E2F1 Regulates Adipocyte Differentiation and Adipogenesis by Activating ICAT
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Lentiviruses Preparation and Generation of Stable Cell Line
2.4. Transient Expression of ICAT in HeLa and HepG2 Cells
2.5. Immunofluorescent Microscopy
2.6. Quantitative Real-Time PCR
2.7. Western Blotting Analysis
2.8. Lipid Droplets’ Staining
2.9. Measurement of TG Content
2.10. siRNA-Mediated Knockdown
2.11. Statistical Analysis
3. Results
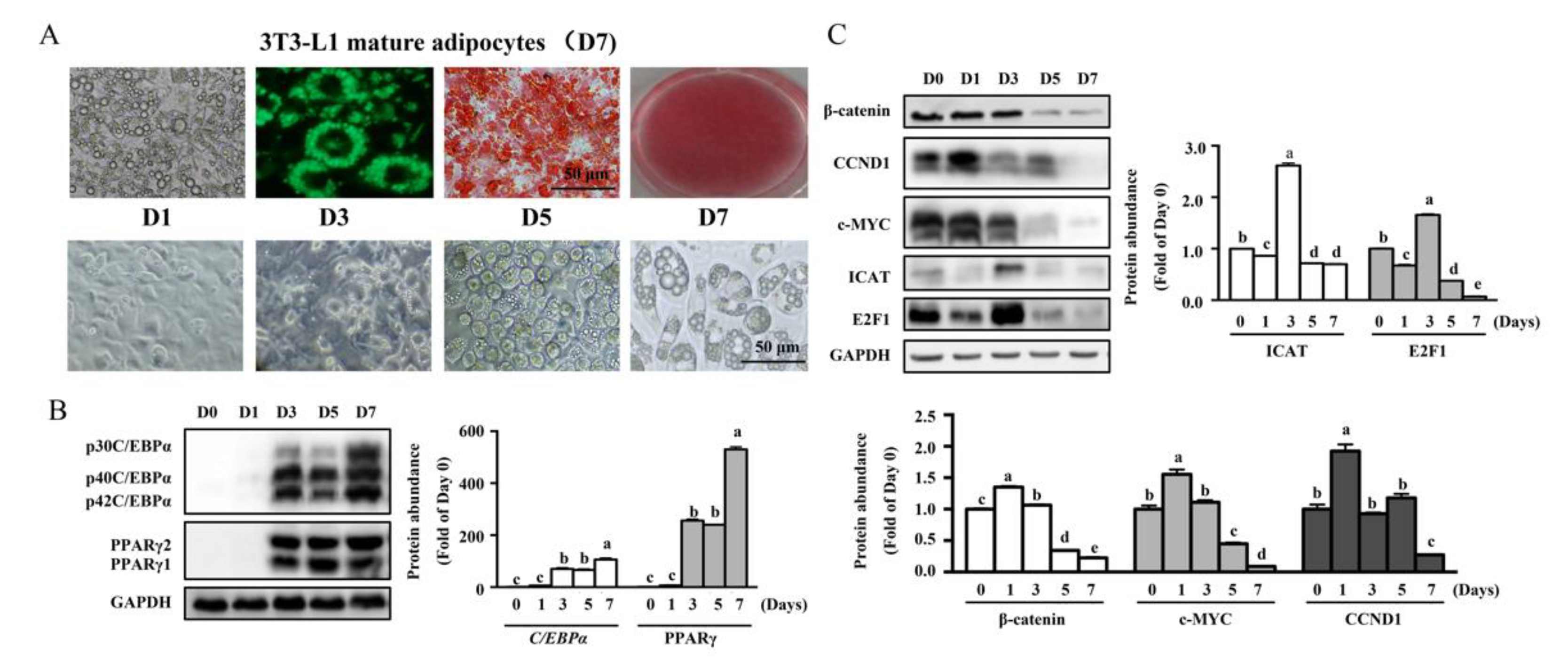
3.1. MDI-Induced Differentiation in 3T3-L1 Cells Was Associated with Increased Protein Levels of E2F1 and ICAT at Day 3 of Differentiation
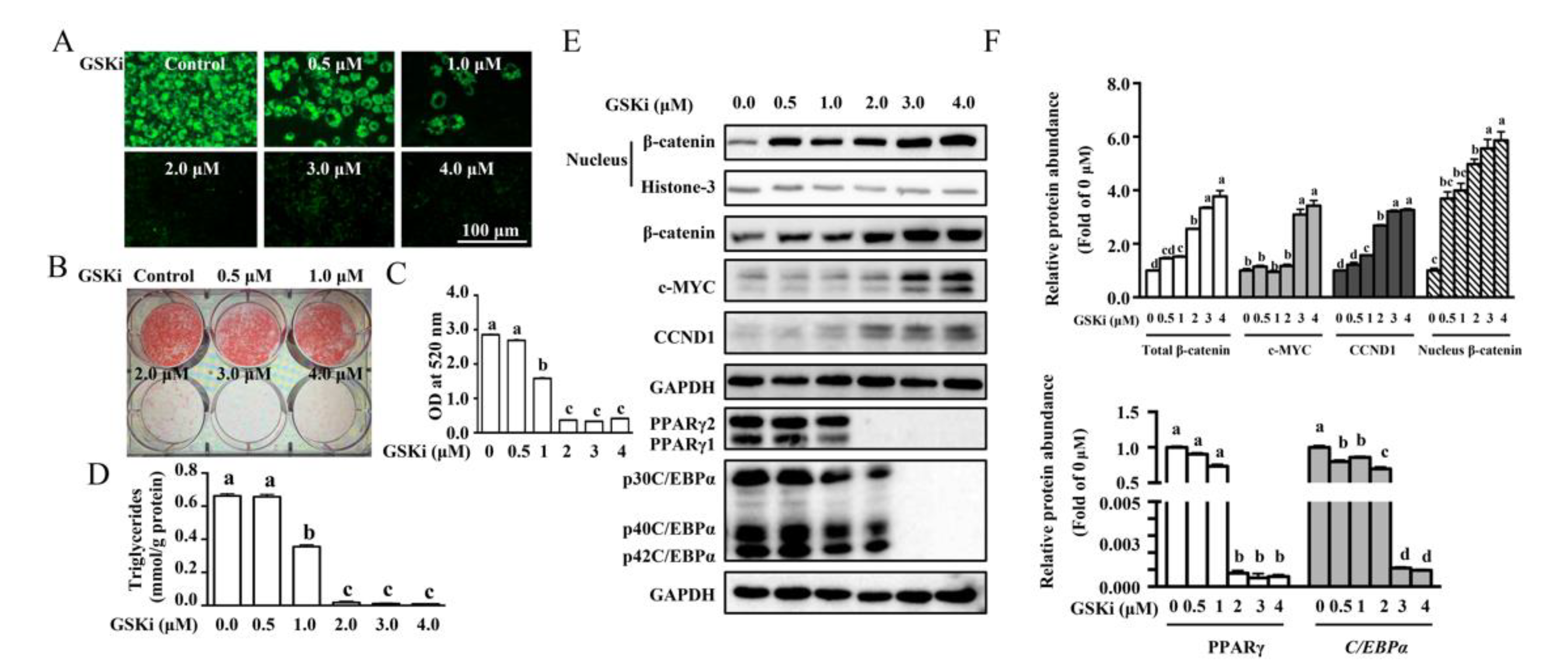
3.2. Activation of Wnt/β-catenin Signaling by GSK3β Inhibitor Blocked Adipogenesis
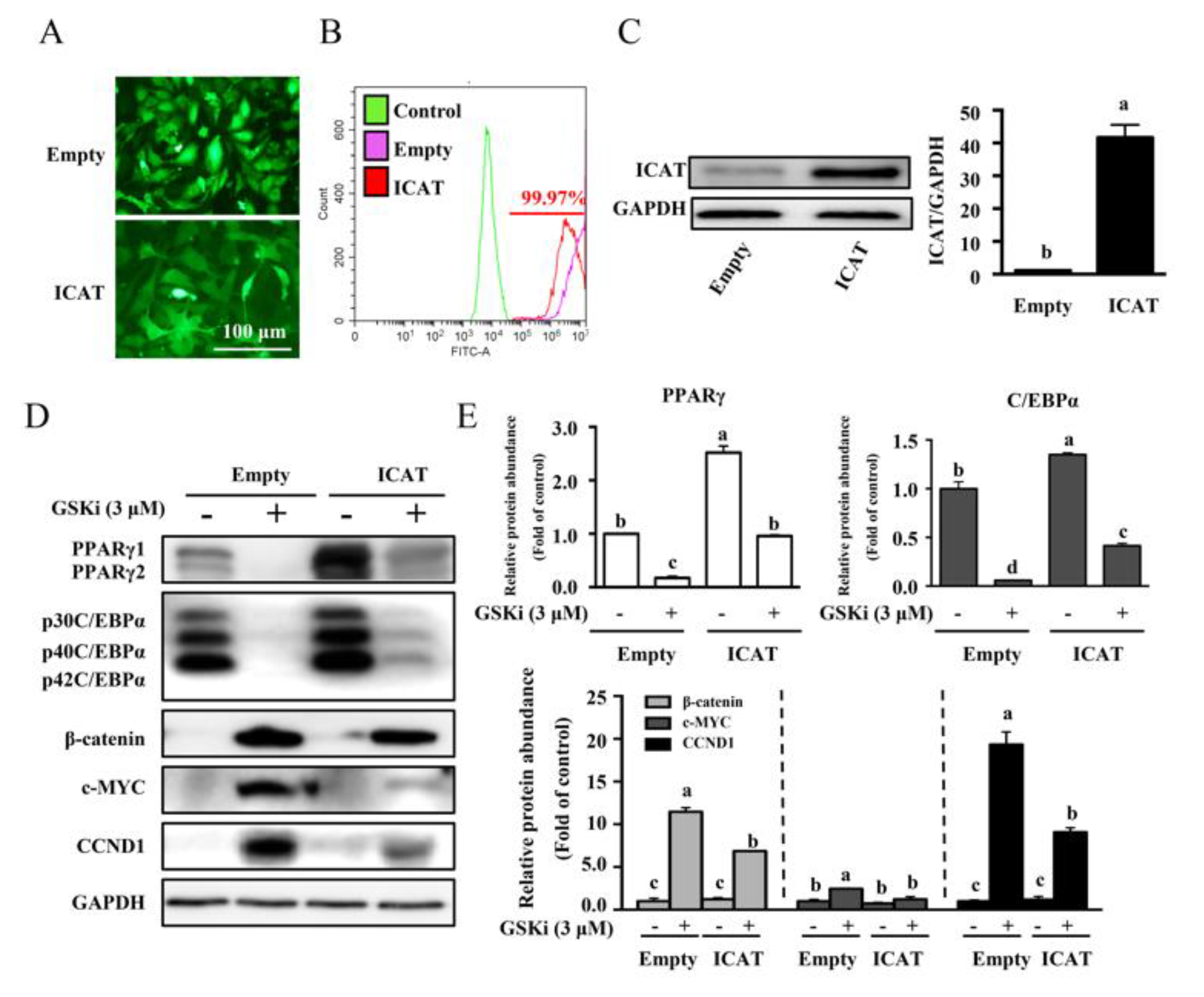
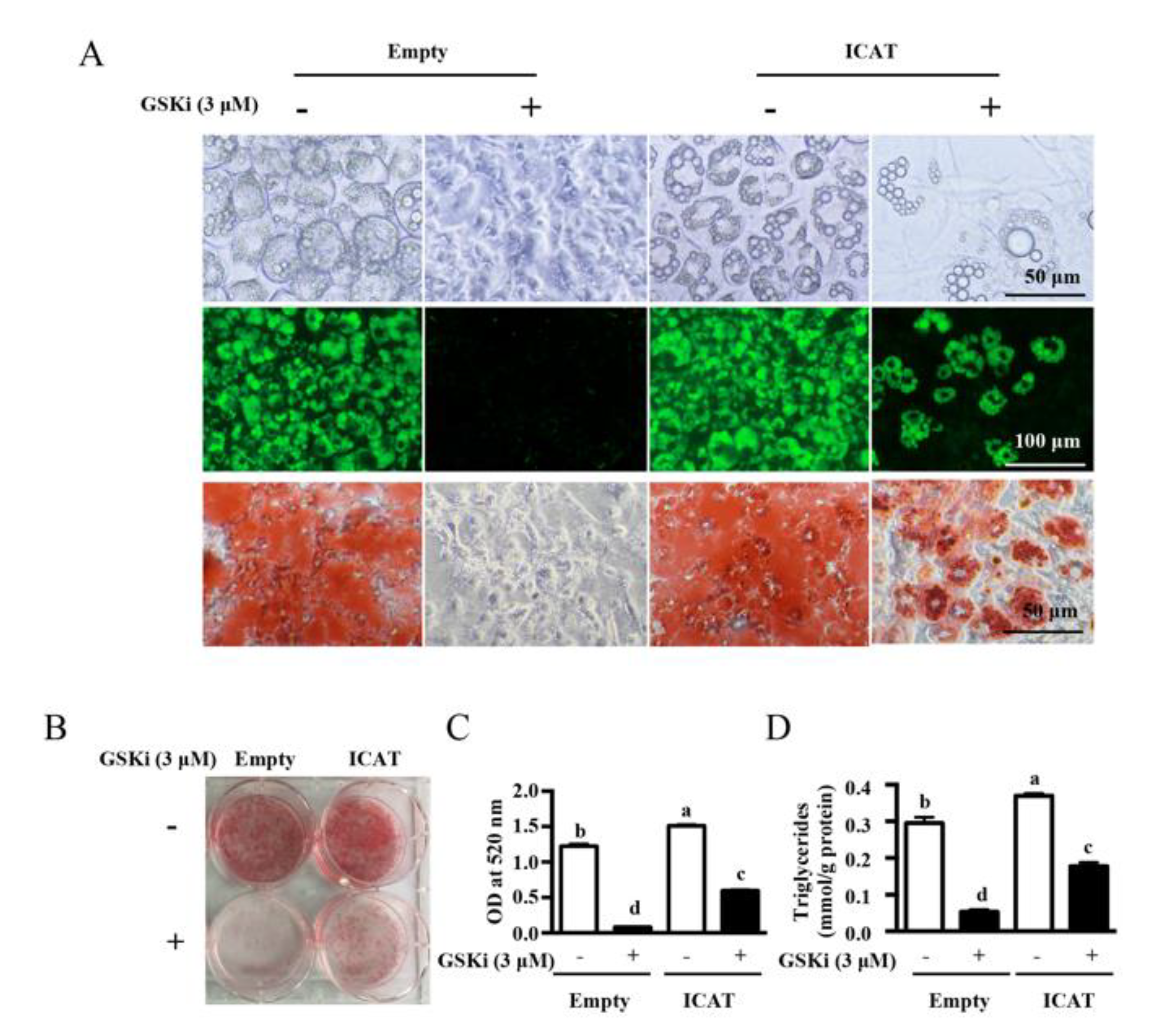
3.3. Overexpression of ICAT Reversed the Effect of GSK3β Inhibitor on Cell Differentiation and Adipogenesis in 3T3-L1 Pre-Adipocytes
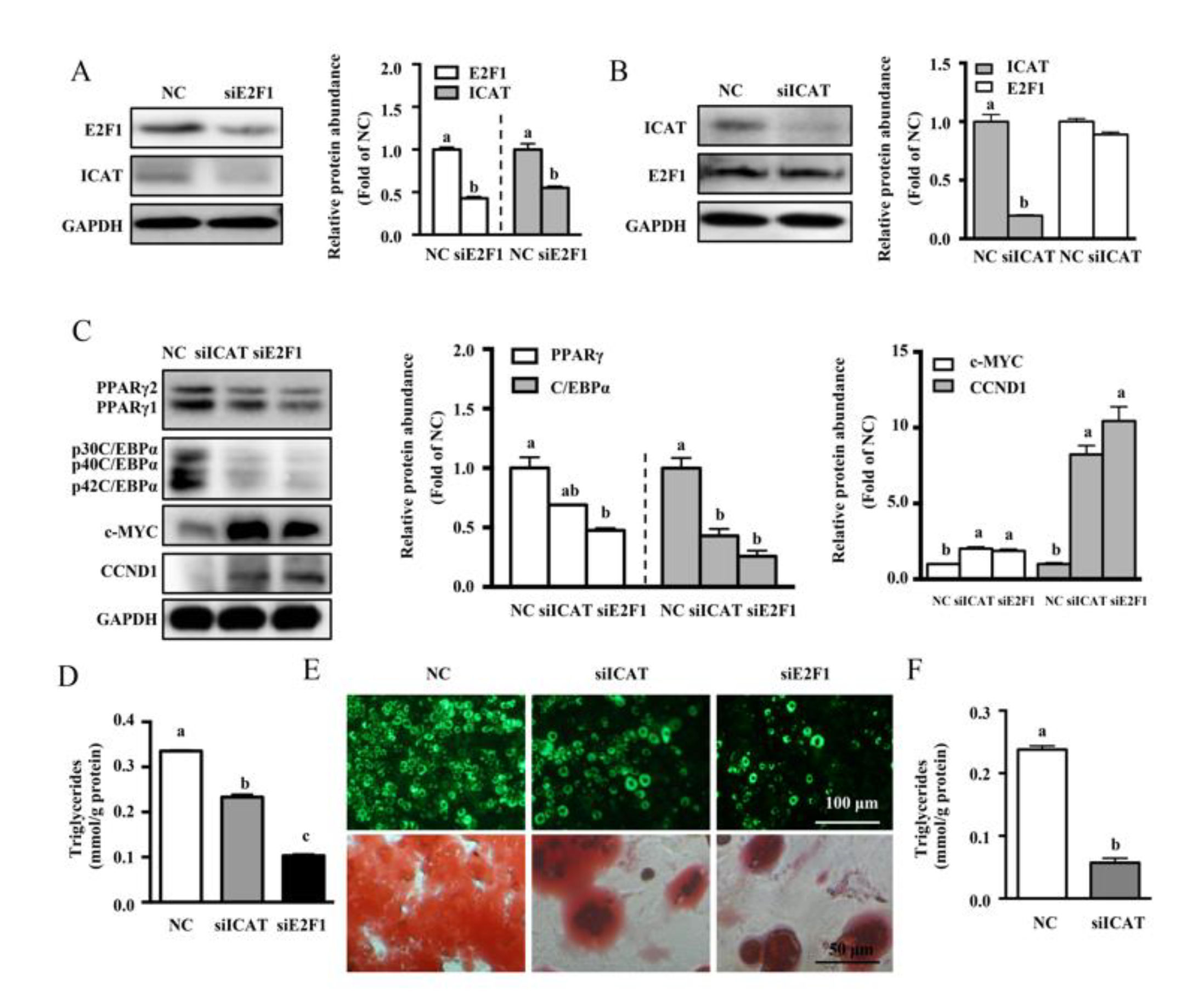
3.4. ICAT or E2F1 Knockdown Inhibited MDI-Induced Differentiation of Pre-Adipocytes
3.5. ICAT Did Not Affect Lipid Accumulation in Hela and HepG2 Cells
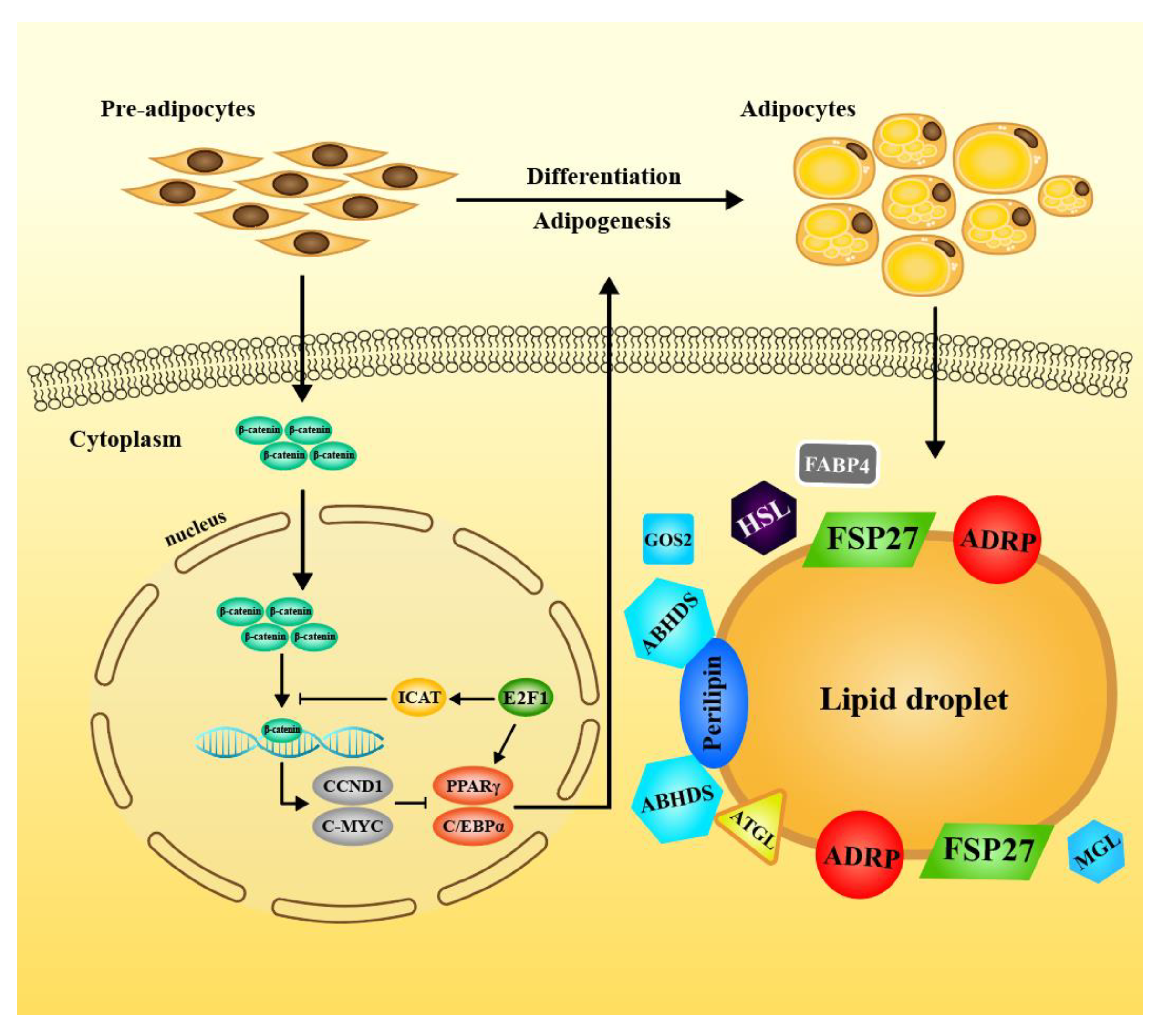
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Scherer, P.E.; Gupta, R.K. Improved methodologies for the study of adipose biology: Insights gained and opportunities ahead. J. Lipid Res. 2014, 55, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Poppy Roworth, A.; Ghari, F.; La Thangue, N.B. To live or let die—complexity within the E2F1 pathway. Mol. Cell Oncol. 2015, 2, e970480. [Google Scholar] [CrossRef]
- Denechaud, P.D.; Fajas, L.; Giralt, A. E2F1, a Novel Regulator of Metabolism. Front. Endocrinol. (Lausanne) 2017, 8, 311. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Petrov, P.; Serrano, M.; Ortega, F.; Garcia-Ruiz, E.; Oliver, P.; Ribot, J.; Ricart, W.; Palou, A.; Bonet, M.L.; et al. Decreased RB1 mRNA, protein, and activity reflect obesity-induced altered adipogenic capacity in human adipose tissue. Diabetes 2013, 62, 1923–1931. [Google Scholar] [CrossRef]
- Haim, Y.; Bluher, M.; Konrad, D.; Goldstein, N.; Kloting, N.; Harman-Boehm, I.; Kirshtein, B.; Ginsberg, D.; Tarnovscki, T.; Gepner, Y.; et al. ASK1 (MAP3K5) is transcriptionally upregulated by E2F1 in adipose tissue in obesity, molecularly defining a human dys-metabolic obese phenotype. Mol. Metab. 2017, 6, 725–736. [Google Scholar] [CrossRef]
- Haim, Y.; Bluher, M.; Slutsky, N.; Goldstein, N.; Kloting, N.; Harman-Boehm, I.; Kirshtein, B.; Ginsberg, D.; Gericke, M.; Guiu Jurado, E.; et al. Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy 2015, 11, 2074–2088. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, S.; Choi, M.S.; Ryoo, Z.Y.; Park, T. Increased expression of FGF1-mediated signaling molecules in adipose tissue of obese mice. J. Physiol. Biochem. 2016, 72, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.D.; Lopez-Mejia, I.C.; Giralt, A.; Lai, Q.; Blanchet, E.; Delacuisine, B.; Nicolay, B.N.; Dyson, N.J.; Bonner, C.; Pattou, F.; et al. E2F1 mediates sustained lipogenesis and contributes to hepatic steatosis. J. Clin. Invest. 2016, 126, 137–150. [Google Scholar] [CrossRef]
- Lai, Q.; Giralt, A.; Le May, C.; Zhang, L.; Cariou, B.; Denechaud, P.D.; Fajas, L. E2F1 inhibits circulating cholesterol clearance by regulating Pcsk9 expression in the liver. JCI Insight. 2017, 2, e89729. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L.; Landsberg, R.L.; Huss-Garcia, Y.; Sardet, C.; Lees, J.A.; Auwerx, J. E2Fs regulate adipocyte differentiation. Dev. Cell. 2002, 3, 39–49. [Google Scholar] [CrossRef]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef]
- Trimarchi, J.M.; Lees, J.A. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002, 3, 11–20. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, S.; Li, Z.; Tan, J.; Yu, Q. E2F1 suppresses Wnt/beta-catenin activity through transactivation of beta-catenin interacting protein ICAT. Oncogene 2011, 30, 3979–3984. [Google Scholar] [CrossRef]
- Tago, K.; Nakamura, T.; Nishita, M.; Hyodo, J.; Nagai, S.; Murata, Y.; Adachi, S.; Ohwada, S.; Morishita, Y.; Shibuya, H.; et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Gene dev. 2000, 14, 1741–1749. [Google Scholar]
- Prestwich, T.C.; Macdougald, O.A. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Pei, Y.; Brun, S.N.; Markant, S.L.; Lento, W.; Gibson, P.; Taketo, M.M.; Giovannini, M.; Gilbertson, R.J.; Wechsler-Reya, R.J. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 2012, 139, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef] [PubMed]
- Abranches, M.V.; Oliveira, F.C.; Conceicao, L.L.; Peluzio, M.D. Obesity and diabetes: The link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, X.; Yang, Y.; Dai, Z.; Wu, Z.; Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids. 2018, 50, 629–640. [Google Scholar] [CrossRef]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wu, W.; Wang, X.; Wang, Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J. Physiol. Biochem. 2015, 71, 405–413. [Google Scholar] [CrossRef]
- Lu, X.; Yang, X.; Liu, J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 2010, 9, 2719–2725. [Google Scholar] [CrossRef]
- Smirnova, E.; Goldberg, E.B.; Makarova, K.a.S.; Lin, L.; Brown, W.J.; Jackson, C.L. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006, 1, 106–113. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Lombes, M.; Rha, G.B.; Chi, Y.I.; Guerin, T.M.; Smart, E.J.; Liu, J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef]
- Klyde, B.J.; Hirsch, J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J. Lipid Res. 1979, 20, 705–715. [Google Scholar]
- Faust, I.M.; Johnson, P.R.; Stern, J.S.; Hirsch, J. Diet-induced adipocyte number increase in adult rats: A new model of obesity. Am. J. Physiol. 1978, 235, E279–E286. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Longo, K.A.; Wright, W.S.; Kang, S.; Gerin, I.; Chiang, S.H.; Lucas, P.C.; Opp, M.R.; MacDougald, O.A. Wnt10b inhibits development of white and brown adipose tissues. J. Biol Chem. 2004, 279, 35503–35509. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Longo, K.A.; Dolinsky, V.W.; Gerin, I.; Kang, S.; Bennett, C.N.; Chiang, S.H.; Prestwich, T.C.; Gress, C.; Burant, C.F.; et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes 2007, 56, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, S.; Kim, K.; Kim, W.; Chung, S.I.; Yang, Y.; Yoon, Y. Shikonin inhibits adipogenesis by modulation of the WNT/beta-catenin pathway. Life Sci. 2011, 88, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, T.; Peng, J.; Zhou, Z.; Wei, H.; Zhou, R.; Jiang, S.; Peng, J. SIRT1 suppresses adipogenesis by activating Wnt/beta-catenin signaling in vivo and in vitro. Oncotarget 2016, 7, 77707–77720. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, S.; Liu, Y.; Dong, X.; Shi, Z.; Zhang, A.; Liu, C.; Chen, L.; Wei, J.; Pu, P.; et al. ICAT inhibits glioblastoma cell proliferation by suppressing Wnt/beta-catenin activity. Cancer Lett. 2015, 357, 404–411. [Google Scholar] [CrossRef]
- Domingues, M.J.; Martinez-Sanz, J.; Papon, L.; Larue, L.; Mouawad, L.; Bonaventure, J. Structure-based mutational analysis of ICAT residues mediating negative regulation of beta-catenin co-transcriptional activity. PLoS ONE 2017, 12, e0172603. [Google Scholar] [CrossRef]
- Freytag, S.O.; Geddes, T.J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science 1992, 256, 379–382. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.; Oh, M.J.; Yoon, J.; Kim, H.Y.; Lee, K.J.; Lee, J.D.; Choi, K.Y. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/beta-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother Res. 2011, 25, 1629–1635. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Jung, E.; Hwang, W.; Kim, Y.S.; Park, D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein. Life Sci. 2010, 86, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Arango, N.A.; Szotek, P.P.; Manganaro, T.F.; Oliva, E.; Donahoe, P.K.; Teixeira, J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 2005, 288, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, P.J.; Lees, J.A. Life and death decisions by the E2F transcription factors. Curr. Opin. Cell Biol. 2007, 19, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Attardi, L.D.; Reczek, E.E.; Cosmas, C.; Demicco, E.G.; McCurrach, M.E.; Lowe, S.W.; Jacks, T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000, 14, 704–718. [Google Scholar]
- Shi, Y.; Tao, T.; Liu, N.; Luan, W.; Qian, J.; Li, R.; Hu, Q.; Wei, Y.; Zhang, J.; You, Y. PPARalpha, a predictor of patient survival in glioma, inhibits cell growth through the E2F1/miR-19a feedback loop. Oncotarget 2016, 7, 84623–84633. [Google Scholar] [CrossRef][Green Version]
- Porse, B.T.; Pedersen, T.A.; Xu, X.; Lindberg, B.; Wewer, U.M.; Friis-Hansen, L.; Nerlov, C. E2F repression by C/EBPalpha is required for adipogenesis and granulopoiesis in vivo. Cell 2001, 107, 247–258. [Google Scholar] [CrossRef]
- Nahle, Z.; Polakoff, J.; Davuluri, R.V.; McCurrach, M.E.; Jacobson, M.D.; Narita, M.; Zhang, M.Q.; Lazebnik, Y.; Bar-Sagi, D.; Lowe, S.W. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 2002, 4, 859–864. [Google Scholar] [CrossRef]
- Morris, E.J.; Ji, J.-Y.; Yang, F.; Di Stefano, L.; Herr, A.; Moon, N.-S.; Kwon, E.-J.; Haigis, K.M.; Näär, A.M.; Dyson, N.J. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8. Nature 2008, 455, 552–556. [Google Scholar] [CrossRef]
- Kim, B.; Woo, M.J.; Park, C.S.; Lee, S.H.; Kim, J.S.; Kim, B.; An, S.; Kim, S.H. Hovenia Dulcis Extract Reduces Lipid Accumulation in Oleic Acid-Induced Steatosis of Hep G2 Cells via Activation of AMPK and PPARalpha/CPT-1 Pathway and in Acute Hyperlipidemia Mouse Model. Phytother Res. 2017, 31, 132–139. [Google Scholar] [CrossRef]
- Li, X.D.; Zhao, M.Y.; Fan, L.Q.; Cao, X.N.; Chen, L.H.; Chen, J.H.; Lo, Y.M.; Zhao, L.M. Chitobiose alleviates oleic acid-induced lipid accumulation by decreasing fatty acid uptake and triglyceride synthesis in HepG2 cells. J. Funct. Foods 2018, 46, 202–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, N.; Xu, J.; Kong, B.; Copple, B.; Guo, G.L.; Wang, L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology 2014, 60, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L.; Blanchet, E.; Annicotte, J.S. The CDK4-pRB-E2F1 pathway: A new modulator of insulin secretion. Islets 2010, 2, 51–53. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, Y.; Li, S.; Yang, Y.; Dai, Z.; Wang, F.; Wu, Z.; Tso, P.; Wu, G. E2F1 Regulates Adipocyte Differentiation and Adipogenesis by Activating ICAT. Cells 2020, 9, 1024. https://doi.org/10.3390/cells9041024
Chen J, Yang Y, Li S, Yang Y, Dai Z, Wang F, Wu Z, Tso P, Wu G. E2F1 Regulates Adipocyte Differentiation and Adipogenesis by Activating ICAT. Cells. 2020; 9(4):1024. https://doi.org/10.3390/cells9041024
Chicago/Turabian StyleChen, Jingqing, Yuchen Yang, Shuai Li, Ying Yang, Zhaolai Dai, Fengchao Wang, Zhenlong Wu, Patrick Tso, and Guoyao Wu. 2020. "E2F1 Regulates Adipocyte Differentiation and Adipogenesis by Activating ICAT" Cells 9, no. 4: 1024. https://doi.org/10.3390/cells9041024
APA StyleChen, J., Yang, Y., Li, S., Yang, Y., Dai, Z., Wang, F., Wu, Z., Tso, P., & Wu, G. (2020). E2F1 Regulates Adipocyte Differentiation and Adipogenesis by Activating ICAT. Cells, 9(4), 1024. https://doi.org/10.3390/cells9041024




