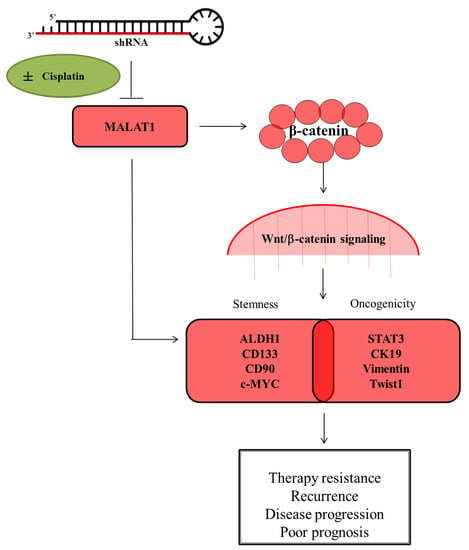
Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma
Abstract
1. Background
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Cell Culture
2.3. MALAT1 Silencing
2.4. Western Blot Analysis
2.5. Immunohistochemistry (IHC) Analysis
2.6. RNA Isolation and Quantitative RT-PCR
2.7. Matrigel Invasion Assay
2.8. Colony Formation Assay
2.9. Tumorsphere Formation Assays
2.10. RNAScope Analysis
2.11. RNA Immunoprecipitation
2.12. Fluorescence-Activated Cell Sorting (FACS) Analysis of ALDH Activity
2.13. TOP/FOP Flash Luciferase Reporter Assays
2.14. Side Population Analysis
2.15. Mice Tumor Xenograft Studies
2.16. Statistical Analysis
3. Results
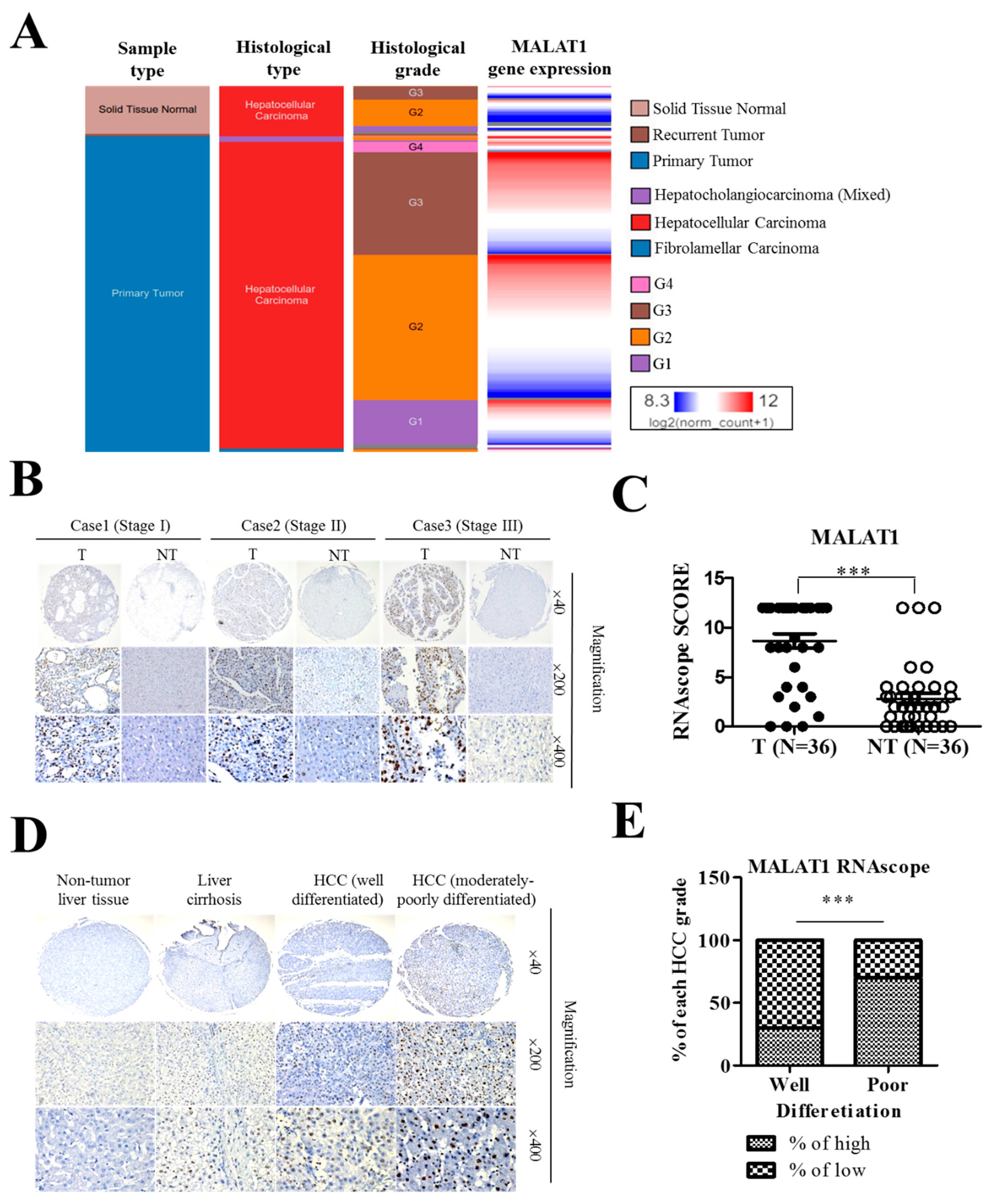
3.1. The lncRNA MALAT1 Is Aberrantly Expressed in HCC Tissues and Cell Lines
3.2. MALAT1 Expression in Liver Cancer Positively Correlates with Poor Cellular Differentiation Status and Disease Progression
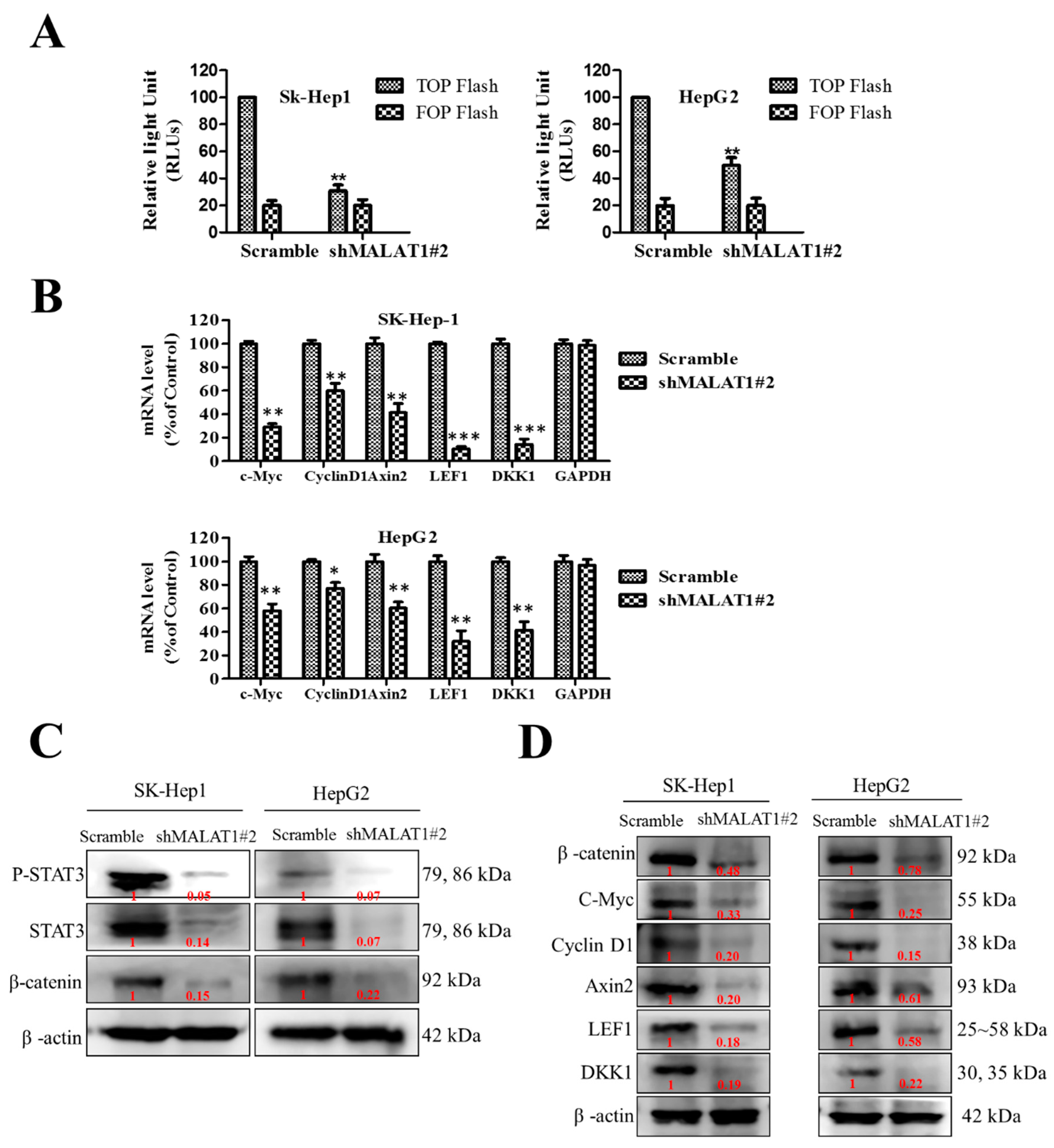
3.3. MALAT1 Expression Modulates HCC Oncogenicity and Stemness via Interaction with Wnt/β-Catenin
3.4. MALAT1 Oncogenic Activity in HCC Is Mediated by the Wnt/β-Catenin Signaling Pathway
3.5. Silencing of MALAT1 Is Associated with a Reduced CD133highCD90high HCC Population with Suppressed HCC Tumorsphere Formation In Vitro
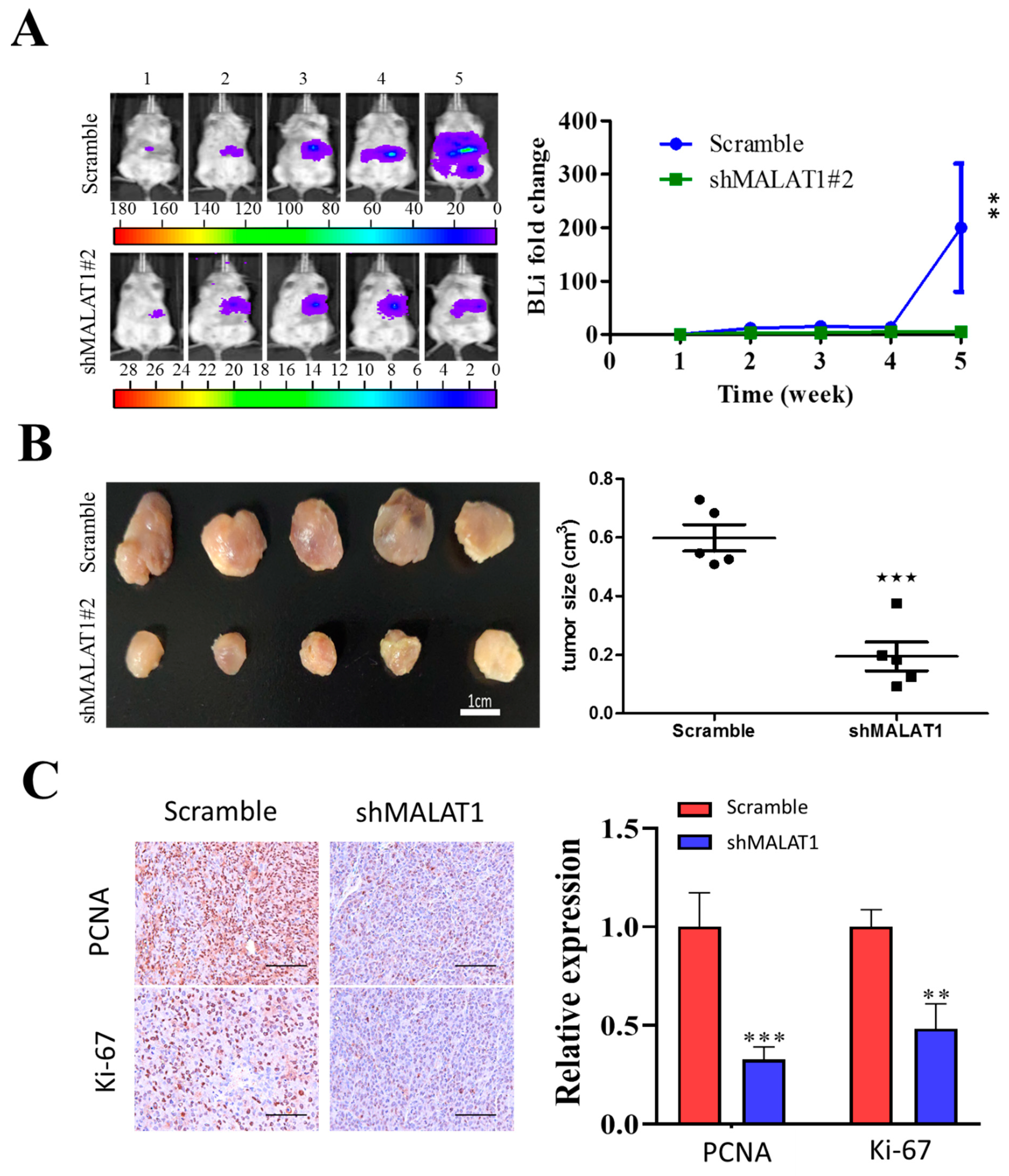
3.6. MALAT1 Is Required for Xenograft Tumorigenesis and Tumor Growth of HCC Cells In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC-SCs | HCC stem cells |
| HCC | Hepatocellular carcinoma |
| lncRNAs | Long non-coding RNAs |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| EpCAM | epithelial cell adhesion molecule |
| Lgr5 | leucine-rich repeat containing G protein-coupled receptor 5 |
| DLK1 | delta-like non-canonical notch ligand 1 |
| FBS | fetal bovine serum |
| PVDF | Polyvinylidene difluoride |
| FACS | Fluorescence-activated cell sorting |
| SP | side population |
| FACS | fluorescence-activated cell sorting |
References
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Shkumatava, A.; Jan, C.H.; Sive, H.L.; Bartel, B. Conserved Function of lincRNAs in Vertebrate Embryonic Development despite Rapid Sequence Evolution. Cell 2011, 147, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Zhang, Q.C.; Georgiev, P.; Ilik, I.A.; Akhtar, A.; Chang, H.Y. Rapid evolutionary turnover underlies conserved lncRNA-genome interactions. Genome Res. 2016, 30, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Fei, T.; Verhaak, R.G.; Su, Z.; Zhang, Y.; Brown, M.; Chen, Y.; Liu, X.S. Intergrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat. Struct. Mol. Biol. 2013, 20, 908–913. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Gutschner, T.; Haemmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2012, 73, 1180–1189. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Wan, X.; Yang, X.; Zhu, C.; Wang, A.; He, L.; Miao, R.; Chen, S.; Zhao, H. Long Noncoding RNA Plays a Key Role in Metastasis and Prognosis of Hepatocellular Carcinoma. BioMed Res. Int. 2014, 2014, 780521. [Google Scholar] [CrossRef]
- Hou, Z.; Xu, X.; Zhou, L.; Fu, X.; Tao, S.; Zhou, J.; Tan, D.; Liu, S. The long non-coding RNA MALAT1 promotes the migration and invasion of hepatocellular carcinoma by sponging miR-204 and releasing SIRT1. Tumor Biol. 2017. [Google Scholar] [CrossRef]
- Jiao, F.; Hu, H.; Han, T.; Yuan, C.; Wang, L.; Jin, Z.; Guo, Z.; Wang, L. Long Noncoding RNA MALAT-1 Enhances Stem Cell-Like Phenotypes in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2015, 16, 6677–6693. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, L.; Wu, T.; Huang, Y.; Cheng, Z.; Li, X.; Sun, T.; Zhou, Y.; Du, Z. Downregulation of lncRNA-MALAT1 Affects Proliferation and the Expression of Stemness Markers in Glioma Stem Cell Line SHG139S. Cell. Mol. Neurobiol. 2015, 36, 1097–1107. [Google Scholar] [CrossRef]
- Nio, K.; Yamashita, T.; Kaneko, S. The evolving concept of liver cancer stem cells. Mol. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.F.Y.; Yip, C.W.; Ng, L.W.-C.; Lo, K.W.; Wong, N.; Choy, K.W.; Chow, C.; Chan, K.F.; Cheung, T.T.; Poon, R.T.P.; et al. Establishment and characterization of a novel primary hepatocellular carcinoma cell line with metastatic ability in vivo. Cancer Cell Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A Novel in Situ RNA Analysis Platform for Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Keskin, F.; Suhre, A.; Kose, K.; Ersahin, T.; Çetin, A.E.; Cetin-Atalay, R. Image Classification of Human Carcinoma Cells Using Complex Wavelet-Based Covariance Descriptors. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Guo, Z.; Li, L.-Q.; Jiang, J.-H.; Ou, C.; Zeng, L.-X.; Xiang, B.-D. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 2098–2106. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Yuan, R.-H.; Yang, W.-C.; Hsu, H.-C.; Jeng, Y.-M. The stem cell E3-ligase Lin-41 promotes liver cancer progression through inhibition of microRNA-mediated gene silencing. J. Pathol. 2013, 229, 486–496. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, S.-Y.; Jun, Y.; Kim, J.-Y.; Nam, J.-S. Roles of Wnt Target Genes in the Journey of Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1604. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2016, 36, 1461–1473. [Google Scholar] [CrossRef]
- Nouso, K.; Miyahara, K.; Uchida, D.; Kuwaki, K.; Izumi, N.; Omata, M.; Ichida, T.; Kudo, M.; Ku, Y.; Kokudo, N.; et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br. J. Cancer 2013, 109, 1904–1907. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chang, Y.-S.; Hsu, H.-P.; Yen, M.-C.; Huang, H.-L.; Cho, C.-Y.; Wang, C.-Y.; Weng, T.-Y.; Lai, P.-T.; Chen, C.-S.; et al. Therapeutics targeting CD90-integrin-AMPK-CD133 signal axis in liver cancer. Oncotarget 2015, 6, 42923–42937. [Google Scholar] [CrossRef]
- Golabi, P.; Fazel, S.; Otgonsuren, M.; Sayiner, M.; Locklear, C.T.; Younossi, Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017. [Google Scholar] [CrossRef] [PubMed]
- Malakar, P.; Shilo, A.; Mogilevsky, A.; Stein, I.; Pikarsky, E.; Nevo, Y.; Benyamini, H.; Elgavish, S.; Zong, X.; Prasanth, K.V.; et al. Long Noncoding RNA MALAT1 Promotes Hepatocellular Carcinoma Development by SRSF1 Upregulation and mTOR Activation. Cancer Res. 2016, 77, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.-C.; Wilkinson, J.E.; Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.R.; Norton, L.; et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2015, 30, 34–51. [Google Scholar] [CrossRef]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nat. Cell Biol. 2013, 15, 338–344. [Google Scholar] [CrossRef]
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef]
- Bamodu, O.A.; Huang, W.-C.; Lee, W.-H.; Wu, A.T.H.; Wang, L.-S.; Hsiao, M.; Yeh, C.-T.; Chao, T.-Y. Aberrant KDM5B expression promotes aggressive breast cancer through MALAT1 overexpression and downregulation of hsa-miR-448. BMC Cancer 2016. [Google Scholar] [CrossRef]
- Yao, Q.; Yang, J.; Liu, T.; Zhang, J.; Zheng, Y. Long noncoding RNA MALAT1 promotes the stemness of esophageal squamous cell carcinoma by enhancing YAP transcriptional activity. FEBS OpenBio 2019, 9, 1392–1402. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, J.; Geng, Q.; Wang, G. LncRNA MALAT1 increases the stemness of gastric cancer cells via enhancing SOX2 mRNA stability. FEBS OpenBio 2019, 9, 1212–1222. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Chang, L.; Wei, C.; Bei, H.; Yin, Y.; Chen, M.; Wang, H.; Liang, J.; Wu, Y. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway. Lipids Heal. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bahnassy, A.A.; Fawzy, M.; El-Wakil, M.; Zekri, A.-R.N.; Abdel-Sayed, A.; Sheta, M. Aberrant expression of cancer stem cell markers (CD44, CD90, and CD133) contributes to disease progression and reduced survival in hepatoblastoma patients: 4-year survival data. Transl. Res. 2015, 165, 396–406. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-L.; Bamodu, O.A.; Ong, J.-R.; Lee, W.-H.; Yeh, C.-T.; Tsai, J.-T. Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma. Cells 2020, 9, 1020. https://doi.org/10.3390/cells9041020
Chang H-L, Bamodu OA, Ong J-R, Lee W-H, Yeh C-T, Tsai J-T. Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma. Cells. 2020; 9(4):1020. https://doi.org/10.3390/cells9041020
Chicago/Turabian StyleChang, Hang-Lung, Oluwaseun Adebayo Bamodu, Jiann-Ruey Ong, Wei-Hwa Lee, Chi-Tai Yeh, and Jo-Ting Tsai. 2020. "Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma" Cells 9, no. 4: 1020. https://doi.org/10.3390/cells9041020
APA StyleChang, H.-L., Bamodu, O. A., Ong, J.-R., Lee, W.-H., Yeh, C.-T., & Tsai, J.-T. (2020). Targeting the Epigenetic Non-Coding RNA MALAT1/Wnt Signaling Axis as a Therapeutic Approach to Suppress Stemness and Metastasis in Hepatocellular Carcinoma. Cells, 9(4), 1020. https://doi.org/10.3390/cells9041020