The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology
Abstract
1. Introduction
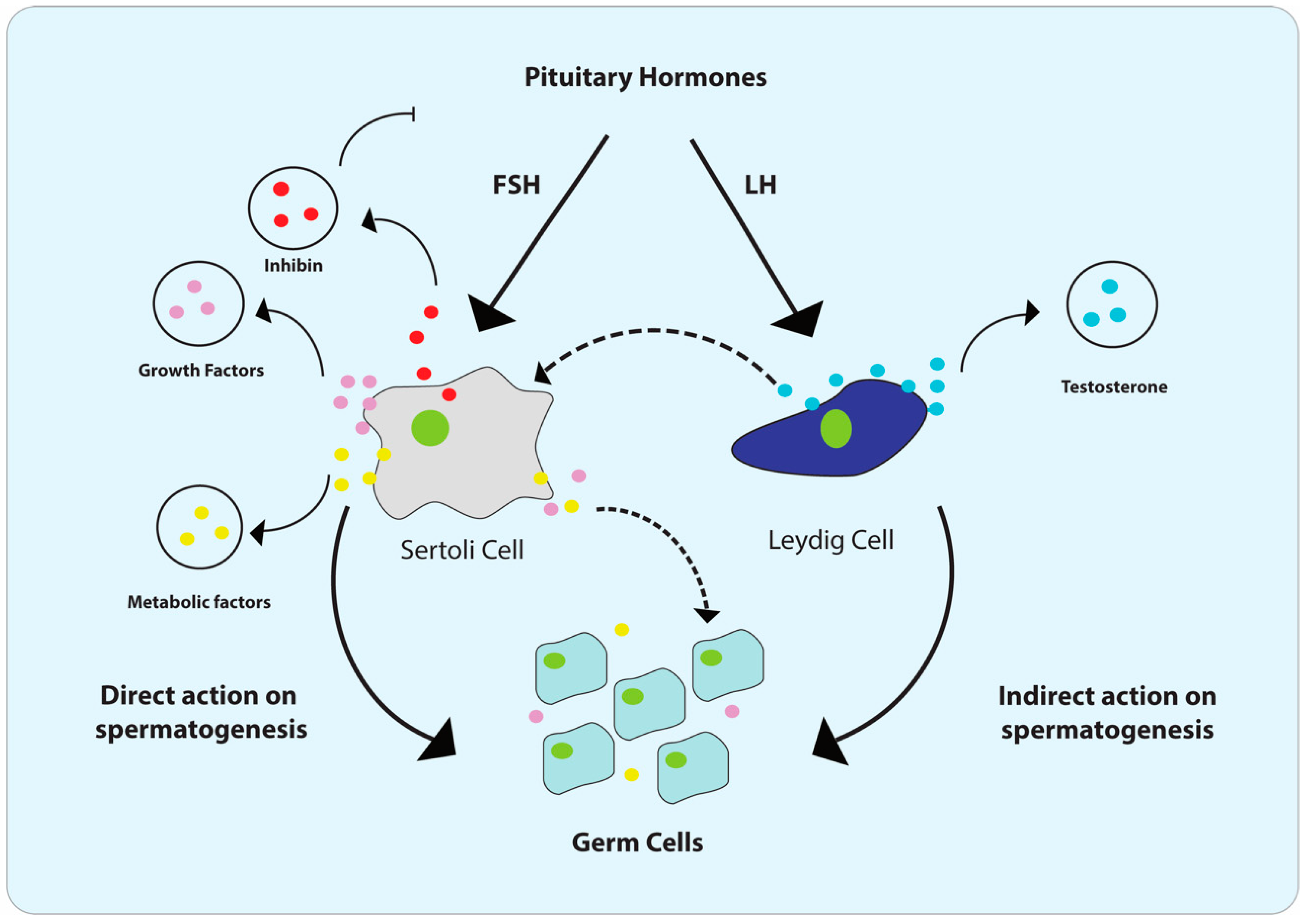
2. Male Reproductive Tract (MRT) and Cellular Composition of Testis
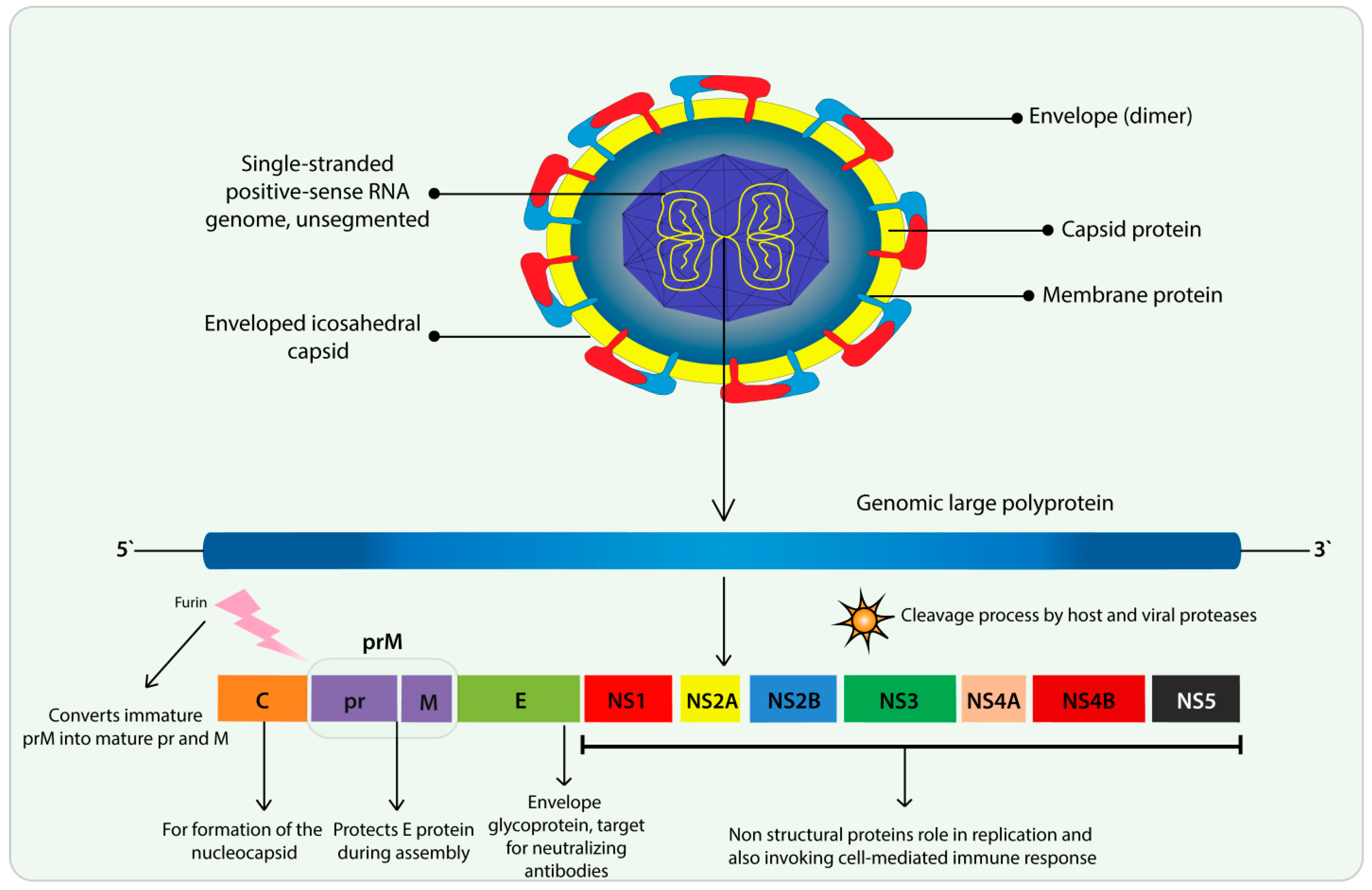
3. Flavivirus and ZIKV Features
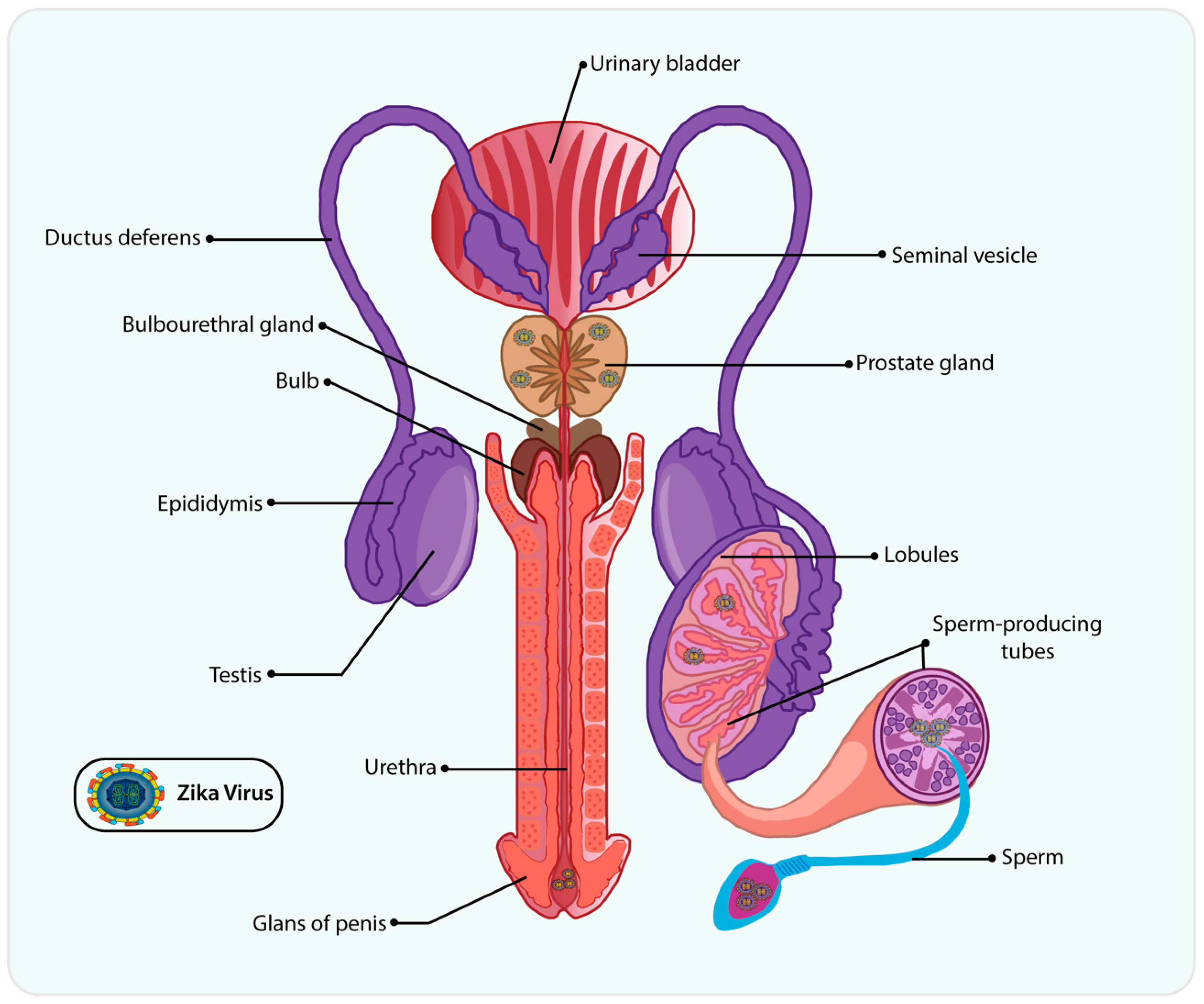
4. ZIKV on Male Reproductive Tract
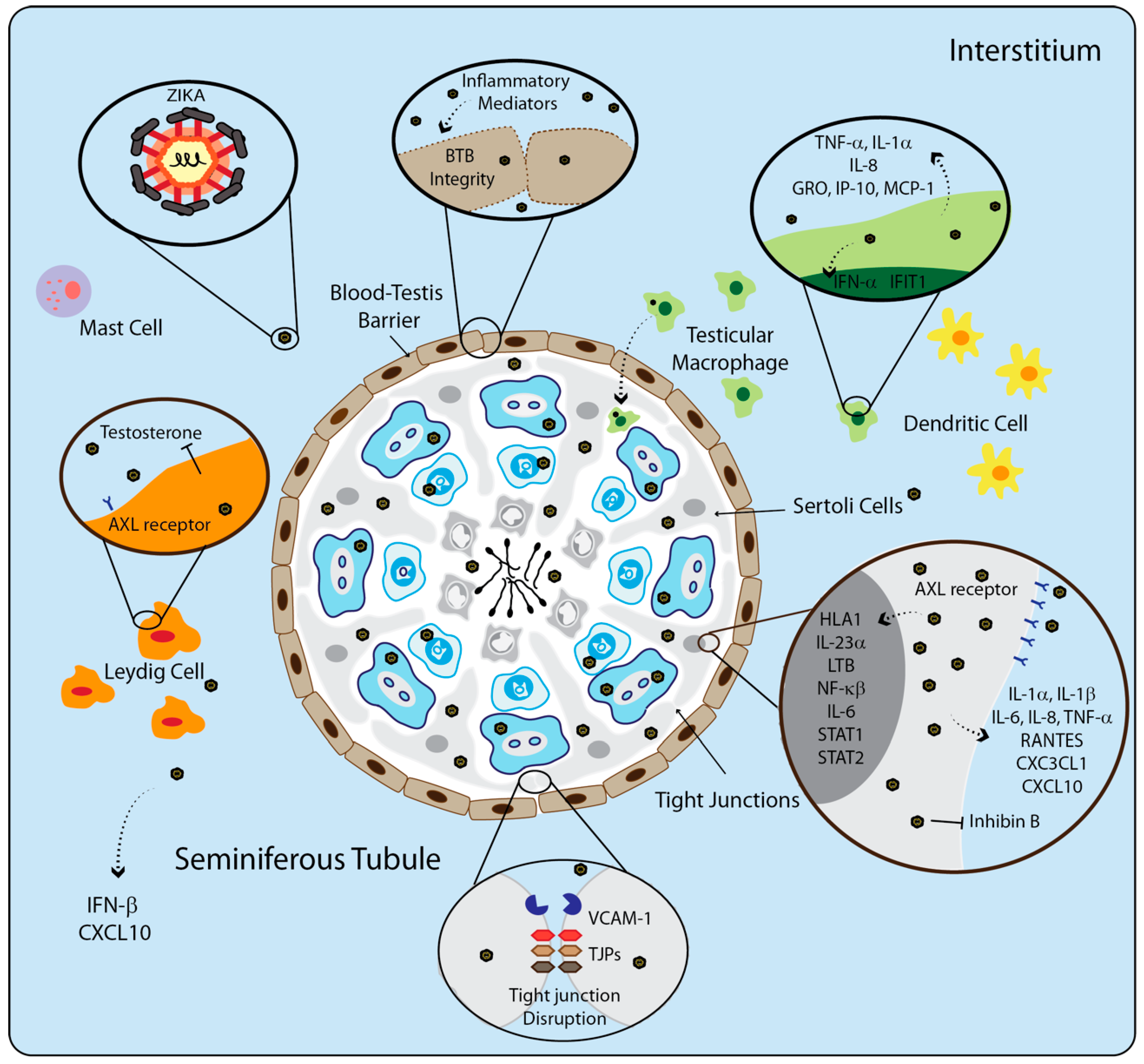
5. The Immune System of Testis during Viral Infection
6. ZIKV Vaccines and Treatment to Improve the Host Response in the MRT
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gurung, P.; Jialal, I. Physiology, Male Reproductive System; StatPearls Publishing [Internet]: Treasure Island, FL, USA, 2019. [Google Scholar]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, W.; Xue, S.; Han, D. Testicular defense systems: Immune privilege and innate immunity. Cell. Mol. Immunol. 2014, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Filippini, A.; Riccioli, A.; Padula, F.; Lauretti, P.; D’Alessio, A.; De Cesaris, P.; Gandini, L.; Lenzi, A.; Ziparo, E. Control and impairment of immune privilege in the testis and in semen. Hum. Reprod. Update 2001, 7, 444–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood—Testis barrier and spermatogenesis: Lessons from genetically-modified mice. Asian J. Androl. 2014, 16, 572–580. [Google Scholar] [PubMed]
- Wang, M.; Fijak, M.; Hossain, H.; Nüsing, R.M.; Lochnit, G.; Michaela, F.; Wudy, S.A.; Zhang, L.; Gu, H.; Konrad, L.; et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J. Immunol. 2017, 198, 4327–4340. [Google Scholar] [CrossRef]
- Mital, P.; Hinton, B.T.; Dufour, J.M. The Blood-Testis and Blood-Epididymis Barriers Are More than Just Their Tight Junctions. Biol. Reprod. 2011, 84, 851–858. [Google Scholar] [CrossRef]
- Luca, G.; Baroni, T.; Arato, I.; Hansen, B.C.; Cameron, D.F.; Calafiore, R. Role of Sertoli Cell Proteins in Immunomodulation. Protein Pept. Lett. 2018, 25, 440–445. [Google Scholar] [CrossRef]
- Keller, N.M.; Gentek, R.; Gimenez, G.; Bigot, S.; Mailfert, S.; Sieweke, M.H. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 2017, 2829–2841. [Google Scholar] [CrossRef]
- Hedger, M.P.; Meinhardt, A. Local regulation of T cell numbers and lymphocyte-inhibiting activity in the interstitial tissue of the adult rat testis. J. Reprod. Immunol. 2000, 48, 69–80. [Google Scholar] [CrossRef]
- Windsch, S. Are testicular mast cells involved in the regulation of germ cells in man? Andrology 2014, 2, 615–622. [Google Scholar] [CrossRef]
- Rival, C.; Lustig, L.; Iosub, R.; Guazzone, V.A.; Schneider, E.; Meinhardt, A.; Fijak, M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006, 324, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Burnett, N.M.; Curtis, K.M. Reproductive Tract Infections. Reproductive Health Epidemiology Series Module 3; CDC, Department of Health and Human Services: Atlanta, GA, USA, 2003; pp. 3–4. [Google Scholar]
- World Health Organization, W. Sexually transmitted infections (STIs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 15 April 2020).
- World Health Organization (WHO). Global Health Sector Strategy on HIV 2016–2021 towards Ending AIDS; WHO: Geneva, Switzerland, 2018; ISBN 9789241501651. [Google Scholar]
- Mackay, I.M.; Arden, K.E. Ebola virus in the semen of convalescent men. Lancet Infect. Dis. 2015, 15, 149–150. [Google Scholar] [CrossRef]
- Oliveira Souto, I.; Alejo-Cancho, I.; Gascón Brustenga, J.; Peiró Mestres, A.; Muñoz Gutiérrez, J.; Martínez Yoldi, M.J. Persistence of Zika virus in semen 93 days after the onset of symptoms. Enferm. Infec. Micr. Cl. 2018, 36, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Pudney, J.; Anderson, D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am. J. Pathol. 1991, 139, 149–160. [Google Scholar]
- Salam, A.P.; Horby, P.W. The breadth of viruses in human semen. Emerg. Infect. Dis. 2017, 23, 1922–1924. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO statement on the first meeting of the International Health Regulations (IHR 2005). In Proceedings of the Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations, Washington, WA, USA, 18 November 2016. [Google Scholar]
- Wang, J.; Counotte, M.J.; Kim, C.R.; Bernstein, K.; Broutet, N.J.N.; Deal, C.D.; Low, N. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018, 15, 30–49. [Google Scholar]
- Kelly, D.A.; Moore, B.C. Integrative and Comparative Biology The Morphological Diversity of Intromittent Organs. Integr. Comp. Biol. 2016, 56, 630–634. [Google Scholar] [CrossRef]
- Jones, R.E.; Lopez, K.H. Human Reproductive Biology, 4th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Fujisawa, M. Cell-to-cell cross talk in the testis. Urol. Res. 2001, 29, 144–151. [Google Scholar] [CrossRef]
- Picut, C.A.; De Rijk, E.P.C.T.; Dixon, D. Immunopathology of the Male Reproductive Tract. In Immunopathology in Toxicology and Drug Development. Molecular and Integrative Toxicology; Parker, G., Ed.; Humana Press: Cham, Switzerland, 2017. [Google Scholar]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, 1–15. [Google Scholar] [CrossRef]
- Kaur, G.; Vadala, S.; Dufour, J.M. An overview of a Sertoli cell transplantation model to study testis morphogenesis and the role of the Sertoli cells in immune privilege. Environ. Epigenet. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sieweke, M.H. Testicular macrophages: Guardians of fertility. Cell. Immunol. 2018, 330, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contaception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.M.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009, 20, 329–338. [Google Scholar] [CrossRef]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: A new regulatory model. J. Reprod. Immunol. 2013, 97, 147–158. [Google Scholar] [CrossRef]
- Matusali, G.; Houzet, L.; Satie, A.-P.; Mahé, D.; Aubry, F.; Couderc, T.; Frouard, J.; Bourgeau, S.; Bensalah, K.; Lavoué, S.; et al. Zika virus infects human testicular tissue and germ cells. J. Clin. Invest. 2018, 128, 4697–4710. [Google Scholar] [CrossRef]
- Nistal, M.; Santamaria, L.; Paniagua, R. Mast Cells in the Human Testis and Epididymis from Birth to Adulthood. Acta Anat. 1984, 119, 155–160. [Google Scholar] [CrossRef]
- Hussein, M.R.; Abou-Deif, E.S.; Bedaiwy, M.A.; Said, T.M.; Mustafa, M.G.; Nada, E.; Ezat, A.; Agarwal, A. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil. Steril. 2005, 83, 1447–1453. [Google Scholar] [CrossRef]
- Schrade, A.; Akinrinade, O.; Pihlajoki, M.; Fischer, S.; Rodriguez, V.M.; Otte, K.; Velagapudi, V.; Toppari, J.; Wilson, D.B.; Heikinheimo, M. GATA4 regulates blood-testis barrier function and lactate metabolism in mouse Sertoli cells. Endocrinology 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 2013, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Yeste, M. The role of miRNAs in male human reproduction: A systematic review. Andrology 2020, 8, 7–26. [Google Scholar]
- Tenorio, F.; Neto, L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in Humans and Its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar]
- Raghupathy, R.; Shaha, C.; Gupta, S.K. Autoimmunity to sperm antigens. Curr. Opin. Immunol. 1990, 2, 757–760. [Google Scholar] [CrossRef]
- Roulet, V.; Satie, A.P.; Ruffault, A.; Le Tortorec, A.; Denis, H.; Patard, J.J.; Rioux-Leclerq, N.; Gicquel, J.; Jégou, B.; Dejucq-Rainsford, N. Susceptibility of Human Testis to Human Immunodeficiency Virus-1 Infection in Situ and in Vitro. Am. J. Pathol. 2006, 169, 2094–2103. [Google Scholar] [CrossRef]
- Robinson, C.L.; Chong, A.C.N.; Ashbrook, A.W.; Jeng, G.; Jin, J.; Chen, H.; Tang, E.I.; Martin, L.A.; Kim, R.S.; Kenyon, R.M.; et al. Male germ cells support long-term propagation of Zika virus. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Holbrook, M.R. Historical perspectives on flavivirus research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; del Carmen Castillo Signor, L. Phylogeny of zika virus in western Hemisphere. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, F.; Latiwesh, O.B.; Hussain, S. A Comprehensive Review of the Manifestations and Pathogenesis of Zika Virus in Neonates and Adults. Cureus 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- MacNamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M. RE: Zika virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; De Melo, V.C.A.; Mosimann, A.L.P.; Dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Enfissi, A.; Codrington, J.; Roosblad, J.; Kazanji, M.; Rousset, D. Zika virus genome from the Americas. Lancet 2016, 387, 227–228. [Google Scholar] [CrossRef]
- Sharma, A.; Lal, S.K. Zika Virus: Transmission, Detection, Control, and Prevention. Front. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Slavov, S.N.; Otaguiri, K.K.; Kashima, S.; Covas, D.T. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz. J. Med. Biol. Res. 2016, 49, 1–11. [Google Scholar] [CrossRef]
- Ayres, C.F.J. Identification of Zika virus vectors and implications for control. Lancet Infect. Dis. 2016, 16, 278–279. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, H.G.; García-Arredondo, J.L.; Rivera, M.D.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, S.M.; Ramírez-Morales, O.J.; Carraquilha, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, G.; Du, F.; Chuang, Y.; Vaughan, R.C.; Sankaran, B.; Kao, C.C.; Li, P. Structure and function of the Zika virus full-length NS5 protein. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.; Vázquez-Calvo, Á.; Blázquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martín-Acebes, M.A. Zika Virus: The Latest Newcomer. Front. Microbiol. 2016, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention, CDC. First female-to-male sexual transmission of Zika virus infection reported in New York City. Available online: https://www.cdc.gov/media/releases/2016/s0715-zika-female-to-male.html (accessed on 15 April 2020).
- Imperato, P.J. The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic. J. Community Health 2016, 41, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Foy, J.L.C.; Blitvich, B.J.; da Rosa, A.T.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable Non–Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Lormeau-Cao, V. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef]
- Guedes, D.R.D.; Paiva, M.H.S.; Donato, M.M.A.; Barbosa, P.P.; Krokovsky, L.; Rocha, S.W.S.; Saraiva, K.L.A.; Crespo, M.M.; Rezende, T.M.T.; Wallau, G.L.; et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Brault, A.C.; Ebel, G.D.; Failloux, A.; Fernandez-Salas, I.; Kitron, U.; Kramer, L.D.; Lourenço-de-Oliveira, R.; Osorio, J.E.; et al. Lack of evidence for Zika virus transmission by Culex mosquitoes. Emerg. Microbes Infect. 2017, 6, 1–2. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016, 21, 1–4. [Google Scholar] [CrossRef]
- Matheron, S.; D’Ortenzio, E.; Leparc-Goffart, I.; Hubert, B.; de Lamballerie, X.; Yazdanpanah, Y. Long-Lasting Persistence of Zika Virus in Semen. Clin. Infect. Dis. 2016, 63, 1264. [Google Scholar] [CrossRef]
- Martin-Blondel, G. Zika virus in semen and spermatozoa. Lancet Infect. Dis. 2016, 16, 1106–1107. [Google Scholar]
- Musso, D.; Richard, V.; Teissier, A.; Stone, M.; Lanteri, M.C.; Latoni, G.; Alsina, J.; Reik, R.; Busch, M.P.; Epidemiology, R.; et al. Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin. Microbiol. Infect. 2017, 23, 1001.e1–1001.e3. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, N.G.; Marras, G.; Jacomo, V.; Bouret, D. Persistence of Zika virus in gradient sperm preparation. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Hearn, P.; Afrough, B.; Lumley, S.; Carter, D.; Aarons, E.J.; Simpson, A.J.; Brooks, T.J. Detection of Zika Virus in Semen. Emerg. Infect. Dis. 2016, 22, 940. [Google Scholar] [CrossRef]
- McDonald, E.M.; Duggal, N.K.; Ritter, J.M.; Brault, A.C. Infection of epididymal epithelial cells and leukocytes drives seminal shedding of Zika virus in a mouse model. PLoS Negl. Trop. Dis. 2018, 12, 1–22. [Google Scholar] [CrossRef]
- Stassen, L.; Armitage, C.; van der Heide, D.; Beagley, K.; Frentiu, F. Zika Virus in the Male Reproductive Tract. Viruses 2018, 10, 198. [Google Scholar] [CrossRef]
- Kumar, A.; Jovel, J.; Lopez-Orozco, J.; Limonta, D.; Airo, A.M.; Hou, S.; Stryapunina, I.; Fibke, C.; Moore, R.B.; Hobman, T.C. Human sertoli cells support high levels of zika virus replication and persistence. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Sheng, Z.-Y.; Gao, N.; Wang, Z.-Y.; Cui, X.-Y.; Zhou, D.-S.; Fan, D.-Y.; Chen, H.; Wang, P.-G.; An, J. Sertoli Cells Are Susceptible to ZIKV Infection in Mouse Testis. Front. Cell. Infect. Microbiol. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Strange, D.P.; Green, R.; Siemann, D.N.; Gale, M.; Verma, S. Immunoprofiles of human Sertoli cells infected with Zika virus reveals unique insights into host-pathogen crosstalk. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Govero, J.; Richner, J.M.; Caine, E.A.; Salazar, V.; Diamond, M.S.; Esakky, P.; Scheaffer, S.M.; Drury, A.; Moley, K.H.; Fernandez, E.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a novel murine model to study zika virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liang, Q. Recent Advances in Animal Models of Zika Virus Infection. Virol. Sin. 2018, 33, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.N.; Strange, D.P.; Maharaj, P.N.; Shi, P.-Y.; Verma, S. Zika Virus Infects Human Sertoli Cells and Modulates the Integrity of the In Vitro Blood-Testis Barrier Model. J. Virol. 2017, 91, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Strange, D.P.; Jiyarom, B.; Pourhabibi Zarandi, N.; Xie, X.; Baker, C.; Sadri-Ardekani, H.; Shi, P.-Y.; Verma, S. Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells. MBio 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Mlera, L.; Bloom, M.E. Differential zika virus infection of testicular cell lines. Viruses 2019, 11, 42. [Google Scholar] [CrossRef]
- Bhushan, S.; Schuppe, H.C.; Fijak, M.; Meinhardt, A. Testicular infection: Microorganisms, clinical implications and host-pathogen interaction. J. Reprod. Immunol. 2009, 83, 164–167. [Google Scholar] [CrossRef]
- Joguet, G.; Mansuy, J.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef]
- Halabi, J.; Jagger, B.W.; Salazar, V.; Winkler, E.S.; White, J.P.; Humphrey, P.A.; Hirsch, A.J.; Streblow, D.N.; Diamond, M.S.; Moley, K. Zika virus causes acute and chronic prostatitis in mice and macaques. J. Infect. Dis. 2020, 221, 1506–1517. [Google Scholar] [CrossRef]
- Uraki, R.; Hwang, J.; Jurado, K.A.; Householder, S.; Yockey, L.J.; Hastings, A.K.; Homer, R.J.; Iwasaki, A.; Fikrig, E. Zika virus causes testicular atrophy. Sci. Adv. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Huits, R.; De Smet, B.; Ariën, K.K.; Van Esbroeck, M.; Bottieau, E.; Cnops, L. Zika virus in semen: A prospective cohort study of symptomatic travellers returning to Belgium. Bull. World Health Organ. 2017, 95, 802–809. [Google Scholar] [CrossRef]
- Wu, S.; Yan, M.; Ge, R.; Cheng, C.Y. Crosstalk between Sertoli and Germ Cells in Male Fertility. Trends Mol. Med. 2020, 26, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.Y.; Cheng, C.Y. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 2004, 26, 978–992. [Google Scholar] [CrossRef] [PubMed]
- McCarrey, J.R.; Berg, W.M.; Paragioudakis, S.J.; Zhang, P.L.; Dilworth, D.D.; Arnold, B.L.; Rossi, J.J. Differential Transcription of Pgk Genes during Spermatogenesis in the Mouse. Dev. Biol. 1992, 168, 160–168. [Google Scholar] [CrossRef]
- Alvarado-Arnez, L.E. International Journal of Infectious Diseases. Int. J. Infect. Dis. 2018, 76, 126–127. [Google Scholar]
- Shamim, M.; Zohaib, S.; Naqvi, G. Case Report Dengue fever associated with acute scrotal oedema: Two case reports. J. Pakistan Med. Assoc. 2011, 61, 601–603. [Google Scholar]
- Molton, J.S.; Low, I.; Ming, M.; Choy, J.; Poh, P.; Aw, K.; Hibberd, M.L.; Tambyah, P.A.; Wilder-Smith, A. Dengue virus not detected in human semen. J. Travel Med. 2018, 25, 1–3. [Google Scholar] [CrossRef]
- Lalle, E.; Colavita, F.; Iannetta, M.; Teklè, S.G.; Carletti, F.; Scorzolini, L.; Vincenti, D.; Castilletti, C.; Ippolito, G.; Capobianchi, M.R.; et al. Prolonged detection of dengue virus RNA in the semen of a man returning from Thailand to Italy, January 2018. Euro Surveill. 2018, 23, 2–4. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Rapid Risk Assessment—Sexual Transmission of Dengue in Spain. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-sexual-transmission-dengue-spain (accessed on 15 April 2020).
- Barbosa, C.M.; Di Paola, N.; Cunha, M.P.; Rodrigues-Jesus, M.J.; Araujo, D.B.; Silveira, V.B.; Leal, F.B.; Mesquita, F.S.; Botosso, V.F.; Zanotto, P.M.A.; et al. Yellow Fever Virus RNA in Urine and Semen of Convalescent Patient, Brazil. Emerg. Infect. Dis. 2018, 24, 176–178. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, X.; Liu, Y.; Li, Y.; Long, S.; Gu, C.; Ye, J.; Xie, S.; Cao, S. Japanese Encephalitis Virus infection induces inflammation of swine testis through RIG-I—NF-ĸB signaling pathway. Vet. Microbiol. 2019, 238, 108430. [Google Scholar] [CrossRef]
- Han, D.; Liu, Z.; Yan, K. Immunology of the Testis and Privileged Sites. Encycl. Immunobiol. 2016, 5, 46–53. [Google Scholar]
- Smith, R.D.; Konoplev, S.; DeCourten-Myers, G.; Brown, T. West Nile Virus Encephalitis with Myositis and Orchitis. Case Stud. Hum. Pathol. 2004, 35, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Moizéis, R.N.C.; Fernandes, T.A.A.M.; Guedes, P.M.D.M.; Pereira, H.W.B.; Lanza, D.C.F.; Azevedo, J.W.V.; Galvão, J.M.A.; Fernandes, J.V. Chikungunya fever: A threat to global public health. Pathog. Glob. Health 2018, 112, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.; Soares, G.; França, V.; Rocha, D.; Solano, B.; Souza, D.F.; Botelho, M.; Soares, P.; Araujo, A.; Carvalho, Y.; et al. IDCases Prolonged shedding of Chikungunya virus in semen and urine: A new perspective for diagnosis and implications for transmission. IDCases 2016, 6, 100–103. [Google Scholar]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le Roux, K.; Prevost, M.C.; Fsihi, H.; et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, 804–817. [Google Scholar] [CrossRef]
- Vouga, M.; Baud, D. Imaging of congenital Zika virus infection: The route to identification of prognostic factors. Prenat. Diagn. 2016, 36, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Das Neves Almeida, R.; Racine, T.; Magalhães, K.; Kobinger, G. Zika Virus Vaccines: Challenges and Perspectives. Vaccines 2018, 6, 62. [Google Scholar] [CrossRef]
- Brault, A.C.; Domi, A.; McDonald, E.M.; Talmi-Fran, D.; McCurley, N.; Basu, R.; Robinson, H.L.; Hellerstein, M.; Duggal, N.K.; Bowen, R.A.; et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R.; Dhama, K.; Sachan, S.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Kumar, D.; Singh, R.K.; Iqbal, H.M.N.; et al. Advances in developing therapies to combat zika virus: Current knowledge and future perspectives. Front. Microbiol. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Morrison, C. DNA vaccines against Zika virus speed into clinical trials. Nat. Publ. Gr. 2016, 15, 521–522. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Q.; Lai, H. Development of Antibody Therapeutics against Flaviviruses. Int. J. Mol. Sci. 2018, 19, 1–26. [Google Scholar]
- Beaver, J.T.; Lelutiu, N.; Habib, R.; Skountzou, I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.S.; Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Bagasra, O.; Addanki, K.C.; Goodwin, G.R.; Hughes, B.W.; Pandey, P.; McLean, E. Cellular Targets and Receptor of Sexual Transmission of Zika Virus. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 679–686. [Google Scholar] [CrossRef]
- Fernandez, E.; Dejnirattisai, W.; Cao, B.; Scheaffer, S.M.; Supasa, P.; Wongwiwat, W.; Esakky, P.; Drury, A.; Mongkolsapaya, J.; Moley, K.H.; et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 2017, 18, 1261–1269. [Google Scholar] [CrossRef]
- Griffin, B.D.; Muthumani, K.; Warner, B.M.; Majer, A.; Hagan, M.; Audet, J.; Stein, D.R.; Ranadheera, C.; Racine, T.; De LaVega, M.A.; et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Zou, J.; Xie, X.; Luo, H.; Shan, C.; Muruato, A.E.; Weaver, S.C.; Wang, T.; Shi, P.Y. A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity. EBioMedicine 2018, 36, 92–102. [Google Scholar] [CrossRef]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Xu, K.; Song, Y.; Dai, L.; Zhang, Y.; Lu, X.; Xie, Y.; Zhang, H.; Cheng, T.; Wang, Q.; Huang, Q.; et al. Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage. J. Virol. 2018, 92, 1–16. [Google Scholar] [CrossRef]
- De La Vega, M.; Piret, J.; Griffin, B.D.; Rhéaume, C.; Venable, M.; Carbonneau, J.; Couture, C.; Almeida, N.; Tremblay, R.R.; Magalhães, K.G.; et al. Zika-Induced Male Infertility in Mice Is Potentially Reversible and Preventable by Deoxyribonucleic Acid Immunization. J. Infect. Dis. 2018, 219, 365–374. [Google Scholar] [CrossRef]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Joshi, A. Recent advances in Zika virus vaccines. Viruses 2018, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Meng, Y.; Tian, F.; Li, W.; Zou, P.; Wang, Q.; Xu, W.; Wang, Y.; Xia, M.; Hu, J.; et al. A Peptide-Based Virus Inactivator Protects Male Mice Against Zika Virus-Induced Damage of Testicular Tissue. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Simanjuntak, Y.; Liang, J.J.; Chen, S.Y.; Li, J.K.; Lee, Y.L.; Wu, H.C.; Lin, Y.L. Ebselen alleviates testicular pathology in mice with Zika virus infection and prevents its sexual transmission. PLoS Pathog. 2018, 14, 1–23. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, R.d.N.; Braz-de-Melo, H.A.; Santos, I.d.O.; Corrêa, R.; Kobinger, G.P.; Magalhaes, K.G. The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells 2020, 9, 1006. https://doi.org/10.3390/cells9041006
Almeida RdN, Braz-de-Melo HA, Santos IdO, Corrêa R, Kobinger GP, Magalhaes KG. The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells. 2020; 9(4):1006. https://doi.org/10.3390/cells9041006
Chicago/Turabian StyleAlmeida, Raquel das Neves, Heloisa Antoniella Braz-de-Melo, Igor de Oliveira Santos, Rafael Corrêa, Gary P. Kobinger, and Kelly Grace Magalhaes. 2020. "The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology" Cells 9, no. 4: 1006. https://doi.org/10.3390/cells9041006
APA StyleAlmeida, R. d. N., Braz-de-Melo, H. A., Santos, I. d. O., Corrêa, R., Kobinger, G. P., & Magalhaes, K. G. (2020). The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells, 9(4), 1006. https://doi.org/10.3390/cells9041006




