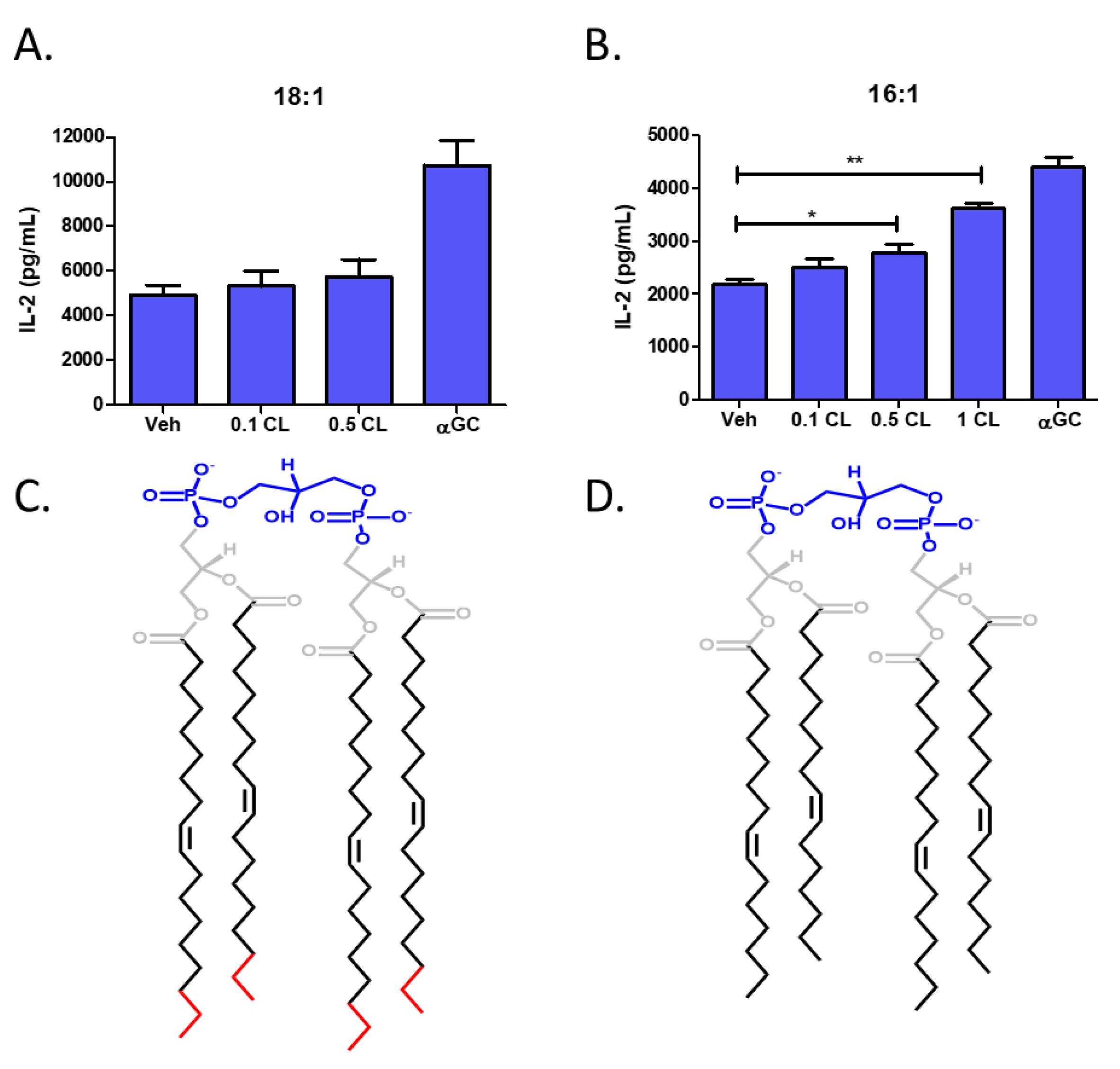
3.5. Cardiolipin Stimulates Type 1 NKT Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez-Galán, P.; Dreyling, M.; Wiestner, A. Mantle cell lymphoma: Biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef]
- Alinari, L.; Christian, B.; Baiocchi, R.A. Novel targeted therapies for mantle cell lymphoma. Oncotarget 2012, 3, 203–211. [Google Scholar] [CrossRef]
- Schieber, M.; Gordon, L.I.; Karmali, R. Current overview and treatment of mantle cell lymphoma. F1000Research 2018, 7, 1136. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Azzimonti, L.; Delfanti, G.; Scarfò, L.; Scielzo, C.; Bertilaccio, M.T.; Ranghetti, P.; Gulino, A.; Doglioni, C.; Di Napoli, A.; et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood 2017, 129, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Nakayama, T.; Kamada, N.; Kaneko, Y.; Harada, M.; Ogura, N.; Akutsu, Y.; Motohashi, S.; Iizasa, T.; Endo, H.; et al. Antitumor Cytotoxicity Mediated by Ligand-activated Human Va24 NKT Cells. Cancer Res. 1999, 59, 5102–5105. [Google Scholar] [PubMed]
- Terabe, M.; Berzofsky, J.A. The Role of NKT Cells in Tumor Immunity. Adv. Cancer Res. 2008, 101, 277–348. [Google Scholar] [CrossRef]
- Illés, Z.; Kondo, T.; Newcombe, J.; Oka, N.; Tabira, T.; Yamamura, T. Differential Expression of NK T Cell Vα24JαQ Invariant TCR Chain in the Lesions of Multiple Sclerosis and Chronic Inflammatory Demyelinating Polyneuropathy. J. Immunol. 2000, 164, 4375–4381. [Google Scholar] [CrossRef]
- Swann, J.B.; Uldrich, A.P.; Van Dommelen, S.; Sharkey, J.; Murray, W.K.; Godfrey, D.I.; Smyth, M.J. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood 2009, 113, 6382–6385. [Google Scholar] [CrossRef]
- Neparidze, N.; Dhodapkar, M.V. Harnessing CD1d-restricted T cells towards anti-tumor immunity in humans. Ann. N. Y. Acad. Sci. 2009, 1174, 61–67. [Google Scholar] [CrossRef]
- Kinjo, Y.; Wu, D.; Kim, G.; Xing, G.W.; Poles, M.A.; Ho, D.D.; Tsuji, M.; Kawahara, K.; Wong, C.H.; Kronenberg, M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005, 434, 520–525. [Google Scholar] [CrossRef]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Harada, M.; Dashtsoodol, N.; Kojo, S. Discovery of NKT cells and development of NKT cell-targeted anti-tumor immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Burdin, N.; Brossay, L.; Koezuka, Y.; Grusby, M.; Taniguchi, M.; Kronenberg, M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates NK T cells. FASEB J. 1998, 161, 3271–3281. [Google Scholar]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Sato, H.; Kondo, E.; Harada, M.; Koseki, H.; Nakayama, T.; et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5690–5693. [Google Scholar] [CrossRef] [PubMed]
- Seino, K.I.; Fujii, S.I.; Harada, M.; Motohashi, S.; Nakayama, T.; Fujisawa, T.; Taniguchi, M. Vα14 NKT cell-mediated anti-tumor responses and their clinical application. Springer Semin. Immunopathol. 2005, 27, 65–74. [Google Scholar] [CrossRef]
- Carnaud, C.; Lee, D.; Donnars, O.; Park, S.H.; Beavis, A.; Koezuka, Y.; Bendelac, A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999, 163, 4647–4650. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10528160 (accessed on 20 January 2020).
- Taniguchi, M.; Harada, M.; Kojo, S.; Nakayama, T.; Wakao, H. The Regulatory Role of Va14 NKT cells in Innate and Aquired Immune Response. Annu. Rev. Immunol. 2003, 21, 483–513. [Google Scholar] [CrossRef]
- Hermans, I.F.; Silk, J.D.; Gileadi, U.; Salio, M.; Mathew, B.; Ritter, G.; Schmidt, R.; Harris, A.L.; Old, L.; Cerundolo, V. NKT Cells Enhance CD4 + and CD8 + T Cell Responses to Soluble Antigen In Vivo through Direct Interaction with Dendritic Cells. J. Immunol. 2003, 171, 5140–5147. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of Natural Killer T Cells by α-Galactosylceramide Rapidly Induces the Full Maturation of Dendritic Cells In Vivo and Thereby Acts as an Adjuvant for Combined CD4 and CD8 T Cell Immunity to a Coadministered Protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef]
- Exley, M.A.; Friedlander, P.; Alatrakchi, N.; Vriend, L.; Yue, S.C.; Sasada, T.; Zeng, W.; Mizukami, Y.; Clark, J.; Nemer, D.; et al. Adoptive Transfer of Invariant NKT Cells as Immunotherapy for Advanced Melanoma: A Phase 1 Clinical Trial. Clin. Cancer Res. 2017, 23, 3510–3519. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Subrahmanyam, P.B.; Page, C.; Younger, K.M.; Tiper, I.V.; Frieman, M.; Kimball, A.S.; Webb, T.J. NKT Cell Responses to B Cell Lymphoma. Med. Sci. Open Access J. 2014, 2, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Punt, C.J.; Ando, Y.; Ruijter, R.; Nishi, N.; Peters, M.; Von Blomberg, B.M.; Scheper, R.J.; Van der Vliet, H.J.J.; Van den Eertwegh, A.J.M.; et al. A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002, 8, 3702–3709. [Google Scholar] [PubMed]
- Nieda, M.; Okai, M.; Tazbirkova, A.; Lin, H.; Yamaura, A.; Ide, K.; Abraham, R.; Juji, T.; Macfarlane, D.J.; Nicol, A.J. Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 2004, 103, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Osman, K.; Connolly, J.; Kukreja, A.; Krasovsky, J.; Pack, M.; Hutchinson, A.; Geller, M.; Liu, N.; Annable, R.; et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 2005, 201, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Molling, J.W.; Kölgen, W.; Van Der Vliet, H.J.J.; Boomsma, M.F.; Kruizenga, H.; Smorenburg, C.H.; Molenkamp, B.G.; Langendijk, J.A.; Leemans, C.R.; Von Blomberg, B.M.E.; et al. Peripheral blood IFN-g-secreting Va24þVb11þ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int. J. Cancer 2005, 116, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Motohashi, S.; Ishikawa, E.; Fuchida, H.; Higashino, K.; Otsuji, M.; Iizasa, T.; Nakayama, T.; Taniguchi, M.; Fujisawa, T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 1910–1917. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2005157427 (accessed on 20 January 2020). [CrossRef]
- Uchida, T.; Horiguchi, S.; Tanaka, Y.; Yamamoto, H.; Kunii, N.; Motohashi, S.; Taniguchi, M.; Nakayama, T.; Okamoto, Y. Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol. Immunother. 2008, 57, 337–345. [Google Scholar] [CrossRef]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef]
- Rolin, J.; Maghazachi, A.A. Effects of lysophospholipids on tumor microenvironment. Cancer Microenviron. 2011, 4, 393–403. [Google Scholar] [CrossRef]
- Xia, P.; Gamble, J.R.; Wang, L.; Pitson, S.M.; Moretti, P.A.B.; Wattenberg, B.W.; D’Andrea, R.J.; Vadas, M.A. An oncogenic role of sphingosine kinase. Curr. Biol. 2000, 10, 1527–1530. [Google Scholar] [CrossRef]
- Wallington-Beddoe, C.T.; Powell, J.A.; Tong, D.; Pitson, S.M.; Bradstock, K.F.; Bendall, L.J. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing myc expression. Cancer Res. 2014, 74, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Liu, L.; Cai, J.; Liu, J.; Ye, C.; Li, M.; Li, Y. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol. Endocrinol. 2011, 25, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Wallington-Beddoe, C.T.; Bradstock, K.F.; Bendall, L.J. Oncogenic properties of sphingosine kinases in haematological malignancies. Br. J. Haematol. 2013, 161, 623–638. [Google Scholar] [CrossRef]
- Kawamori, T.; Osta, W.; Johnson, K.R.; Pettus, B.J.; Bielawski, J.; Tanaka, T.; Wargovich, M.J.; Reddy, B.S.; Hannun, Y.A.; Obeid, L.M.; et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2005, 20, 386–388. [Google Scholar] [CrossRef]
- Chen, J.; Qi, Y.; Zhao, Y.; Kaczorowski, D.; Couttas, T.A.; Coleman, P.R.; Don, A.S.; Bertolino, P.; Gamble, J.R.; Vadas, M.A.; et al. Deletion of sphingosine kinase 1 inhibits liver tumorigenesis in diethylnitrosamine-treated mice. Oncotarget 2018, 9, 15635–15649. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, B.; Huang, J.; Kong, W.; Xue, W.; Zhu, Y.; Zhang, J.; Huang, Y. Sphingosine kinase 1 is overexpressed and promotes adrenocortical carcinoma progression. Oncotarget 2016, 7, 3233–3244. [Google Scholar] [CrossRef]
- Li, W.; Tian, Z.; Qin, H.; Li, N.; Zhou, X.; Li, J.; Ni, B.; Ruan, Z. High expression of sphingosine kinase 1 is associated with poor prognosis in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2015, 460, 341–347. [Google Scholar] [CrossRef]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef]
- Liu, Q.; Alinari, L.; Chen, C.S.; Yan, F.; Dalton, J.T.; Lapalombella, R.; Zhang, R.; Mani, R.; Lin, T.; Byrd, J.C.; et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin. Cancer Res. 2010, 16, 3182–3192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, T.J.; Sriram, V.; Spence, P.M.; Gui, M.; Hayakawa, K.; Bacik, I.; Bennink, J.R.; Yewdell, J.W.; Brutkiewicz, R.R. Recycling CD1d1 Molecules Present Endogenous Antigens Processed in an Endocytic Compartment to NKT Cells. J. Immunol. 2002, 168, 5409–5414. [Google Scholar] [CrossRef] [PubMed]
- Sriram, V.; Willard, C.A.; Liu, J.; Brutkiewicz, R.R. Importance of N-linked glycosylation in the functional expression of murine CD1d1. Immunology 2008, 123, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Tupin, E.; Kronenberg, M. Activation of Natural Killer T Cells by Glycolipids. Methods Enzymol. 2006, 417, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Swaney, J.S.; Moreno, K.M.; Gentile, A.M.; Sabbadini, R.A.; Stoller, G.L. Sphingosine-1-phosphate (S1P) is a novel fibrotic mediator in the eye. Exp. Eye Res. 2008, 87, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Kitatani, K.; El-Alwani, M.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: Modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2005, 20, 482–484. [Google Scholar] [CrossRef]
- Yasui, H.; Hideshima, T.; Raje, N.; Roccaro, A.M.; Shiraishi, N.; Kumar, S.; Hamasaki, M.; Ishitsuka, K.; Tai, Y.-T.; Podar, K.; et al. FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res. 2005, 65, 7478–7484. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Frissora, F.; Ma, Y.; Santhanam, R.; Jarjoura, D.; Lehman, A.; Perotti, D.; Dalton, J.T.; Muthusamy, N.; et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood 2008, 111, 275–284. [Google Scholar] [CrossRef]
- Rolin, J.; Sand, K.L.; Knudsen, E.; Maghazachi, A.A. FTY720 and SEW2871 reverse the inhibitory effect of S1P on natural killer cell mediated lysis of K562 tumor cells and dendritic cells but not on cytokine release. Cancer Immunol. Immunother. 2010, 59, 575–586. [Google Scholar] [CrossRef]
- Furuya, H.; Shimizu, Y.; Kawamori, T. Sphingolipids in cancer. Cancer Metastasis Rev. 2011, 30, 567–576. [Google Scholar] [CrossRef]
- Wu, D.; Xing, G.W.; Poles, M.A.; Horowitz, A.; Kinjo, Y.; Sullivan, B.; Bodmer-Narkevitch, V.; Plettenburg, O.; Kronenberg, M.; Tsuji, M.; et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1351–1356. [Google Scholar] [CrossRef]
- Rauch, J.; Gumperz, J.; Robinson, C.; Sköld, M.; Roy, C.; Young, D.C.; Lafleur, M.; Branch Moody, D.; Brenner, M.B.; Costello, C.E.; et al. Structural Features of the Acyl Chain Determine Self-phospholipid Antigen Recognition by a CD1d-restricted Invariant NKT (iNKT) Cell. J. Biol. Chem. 2003, 278, 47508–47515. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena-Wolf, J.; Benlagha, K.; Chiu, Y.H.; Mehr, R.; Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 2001, 15, 897–908. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Park, S.H.; Benlagha, K.; Forestier, C.; Jayawardena-Wolf, J.; Savage, P.B.; Teyton, L.; Bendelac, A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CDld. Nat. Immunol. 2002, 3, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Khan, M.A.; Vieira, M.; Du, W.; Gervay-hague, J.; Brutkiewicz, R.R.; Dc, W. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood J. Am. Soc. Hematol. 2011, 111, 5637–5645. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Rosenthal, J.; Spiegel, S. Effect of acidic phospholipids on sphingosine kinase. J. Cell. Biochem. 1996, 60, 529–537. [Google Scholar] [CrossRef]
- Dieudé, M.; Striegl, H.; Tyznik, A.J.; Wang, J.; Behar, S.M.; Piccirillo, C.A.; Levine, J.S.; Zajonc, D.M.; Rauch, J. Cardiolipin Binds to CD1d and Stimulates CD1d-Restricted γδ T cells in the Normal Murine Repertoire. J. Immunol. 2011, 186, 4771–4781. [Google Scholar] [CrossRef]
- Cox, D.; Fox, L.; Tian, R.; Bardet, W.; Skaley, M.; Mojsilovic, D.; Gumperz, J.; Hildebrand, W. Determination of Cellular Lipids Bound to Human CD1d Molecules. PLoS ONE 2009, 4, e5325. [Google Scholar] [CrossRef]
- Tatituri, R.V.V.; Watts, G.F.M.; Bhowruth, V.; Barton, N.; Rothchild, A.; Hsu, F.-F.; Almeida, C.F.; Cox, L.R.; Eggeling, L.; Caardell, S.; et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc. Natl. Acad. Sci. USA 2013, 110, 1827–1832. [Google Scholar] [CrossRef]
- Fox, L.M.; Cox, D.G.; Lockridge, J.L.; Wang, X.; Chen, X.; Scharf, L.; Trott, D.L.; Ndonye, R.M.; Veerapen, N.; Besra, G.S.; et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef]
- Pizzuto, M.; Lonez, C.; Baroja-Mazo, A.; Martínez-Banaclocha, H.; Tourlomousis, P.; Gangloff, M.; Pelegrin, P.; Ruysschaert, J.-M.; Gay, N.J.; Bryant, C.E. Saturation of acyl chains converts cardiolipin from an antagonist to an activator of Toll-like receptor-4. Cell. Mol. Life Sci. 2019, 76, 3667–3678. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.S.; Sun, W.; Webb, T.J. Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma. Cells 2020, 9, 1030. https://doi.org/10.3390/cells9041030
Lee MS, Sun W, Webb TJ. Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma. Cells. 2020; 9(4):1030. https://doi.org/10.3390/cells9041030
Chicago/Turabian StyleLee, Michael S., Wenji Sun, and Tonya J. Webb. 2020. "Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma" Cells 9, no. 4: 1030. https://doi.org/10.3390/cells9041030
APA StyleLee, M. S., Sun, W., & Webb, T. J. (2020). Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma. Cells, 9(4), 1030. https://doi.org/10.3390/cells9041030




