Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents
2.3. AEC Isolation
2.4. Cell Culture and CB Agonist/Antagonist Treatments
2.5. Real-Time qPCR
2.6. Cannabinoid Receptor Binding Assay
2.7. ELISA Detection of Interleukins in AEC CM
2.8. Statistical Analyses
3. Results
3.1. The ECS Was Modulated in AEC during Pregnancy
3.2. Cannabinoid Receptor Binding Activity of AEC Was Higher at Middle and Late Stage of Gestation
3.3. Interleukin Expression Profile Was Strictly LPS- and Gestational-Dependent
3.4. ECS Controlled the Immune Activities of AEC at Middle Stage of Gestation
Interleukin Gene Expression Profile in AEC Was Mainly Modulated by CB2
3.5. Interleukin Release Was CB-Dependent
3.6. AEC’s Immunomodulatory Activity Was Dependent on CB Receptor Signaling
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Cole, B.J. Human Amniotic Membrane-Derived Products in Sports Medicine: Basic Science, Early Results, and Potential Clinical Applications. Am. J. Sports Med. 2016, 44, 2425–2434. [Google Scholar] [CrossRef]
- Ananth, C.V.; Oyelese, Y.; Srinivas, N.; Yeo, L.; Vintzileos, A.M. Preterm premature rupture of membranes, intrauterine infection, and oligohydramnios: Risk factors for placental abruption. Obstet. Gynecol. 2004, 104, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Romero, R.; Gervasi, M.T.; Kim, J.S.; Yoo, W.; Lee, D.C.; Mittal, P.; Erez, O.; Kusanovic, J.P.; Hassan, S.S.; et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab. Investig. 2009, 89, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Grzela, T. Amniotic membrane: New concepts for an old dressing. Wound Repair Regen. 2014, 22, 451–456. [Google Scholar] [CrossRef]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 162. [Google Scholar] [CrossRef]
- Favaron, P.O.; Carvalho, R.C.; Borghesi, J.; Anunciação, A.R.A.; Miglino, M.A. The Amniotic Membrane: Development and Potential Applications—A Review. Reprod. Domest. Anim. 2015, 50, 881–892. [Google Scholar] [CrossRef]
- Eskandarlou, M.; Azimi, M.; Rabiee, S.; Seif Rabiee, M.A. The Healing Effect of Amniotic Membrane in Burn Patients. World J. Plast. Surg. 2016, 5, 39–44. [Google Scholar]
- Lo, V.; Pope, E. Amniotic membrane use in dermatology. Int. J. Dermatol. 2009, 48, 935–940. [Google Scholar] [CrossRef]
- Parolini, O.; Caruso, M. Review: Preclinical studies on placenta-derived cells and amniotic membrane: An update. Placenta 2011, 32, S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Miki, T. Amnion-derived stem cells: In quest of clinical applications. Stem Cell Res. Ther. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Berardinelli, P.; Mauro, A.; Valbonetti, L.; Sanyal, H.; Canciello, A.; Greco, L.; Muttini, A.; Gatta, V.; et al. Placental Stem Cells from Domestic Animals. Cell Transplant. 2018, 27, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: Potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006, 2, 133–141. [Google Scholar] [CrossRef]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The Immunomodulatory Properties of Amniotic Cells. Cell Transplant. 2018, 27, 31–44. [Google Scholar] [CrossRef]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Mayhew, E.; Word, R.A.; McCulley, J.P.; Alizadeh, H. Immunosuppressive Factors Secreted by Human Amniotic Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2005, 46, 900. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.V.; Parolini, O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32, S320–S325. [Google Scholar] [CrossRef]
- Insausti, C.L.; Blanquer, M.; García-Hernández, A.M.; Castellanos, G.; Moraleda, J.M. Amniotic membrane-derived stem cells: Immunomodulatory properties and potential clinical application. Stem Cells Cloning Adv. Appl. 2014, 7, 53–63. [Google Scholar] [CrossRef]
- Silini, A.R.; Magatti, M.; Cargnoni, A.; Parolini, O. Is Immune Modulation the Mechanism Underlying the Beneficial Effects of Amniotic Cells and Their Derivatives in Regenerative Medicine? Cell Transplant. 2017, 26, 531–539. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Qian, B.Z. Inflammation fires up cancer metastasis. Semin. Cancer Biol. 2017, 47, 170–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of cancer-promoting inflammation: A new approach for anticancer therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Flores-Espinosa, P.; Pineda-Torres, M.; Vega-Sánchez, R.; Estrada-Gutiérrez, G.; Espejel-Nuñez, A.; Flores-Pliego, A.; Maida-Claros, R.; Paredes-Vivas, Y.; Morales-Méndez, I.; Sosa-González, I.; et al. Progesterone Elicits an Inhibitory Effect upon LPS-Induced Innate Immune Response in Pre-Labor Human Amniotic Epithelium. Am. J. Reprod. Immunol. 2014, 71, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Wolbank, S.; Hildner, F.; Redl, H.; van Griensven, M.; Gabriel, C.; Hennerbichler, S. Impact of human amniotic membrane preparation on release of angiogenic factors. J. Tissue Eng. Regen. Med. 2009, 3, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Grzywocz, Z.; Pius-Sadowska, E.; Klos, P.; Gryzik, M.; Wasilewska, D.; Aleksandrowicz, B.; Dworczynska, M.; Sabalinska, S.; Hoser, G.; Machalinski, B.; et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem. Cytobiol. 2014, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Pianta, S.; Magatti, M.; Sedlmayr, P.; Parolini, O. Characterization of the Conditioned Medium from Amniotic Membrane Cells: Prostaglandins as Key Effectors of Its Immunomodulatory Activity. PLoS ONE 2012, 7, e46956. [Google Scholar] [CrossRef]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in culture expanded human amniotic epithelial cells: Implications for potential therapeutic applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human Mesenchymal Stem Cells from Adipose Tissue and Amnion Influence T-Cells Depending on Stimulation Method and Presence of Other Immune Cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef]
- Kranz, A.; Wagner, D.-C.; Kamprad, M.; Scholz, M.; Schmidt, U.R.; Nitzsche, F.; Aberman, Z.; Emmrich, F.; Riegelsberger, U.-M.; Boltze, J. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010, 1315, 128–136. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Gatta, V.; Bernabò, N.; Berardinelli, P.; Mauro, A.; Martelli, A.; Valbonetti, L.; Muttini, A.; Di Giacinto, O.; et al. Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1594–e1608. [Google Scholar] [CrossRef]
- Canciello, A.; Russo, V.; Berardinelli, P.; Bernabò, N.; Muttini, A.; Mattioli, M.; Barboni, B. Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties. Sci. Rep. 2017, 7, 3761. [Google Scholar] [CrossRef] [PubMed]
- Canciello, A.; Teti, G.; Mazzotti, E.; Falconi, M.; Russo, V.; Giordano, A.; Barboni, B. Progesterone Prolongs Viability and Anti-inflammatory Functions of Explanted Preterm Ovine Amniotic Membrane. Front. Bioeng. Biotechnol. 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Blach-Olszewska, Z.; Gejdel, E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta 1997, 18, 441–446. [Google Scholar] [CrossRef]
- Simpson, K.L.; Keelan, J.A.; Mitchell, M.D. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J. Clin. Endocrinol. Metab. 1998, 83, 4332–4337. [Google Scholar] [CrossRef][Green Version]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Gowran, A.; McKayed, K.; Campbell, V.A. The cannabinoid receptor type 1 is essential for mesenchymal stem cell survival and differentiation: Implications for bone health. Stem Cells Int. 2013. [Google Scholar] [CrossRef]
- Chiurchiù, V. Endocannabinoids and Immunity. Cannabis Cannabinoid Res. 2016, 1, 59–66. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Chiurchiù, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE 2010, 5, e8688. [Google Scholar] [CrossRef]
- Kaplan, B.L.F. The role of CB1 in immune modulation by cannabinoids. Pharmacol. Ther. 2013, 137, 365–374. [Google Scholar] [CrossRef]
- Rossi, F.; Bernardo, M.E.; Bellini, G.; Luongo, L.; Conforti, A.; Manzo, I.; Guida, F.; Cristino, L.; Imperatore, R.; Petrosino, S.; et al. The Cannabinoid Receptor Type 2 as Mediator of Mesenchymal Stromal Cell Immunosuppressive Properties. PLoS ONE 2013, 8, e80022. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xiao, D.; Xu, Y.; Zhao, J.; Jiang, L.; Hu, X.; Zhang, Y.; Yu, L. Up-regulation of immunomodulatory effects of mouse bone-marrow derived mesenchymal stem cells by tetrahydrocannabinol pre-treatment involving cannabinoid receptor CB2. Oncotarget 2016, 7, 6436–6447. [Google Scholar] [CrossRef] [PubMed]
- Barone, R. Comparative Anatomy of Domestic Mammals; Edagricole, Ed.; Vigot: Bologna, Italy, 2003. [Google Scholar]
- Gomez, O.; Sanchez-Rodriguez, A.; Le, M.Q.U.; Sanchez-Caro, C.; Molina-Holgado, F.; Molina-Holgado, E. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 2011, 163, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Bort, A.; Alvarado-Vazquez, P.A.; Moracho-Vilrriales, C.; Virga, K.G.; Gumina, G.; Romero-Sandoval, A.; Asbill, S. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol. Pain 2017, 13, 1744806916688220. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Christou, I.; Kapellos, T.S.; Buchan, A.; Brodermann, M.H.; Gianella-Borradori, M.; Russell, A.; Iqbal, A.J.; Greaves, D.R. Primary macrophage chemotaxis induced by cannabinoid receptor 2 agonists occurs independently of the CB2 receptor. Sci. Rep. 2015, 5, 10682. [Google Scholar] [CrossRef]
- Morten, V. Comparative Placentation. In Essentials of Domestic Animal Embryology; Saunders Elsevier, Ed.; Saunders: Oxford, UK, 2010; Volume 1, pp. 104–119. ISBN 978-0-7020-2899-1. [Google Scholar]
- Barboni, B.; Russo, V.; Curini, V.; Martelli, A.; Berardinelli, P.; Mauro, A.; Mattioli, M.; Marchisio, M.; Bonassi Signoroni, P.; Parolini, O.; et al. Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell Rev. Rep. 2014, 10, 725–741. [Google Scholar] [CrossRef]
- Cecconi, S.; Rapino, C.; Di Nisio, V.; Rossi, G.; Maccarrone, M. The (endo)cannabinoid signaling in female reproduction: What are the latest advances? Prog. Lipid Res. 2020, 77, 101019. [Google Scholar] [CrossRef]
- Correa, F.; Wolfson, M.L.; Valchi, P.; Aisemberg, J.; Franchi, A.M. Endocannabinoid system and pregnancy. Reproduction 2016, 152, R191–R200. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Almada, M.; Costa, M.A.; Teixeira, N.A. The Endocannabinoid System in the Postimplantation Period: A Role during Decidualization and Placentation. Int. J. Endocrinol. 2013, 2013, 510540. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Taylor, A.H.; Lam, P.M.W.; Marczylo, T.H.; Bell, S.C.; Konje, J.C.; Teixeira, N.A. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: A role in uterine remodelling. Int. J. Biochem. Cell Biol. 2010, 42, 1884–1892. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat. 2005, 77, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dey, S.K. Endocannabinoid Signaling in Female Reproduction. ACS Chem. Neurosci. 2012, 3, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Trabucco, E.; Acone, G.; Marenna, A.; Pierantoni, R.; Cacciola, G.; Chioccarelli, T.; Mackie, K.; Fasano, S.; Colacurci, N.; Meccariello, R.; et al. Endocannabinoid System in First Trimester Placenta: Low FAAH and High CB1 Expression Characterize Spontaneous Miscarriage. Placenta 2009, 30, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Finney, M.; Lam, P.M.W.; Konje, J.C. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod. Biol. Endocrinol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, H.; Dey, S.K. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS ONE 2008, 3, e3320. [Google Scholar] [CrossRef]
- Habayeb, O.M.H.; Taylor, A.H.; Finney, M.; Evans, M.D.; Konje, J.C. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. J. Am. Med. Assoc. 2008, 299, 1135–1136. [Google Scholar]
- Keelan, J.A.; Blumenstein, M.; Helliwell, R.J.A.; Sato, T.A.; Marvin, K.W.; Mitchell, M.D. Cytokines, prostaglandins and parturition—A review. Placenta 2003, 24, S33–S46. [Google Scholar] [CrossRef] [PubMed]
- Dealtry, G.B.; O’Farrell, M.K.; Fernandez, N. The Th2 cytokine environment of the placenta. Int. Arch. Allergy Immunol. 2000, 123, 107–119. [Google Scholar] [CrossRef]
- Romero, R.; Parvizi, S.T.; Oyarzun, E.; Mazor, M.; Wu, Y.K.; Avila, C.; Athanassiadis, A.P.; Mitchell, M.D. Amniotic fluid interleukin-1 in spontaneous labor at term. J. Reprod. Med. Obstet. Gynecol. 1990, 35, 235–238. [Google Scholar]
- Laham, N.; Brennecke, S.P.; Bendtzen, K.; Rice, G.E. Tumour necrosis factor α during human pregnancy and labour: Maternal plasma and amniotic fluid concentrations and release from intrauterine tissues. Eur. J. Endocrinol. 1994, 131, 607–614. [Google Scholar] [CrossRef]
- Bowen, J.M.; Chamley, L.; Keelan, J.A.; Mitchell, M.D. Cytokines of the placenta and extra-placental membranes: Roles and regulation during human pregnancy and parturition. Placenta 2002, 23, 257–273. [Google Scholar] [CrossRef]
- Erlebacher, A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013, 13, 23–33. [Google Scholar] [CrossRef]
- Kuon, R.J.; Strowitzki, T.; Sohn, C.; Daniel, V.; Toth, B. Immune profiling in patients with recurrent miscarriage. J. Reprod. Immunol. 2015, 108, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and Clinical Significance of Sterile Intra-amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Dietl, J. The pathogenesis of pre-eclampsia: New aspects. J. Perinat. Med. 2000, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A Multi-Faceted Agent of Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Hanna, N.N.; Fast, L.D.; Shaw, S.K.; Berg, G.; Padbury, J.F.; Romero, R.; Sharma, S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am. J. Obstet. Gynecol. 2009, 200, 308.e1–308.e9. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, D.W.; Novy, M.J.; Witkin, S.S.; Gravett, M.G. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am. J. Obstet. Gynecol. 2003, 188, 252–263. [Google Scholar] [CrossRef]
- Fortunato, S.J.; Menon, R.; Lombardi, S.J.; LaFleur, B. Interleukin-10 inhibition of gelatinases in fetal membranes: Therapeutic implications in preterm premature rupture of membranes. Obstet. Gynecol. 2001, 98, 284–288. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Lazzari, B.; Perrini, C.; Pizzi, F.; Stella, A.; Cremonesi, F.; Capra, E. MicroRNAs of Equine Amniotic Mesenchymal Cell-derived Microvesicles and Their Involvement in Anti-inflammatory Processes. Cell Transplant. 2018, 27, 45–54. [Google Scholar] [CrossRef]
- Cargnoni, A.; Gibelli, L.; Tosini, A.; Signoroni, P.B.; Nassuato, C.; Arienti, D.; Lombardi, G.; Albertini, A.; Wengler, G.S.; Parolini, O. Transplantation of Allogeneic and Xenogeneic Placenta-Derived Cells Reduces Bleomycin-Induced Lung Fibrosis. Cell Transplant. 2009, 18, 405–422. [Google Scholar] [CrossRef]
- Muttini, A.; Valbonetti, L.; Abate, M.; Colosimo, A.; Curini, V.; Mauro, A.; Berardinelli, P.; Russo, V.; Cocciolone, D.; Marchisio, M.; et al. Ovine amniotic epithelial cells: In vitro characterization and transplantation into equine superficial digital flexor tendon spontaneous defects. Res. Vet. Sci. 2013, 94, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Chan, S.T.; Lo, C.Y.; Deane, J.A.; McDonald, C.A.; Bernard, C.C.; Wallace, E.M.; Lim, R. Amnion cell-mediated immune modulation following bleomycin challenge: Controlling the regulatory T cell response. Stem Cell Res. Ther. 2015, 6, 8. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Williams, E.D.; Sievert, W. Transplantation of Human Amnion Epithelial Cells Reduces Hepatic Fibrosis in Immunocompetent CCl 4 -Treated Mice. Cell Transplant. 2010, 19, 1157–1168. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.-Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human Amniotic Epithelial Cell Transplantation Induces Markers of Alternative Macrophage Activation and Reduces Established Hepatic Fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef]
- Moodley, Y.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.; Trounson, A.; Manuelpillai, U. Human Amnion Epithelial Cell Transplantation Abrogates Lung Fibrosis and Augments Repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef]
- Murphy, S.; Lim, R.; Dickinson, H.; Acharya, R.; Rosli, S.; Jenkin, G.; Wallace, E. Human Amnion Epithelial Cells Prevent Bleomycin-Induced Lung Injury and Preserve Lung Function. Cell Transplant. 2011, 20, 909–924. [Google Scholar] [CrossRef]
- Murphy, S.V.; Shiyun, S.C.; Tan, J.L.; Chan, S.; Jenkin, G.; Wallace, E.M.; Lim, R. Human Amnion Epithelial Cells do Not Abrogate Pulmonary Fibrosis in Mice with Impaired Macrophage Function. Cell Transplant. 2012, 21, 1477–1492. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles Tendon Regeneration can be Improved by Amniotic Epithelial Cell Allotransplantation. Cell Transplant. 2012, 21, 2377–2395. [Google Scholar] [CrossRef]
- Vosdoganes, P.; Wallace, E.M.; Chan, S.T.; Acharya, R.; Moss, T.J.M.; Lim, R. Human Amnion Epithelial Cells Repair Established Lung Injury. Cell Transplant. 2013, 22, 1337–1349. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Rossi, D.; Tassan, S.; Perego, R.; Cremonesi, F.; Parolini, O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: Immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013, 22, 3015–3024. [Google Scholar] [CrossRef]
- Parolini, O.; Souza-Moreira, L.; O’Valle, F.; Magatti, M.; Hernandez-Cortes, P.; Gonzalez-Rey, E.; Delgado, M. Therapeutic Effect of Human Amniotic Membrane-Derived Cells on Experimental Arthritis and Other Inflammatory Disorders. Arthritis Rheumatol. 2014, 66, 327–339. [Google Scholar] [CrossRef]
- Pischiutta, F.; Brunelli, L.; Romele, P.; Silini, A.; Sammali, E.; Paracchini, L.; Marchini, S.; Talamini, L.; Bigini, P.; Boncoraglio, G.B.; et al. Protection of Brain Injury by Amniotic Mesenchymal Stromal Cell-Secreted Metabolites. Crit. Care Med. 2016, 44, e1118–e1131. [Google Scholar] [CrossRef]
- Yan, Z.-J.; Zhang, P.; Hu, Y.-Q.; Zhang, H.-T.; Hong, S.-Q.; Zhou, H.-L.; Zhang, M.-Y.; Xu, R.-X. Neural Stem-Like Cells Derived from Human Amnion Tissue are Effective in Treating Traumatic Brain Injury in Rat. Neurochem. Res. 2013, 38, 1022–1033. [Google Scholar] [CrossRef]
- Ventura, C.; Cantoni, S.; Bianchi, F.; Lionetti, V.; Cavallini, C.; Scarlata, I.; Foroni, L.; Maioli, M.; Bonsi, L.; Alviano, F.; et al. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J. Biol. Chem. 2007, 282, 14243–14252. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef]
- Fang, C.-H.; Jin, J.; Joe, J.-H.; Song, Y.-S.; So, B.-I.; Lim, S.M.; Cheon, G.J.; Woo, S.-K.; Ra, J.-C.; Lee, Y.-Y.; et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: Comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef]
- Ichim, C. V Revisiting immunosurveillance and immunostimulation: Implications for cancer immunotherapy. J. Transl. Med. 2005, 3, 8. [Google Scholar] [CrossRef]
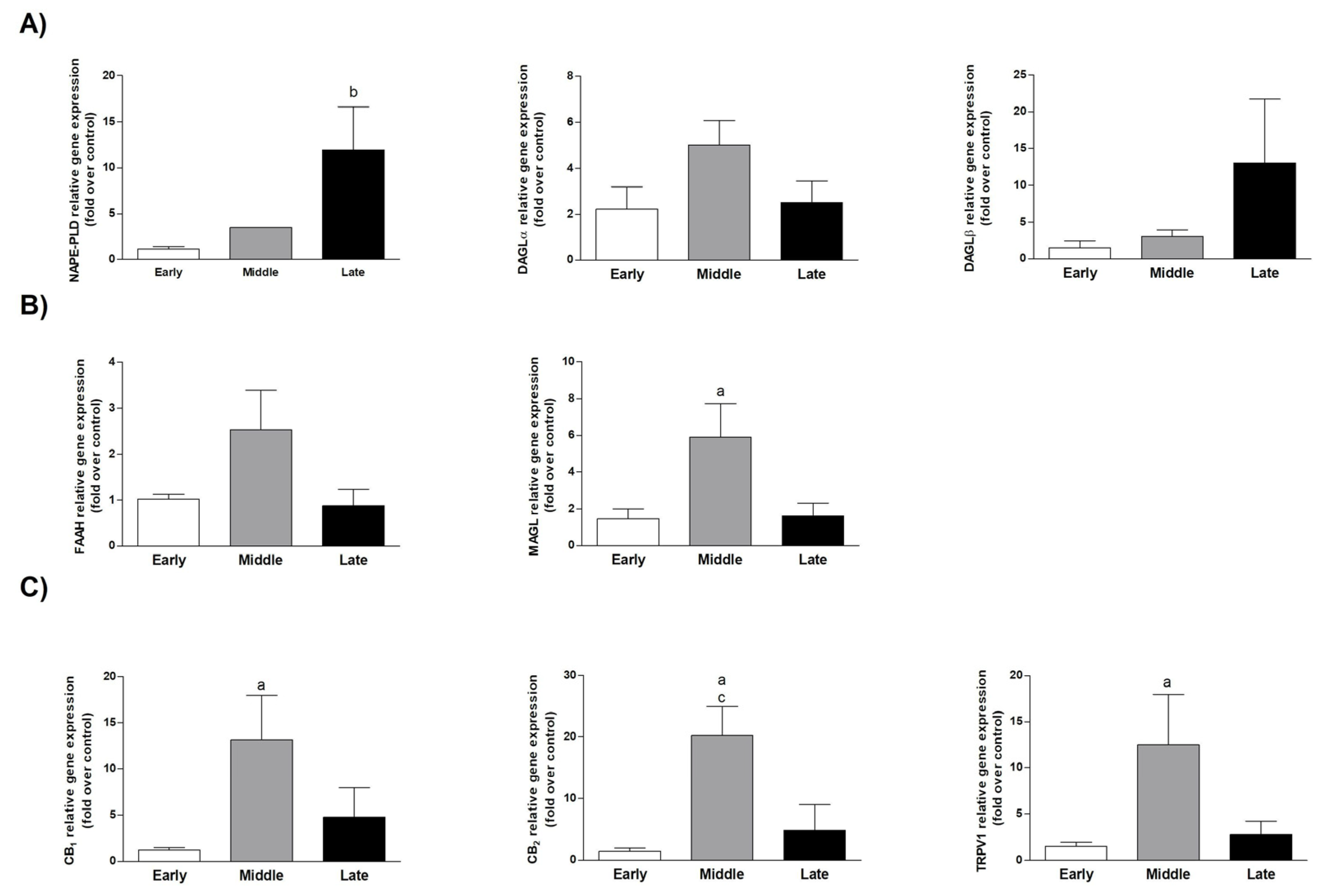
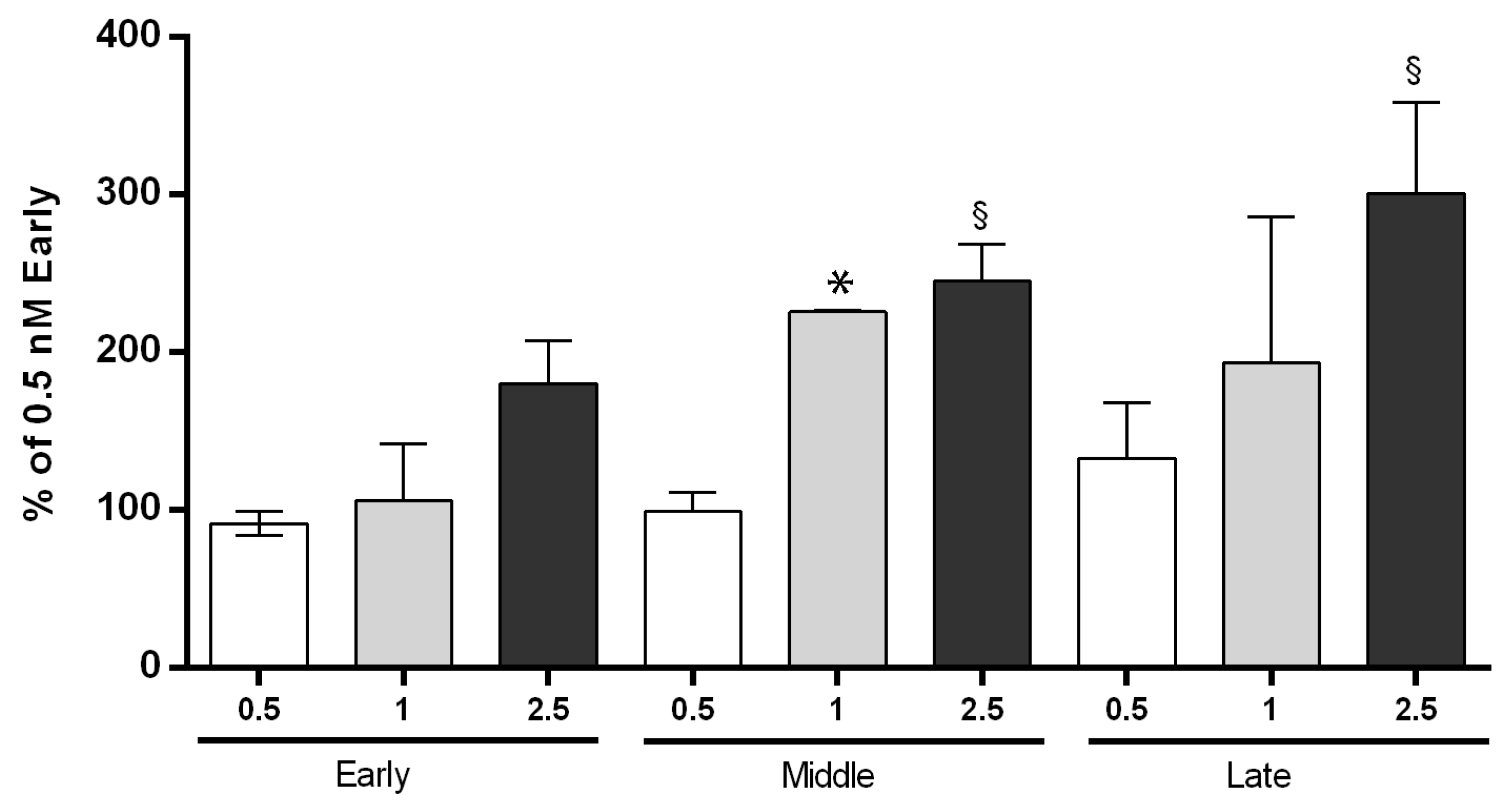


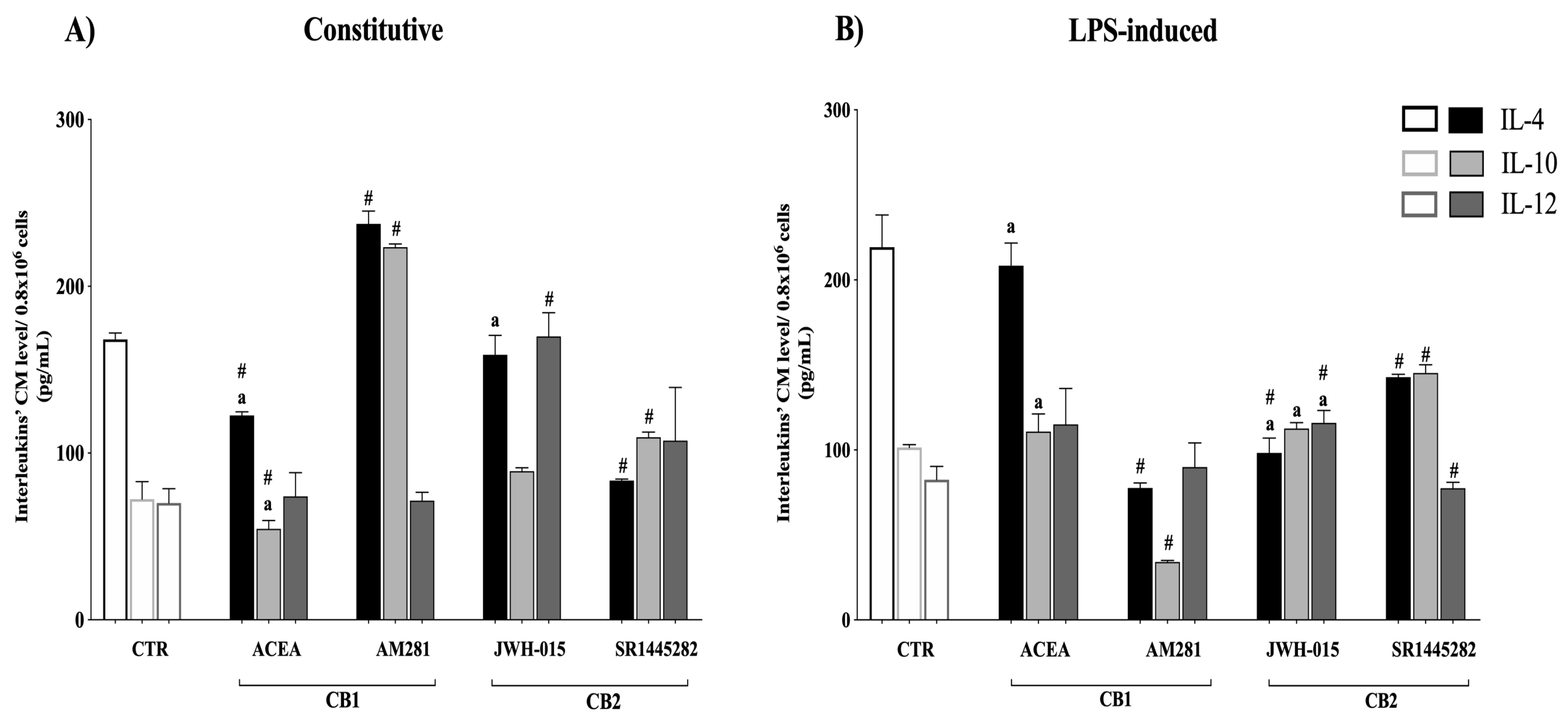


| Sheep Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| NAPE-PLD | CTGTCTTGGGGCCTTGGAAC | GGCTCTAAATAATGCTCACTTGC |
| FAAH | CCTTGGGAGCAGAGGTTTCA | AGAGACTTGAGGTTGCTGGC |
| DAGLα | TGCTGAGCGAGGATGCTATG | CAAGTCACTGGGGTGAGTCC |
| DAGLβ | CACCGAGGTAGTCACCCACT | CTGATCACCTCCGACCAAGT |
| MAGL | GAAGCGACGTTCACAGGAGA | GCAGCAGTCCTGGAAGATCC |
| CB1 | TGACCATGTCTGTGTCCACG | AGACGCTTCTGGGTTTCGAG |
| CB2 | CATCGACCGCTACCTCTGTC | AGGTAGGACACCAATGCAGC |
| TRPV1 | AACCAAGCCCCACAGCTTC | GGACAGCTGCCTGACACAC |
| IL-4 | AAGCCCTCAGCTAAGCTCAAGTC | AGGCATCACAGGCTCAAGTC |
| IL-10 | CCAGGATGGTGACTCGACTAG | TGGCTCTGCTCTCCCAGAAC |
| IL-12 | TCAAACCAGACCCACCCAAG | CACAGATGCCCATTCACTCC |
| GAPDH | TCGGAGTGAACGGATTTGGC | CCGTTCTCTGCCTTGACTGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, L.; Russo, V.; Rapino, C.; Di Germanio, C.; Fezza, F.; Bernabò, N.; Berardinelli, P.; Peserico, A.; Fazio, D.; Maccarrone, M.; et al. Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response. Cells 2020, 9, 1008. https://doi.org/10.3390/cells9041008
Greco L, Russo V, Rapino C, Di Germanio C, Fezza F, Bernabò N, Berardinelli P, Peserico A, Fazio D, Maccarrone M, et al. Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response. Cells. 2020; 9(4):1008. https://doi.org/10.3390/cells9041008
Chicago/Turabian StyleGreco, Luana, Valentina Russo, Cinzia Rapino, Clara Di Germanio, Filomena Fezza, Nicola Bernabò, Paolo Berardinelli, Alessia Peserico, Domenico Fazio, Mauro Maccarrone, and et al. 2020. "Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response" Cells 9, no. 4: 1008. https://doi.org/10.3390/cells9041008
APA StyleGreco, L., Russo, V., Rapino, C., Di Germanio, C., Fezza, F., Bernabò, N., Berardinelli, P., Peserico, A., Fazio, D., Maccarrone, M., Mattioli, M., & Barboni, B. (2020). Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response. Cells, 9(4), 1008. https://doi.org/10.3390/cells9041008










