The Solo Play of TERT Promoter Mutations
Abstract
1. Introduction
2. Telomerase Reverse Transcriptase Promoter (TERTp) Mutations
3. Cancer Distribution of TERTp Mutations
3.1. Gliomas and Glioblastoma (GBM)
3.2. Melanoma and Non-Melanoma Skin Carcinoma
3.3. Urothelial Bladder Cancer
3.4. Thyroid
3.5. Hepatocellular Carcinoma (HCC)
3.6. Cervical and Oral Head and Neck Squamous Cell Carcinoma (HNSCC)
3.7. The rs2853669 Polymorphism
4. Cancer Bias of TERTp Mutations
5. Exclusiveness of TERTp Mutations
6. Discussion
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alternative lengthening of telomeres |
| ATC | Anaplastic thyroid carcinoma |
| ATRX | α-Thalassemia/mental retardation syndrome X-linked |
| BCC | Basal cell carcinoma |
| CNS | Central nervous system |
| CNV | Copy number variant |
| DAXX | Death-domain-associated protein |
| DFS | Disease-free survival |
| DTC | Differentiated thyroid carcinoma |
| EGFR | Epidermal growth factor receptor |
| FTC | Follicular thyroid carcinoma |
| GBM | Glioblastoma multiforme |
| HBV | Hepatitis B virus |
| HBx | Hepatitis B X protein |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| IDH | Isocytrate dehydrogenase |
| OS | Overall survival |
| PDTC | Poorly differentiated thyroid carcinoma |
| PTC | Papillary thyroid carcinoma |
| ROS | Reactive oxygen species |
| SCC | Squamous cell carcinoma |
| TERT | Telomerase reverse transcriptase |
| TERTp | TERT promoter |
| TF | Transcription factor |
| TMZ | Temozolomide |
| TSS | Translational start site |
References
- Greider, C.W. Telomere length regulation. Annu. Rev. Biochem. 1996, 65, 337–365. [Google Scholar] [CrossRef]
- Robles-Espinoza, C.D.; Velasco-Herrera Mdel, C.; Hayward, N.K.; Adams, D.J. Telomere-regulating genes and the telomere interactome in familial cancers. Mol. Cancer Res. 2015, 13, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Kumar, R. TERT promoter mutations in telomere biology. Mutat. Res. 2017, 771, 15–31. [Google Scholar] [CrossRef]
- Leao, R.; Apolonio, J.D.; Lee, D.; Figueiredo, A.; Tabori, U.; Castelo-Branco, P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: Clinical impacts in cancer. J. Biomed. Sci. 2018, 25, 22. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Liu, L.; Lai, S.; Andrews, L.G.; Tollefsbol, T.O. Genetic and epigenetic modulation of telomerase activity in development and disease. Gene 2004, 340, 1–10. [Google Scholar] [CrossRef]
- Holt, S.E.; Wright, W.E.; Shay, J.W. Regulation of telomerase activity in immortal cell lines. Mol. Cell Biol. 1996, 16, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, S.; Kunimura, C.; Kikuchi, K.; Tahara, H.; Ohji, H.; Yamamoto, H.; Ide, T.; Utakoji, T. Telomerase activity in normal human epithelial cells. Oncogene 1996, 13, 433–439. [Google Scholar] [PubMed]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of telomerase in cancer. Cell Mol. Life Sci. 2016, 73, 1659–1670. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.L.; Westra, W.H.; et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef]
- Redon, S.; Reichenbach, P.; Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010, 38, 5797–5806. [Google Scholar] [CrossRef]
- Feng, J.; Funk, W.D.; Wang, S.S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA component of human telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Stewart, S.A.; Hahn, W.C.; O’Connor, B.F.; Banner, E.N.; Lundberg, A.S.; Modha, P.; Mizuno, H.; Brooks, M.W.; Fleming, M.; Zimonjic, D.B.; et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 12606–12611. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Fujiwara, T.; Inoue, M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008, 99, 1528–1538. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, X.; Xu, D. Cancer-specific telomerase reverse transcriptase (tert) promoter mutations: Biological and clinical implications. Genes 2016, 7, 38. [Google Scholar] [CrossRef]
- Bell, R.J.; Rube, H.T.; Xavier-Magalhaes, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding tert promoter mutations: A common path to immortality. Mol. Cancer Res. 2016, 14, 315–323. [Google Scholar] [CrossRef]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting telomere shortening in cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Hu, J.F.; Vu, T.H.; Giudice, L.C.; Hoffman, A.R. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998, 58, 4168–4172. [Google Scholar] [PubMed]
- Ulaner, G.A.; Hu, J.F.; Vu, T.H.; Giudice, L.C.; Hoffman, A.R. Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. Int. J. Cancer 2001, 91, 644–649. [Google Scholar] [CrossRef]
- Kang, S.S.; Kwon, T.; Kwon, D.Y.; Do, S.I. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 1999, 274, 13085–13090. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood 2008, 112, 2946–2955. [Google Scholar] [CrossRef]
- Heeg, S.; Hirt, N.; Queisser, A.; Schmieg, H.; Thaler, M.; Kunert, H.; Quante, M.; Goessel, G.; von Werder, A.; Harder, J.; et al. EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011, 102, 351–360. [Google Scholar] [CrossRef]
- Yang, K.; Zheng, D.; Deng, X.; Bai, L.; Xu, Y.; Cong, Y.S. Lysophosphatidic acid activates telomerase in ovarian cancer cells through hypoxia-inducible factor-1alpha and the PI3K pathway. J. Cell Biochem. 2008, 105, 1194–1201. [Google Scholar] [CrossRef]
- Pestana, A.; Vinagre, J.; Sobrinho-Simoes, M.; Soares, P. TERT biology and function in cancer: Beyond immortalisation. J. Mol. Endocrinol. 2017, 58, R129–R146. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Foster, S.A.; McDougall, J.K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996, 380, 79–82. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, C.; Lindvall, C.; Hou, M.; Ekedahl, J.; Lewensohn, R.; Yan, Z.; Yang, X.; Henriksson, M.; Blennow, E.; et al. Frequent amplification of the telomerase reverse transcriptase gene in human tumors. Cancer Res. 2000, 60, 6230–6235. [Google Scholar]
- Oh, S.T.; Kyo, S.; Laimins, L.A. Telomerase activation by human papillomavirus type 16 E6 protein: Induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 2001, 75, 5559–5566. [Google Scholar] [CrossRef]
- Veldman, T.; Horikawa, I.; Barrett, J.C.; Schlegel, R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 2001, 75, 4467–4472. [Google Scholar] [CrossRef]
- Paterlini-Brechot, P.; Saigo, K.; Murakami, Y.; Chami, M.; Gozuacik, D.; Mugnier, C.; Lagorce, D.; Brechot, C. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene 2003, 22, 3911–3916. [Google Scholar] [CrossRef]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Kramer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Arunkumar, G.; Revathidevi, S.; Arun, K.; Manikandan, M.; Rao, A.K.; Rajkumar, K.S.; Ajay, C.; Rajaraman, R.; Ramani, R.; et al. Erratum to: TERT promoter hot spot mutations are frequent in Indian cervical and oral squamous cell carcinomas. Tumour Biol. 2016, 37, 7005. [Google Scholar] [CrossRef]
- Annunziata, C.; Pezzuto, F.; Greggi, S.; Ionna, F.; Losito, S.; Botti, G.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Distinct profiles of TERT promoter mutations and telomerase expression in head and neck cancer and cervical carcinoma. Int. J. Cancer 2018, 143, 1153–1161. [Google Scholar] [CrossRef]
- Gaspar, T.B.; Sa, A.; Lopes, J.M.; Sobrinho-Simoes, M.; Soares, P.; Vinagre, J. Telomere maintenance mechanisms in cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 69, 8. [Google Scholar] [CrossRef]
- Arita, H.; Narita, Y.; Takami, H.; Fukushima, S.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013, 126, 939–941. [Google Scholar] [CrossRef]
- Guilleret, I.; Yan, P.; Grange, F.; Braunschweig, R.; Bosman, F.T.; Benhattar, J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int. J. Cancer 2002, 101, 335–341. [Google Scholar] [CrossRef]
- Guilleret, I.; Benhattar, J. Demethylation of the human telomerase catalytic subunit (hTERT) gene promoter reduced hTERT expression and telomerase activity and shortened telomeres. Exp. Cell Res. 2003, 289, 326–334. [Google Scholar] [CrossRef]
- Renaud, S.; Loukinov, D.; Abdullaev, Z.; Guilleret, I.; Bosman, F.T.; Lobanenkov, V.; Benhattar, J. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007, 35, 1245–1256. [Google Scholar] [CrossRef]
- Renaud, S.; Loukinov, D.; Bosman, F.T.; Lobanenkov, V.; Benhattar, J. CTCF binds the proximal exonic region of hTERT and inhibits its transcription. Nucleic Acids Res. 2005, 33, 6850–6860. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, J.; Kooter, J.M.; Overmeer, R.M.; Claassen-Kramer, D.; Meijer, C.J.; Snijders, P.J.; Steenbergen, R.D. hTERT promoter activity and CpG methylation in HPV-induced carcinogenesis. BMC Cancer 2010, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Tollefsbol, T.O. Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Front. Genet. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Seo, H.W.; Jung, E.S.; Kim, B.H.; Jung, G. The TERT promoter SNP rs2853669 decreases E2F1 transcription factor binding and increases mortality and recurrence risks in liver cancer. Oncotarget 2016, 7, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, D. Telomerase reverse transcriptase (TERT) in action: Cross-talking with epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef]
- Andres-Lencina, J.J.; Rachakonda, S.; Garcia-Casado, Z.; Srinivas, N.; Skorokhod, A.; Requena, C.; Soriano, V.; Kumar, R.; Nagore, E. TERT promoter mutation subtypes and survival in stage I and II melanoma patients. Int. J. Cancer 2018, 144, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Bielski, C.M.; Rinne, M.L.; Hahn, W.C.; Sellers, W.R.; Stegmeier, F.; Garraway, L.A.; Kryukov, G.V. TERT promoter mutations and monoallelic activation of TERT in cancer. Oncogenesis 2015, 4, e176. [Google Scholar] [CrossRef]
- Griewank, K.G.; Murali, R.; Schilling, B.; Schimming, T.; Moller, I.; Moll, I.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Schadendorf, D.; et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE 2013, 8, e80354. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef]
- Huang, D.S.; Wang, Z.; He, X.J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.Y.; Wang, W.; Jiang, X.T.; et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef]
- Johanns, T.M.; Fu, Y.; Kobayashi, D.K.; Mei, Y.; Dunn, I.F.; Mao, D.D.; Kim, A.H.; Dunn, G.P. High incidence of TERT mutation in brain tumor cell lines. Brain Tumor Pathol. 2016, 33, 222–227. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.L.; Sun, W.; Chandrasekharan, P.; Cheng, H.S.; Ying, Z.; Lakshmanan, M.; Raju, A.; Tenen, D.G.; Cheng, S.Y.; et al. Non-canonical NF-kappaB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat. Cell Biol. 2015, 17, 1327–1338. [Google Scholar] [CrossRef]
- Chen, C.; Han, S.; Meng, L.; Li, Z.; Zhang, X.; Wu, A. TERT promoter mutations lead to high transcriptional activity under hypoxia and temozolomide treatment and predict poor prognosis in gliomas. PLoS ONE 2014, 9, e100297. [Google Scholar] [CrossRef]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, X.; Chen, Y.; Chen, G.; Ma, Y.; Huang, K.; Zhang, Y.; Zhao, Q.; Winkler, C.A.; An, P.; et al. Telomerase reverse transcriptase promoter mutations in hepatitis B virus-associated hepatocellular carcinoma. Oncotarget 2016, 7, 27838–27847. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, S.H.; Kim, J.Y.; Kim, J.E.; Kim, T.M.; Lee, S.T.; Choi, S.H.; Park, S.H.; Kim, I.H. Expression level of hTERT is regulated by somatic mutation and common single nucleotide polymorphism at promoter region in glioblastoma. Oncotarget 2014, 5, 3399–3407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spiegl-Kreinecker, S.; Lotsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015, 17, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef]
- Li, C.; Wu, S.; Wang, H.; Bi, X.; Yang, Z.; Du, Y.; He, L.; Cai, Z.; Wang, J.; Fan, Z. The C228T mutation of TERT promoter frequently occurs in bladder cancer stem cells and contributes to tumorigenesis of bladder cancer. Oncotarget 2015, 6, 19542–19551. [Google Scholar] [CrossRef]
- Sizemore, G.M.; Pitarresi, J.R.; Balakrishnan, S.; Ostrowski, M.C. The ETS family of oncogenic transcription factors in solid tumours. Nat. Rev. Cancer 2017, 17, 337–351. [Google Scholar] [CrossRef]
- Bell, R.J.; Rube, H.T.; Kreig, A.; Mancini, A.; Fouse, S.D.; Nagarajan, R.P.; Choi, S.; Hong, C.; He, D.; Pekmezci, M.; et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015, 348, 1036–1039. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Khattar, E.; Boon, P.L.; Unal, B.; Fullwood, M.J.; Tergaonkar, V. Long-range chromatin interactions drive mutant tert promoter activation. Cancer Discov. 2016, 6, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Xavier-Magalhaes, A.; Woods, W.S.; Nguyen, K.T.; Amen, A.M.; Hayes, J.L.; Fellmann, C.; Gapinske, M.; McKinney, A.M.; Hong, C.; et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer Cell 2018, 34, 513–528. [Google Scholar] [CrossRef]
- Thompson, C.C.; Brown, T.A.; McKnight, S.L. Convergence of Ets-and notch-related structural motifs in a heteromeric DNA binding complex. Science 1991, 253, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Yamada, T. Molecular biology of the Ets family of transcription factors. Gene 2003, 303, 11–34. [Google Scholar] [CrossRef]
- LaMarco, K.; Thompson, C.C.; Byers, B.P.; Walton, E.M.; McKnight, S.L. Identification of Ets- and notch-related subunits in GA binding protein. Science 1991, 253, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.L.; Theodorescu, D.; Vogelstein, B.; Papadopoulos, N.; Cech, T.R. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015, 29, 2219–2224. [Google Scholar] [CrossRef]
- Stern, J.L.; Paucek, R.D.; Huang, F.W.; Ghandi, M.; Nwumeh, R.; Costello, J.C.; Cech, T.R. Allele-specific DNA methylation and its interplay with repressive histone marks at promoter-mutant tert genes. Cell Rep. 2017, 21, 3700–3707. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef]
- Liu, X.; Wu, G.; Shan, Y.; Hartmann, C.; von Deimling, A.; Xing, M. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle 2013, 12, 1637–1638. [Google Scholar] [CrossRef]
- Pekmezci, M.; Rice, T.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Hansen, H.; Sicotte, H.; Kollmeyer, T.M.; McCoy, L.S.; Sarkar, G.; et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017, 133, 1001–1016. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Yamasaki, K.; Matsushita, Y.; Nakamura, T.; Shimokawa, A.; Takami, H.; Tanaka, S.; Mukasa, A.; Shirahata, M.; Shimizu, S.; et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol. Commun. 2016, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cai, J.; Yan, W.; Zhang, W.; Wang, Y.; Chen, B.; Li, G.; Li, S.; Wu, C.; Yao, K.; et al. Classification based on mutations of TERT promoter and IDH characterizes subtypes in grade II/III gliomas. Neuro Oncol. 2016, 18, 1099–1108. [Google Scholar] [CrossRef]
- Mosrati, M.A.; Malmstrom, A.; Lysiak, M.; Krysztofiak, A.; Hallbeck, M.; Milos, P.; Hallbeck, A.L.; Bratthall, C.; Strandeus, M.; Stenmark-Askmalm, M.; et al. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget 2015, 6, 16663–16673. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Cruvinel-Carloni, A.; Vinagre, J.; Peixoto, J.; Catarino, T.A.; Campanella, N.C.; Menezes, W.; Becker, A.P.; de Almeida, G.C.; Matsushita, M.M.; et al. The prognostic impact of TERT promoter mutations in glioblastomas is modified by the rs2853669 single nucleotide polymorphism. Int. J. Cancer 2016, 139, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Miller, J.J.; Tummala, S.S.; Penson, T.; Iafrate, A.J.; Juratli, T.A.; Cahill, D.P. TERT promoter wild-type glioblastomas show distinct clinical features and frequent PI3K pathway mutations. Acta Neuropathol. Commun. 2018, 6, 106. [Google Scholar] [CrossRef]
- Griewank, K.G.; Murali, R.; Schilling, B.; Scholz, S.; Sucker, A.; Song, M.; Susskind, D.; Grabellus, F.; Zimmer, L.; Hillen, U.; et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br. J. Cancer 2013, 109, 497–501. [Google Scholar] [CrossRef]
- Heidenreich, B.; Nagore, E.; Rachakonda, P.S.; Garcia-Casado, Z.; Requena, C.; Traves, V.; Becker, J.; Soufir, N.; Hemminki, K.; Kumar, R. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat. Commun. 2014, 5, 3401. [Google Scholar] [CrossRef]
- Populo, H.; Boaventura, P.; Vinagre, J.; Batista, R.; Mendes, A.; Caldas, R.; Pardal, J.; Azevedo, F.; Honavar, M.; Guimaraes, I.; et al. TERT promoter mutations in skin cancer: The effects of sun exposure and X-irradiation. J. Invest. Dermatol. 2014, 134, 2251–2257. [Google Scholar] [CrossRef]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef]
- Ofner, R.; Ritter, C.; Heidenreich, B.; Kumar, R.; Ugurel, S.; Schrama, D.; Becker, J.C. Distribution of TERT promoter mutations in primary and metastatic melanomas in Austrian patients. J. Cancer Res. Clin. Oncol. 2017, 143, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Taheri, D.; Springer, S.; Cowan, M.; Guner, G.; Mendoza Rodriguez, M.A.; Wang, Y.; Kinde, I.; VandenBussche, C.J.; Olson, M.T.; et al. High prevalence of TERT promoter mutations in micropapillary urothelial carcinoma. Virchows Arch. 2016, 469, 427–434. [Google Scholar] [CrossRef]
- Cowan, M.; Springer, S.; Nguyen, D.; Taheri, D.; Guner, G.; Rodriguez, M.A.; Wang, Y.; Kinde, I.; VandenBussche, C.J.; Olson, M.T.; et al. High prevalence of TERT promoter mutations in primary squamous cell carcinoma of the urinary bladder. Mod. Pathol. 2016, 29, 511–515. [Google Scholar] [CrossRef]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flandez, M.; Marques, M.; Marquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef]
- Pezzuto, F.; Izzo, F.; Buonaguro, L.; Annunziata, C.; Tatangelo, F.; Botti, G.; Buonaguro, F.M.; Tornesello, M.L. Tumor specific mutations in TERT promoter and CTNNB1 gene in hepatitis B and hepatitis C related hepatocellular carcinoma. Oncotarget 2016, 7, 54253–54262. [Google Scholar] [CrossRef]
- Chen, Y.L.; Jeng, Y.M.; Chang, C.N.; Lee, H.J.; Hsu, H.C.; Lai, P.L.; Yuan, R.H. TERT promoter mutation in resectable hepatocellular carcinomas: A strong association with hepatitis C infection and absence of hepatitis B infection. Int. J. Surg. 2014, 12, 659–665. [Google Scholar] [CrossRef]
- Cevik, D.; Yildiz, G.; Ozturk, M. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J. Gastroenterol. 2015, 21, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, N.; Cao, J.; Sofiadis, A.; Dinets, A.; Zedenius, J.; Larsson, C.; Xu, D. The age-and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014, 33, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 9, E1130–E1136. [Google Scholar] [CrossRef]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Muzza, M.; Colombo, C.; Rossi, S.; Tosi, D.; Cirello, V.; Perrino, M.; De Leo, S.; Magnani, E.; Pignatti, E.; Vigo, B.; et al. Telomerase in differentiated thyroid cancer: Promoter mutations, expression and localization. Mol. Cell Endocrinol. 2015, 399, 288–295. [Google Scholar] [CrossRef] [PubMed]
- George, J.R.; Henderson, Y.C.; Williams, M.D.; Roberts, D.B.; Hei, H.; Lai, S.Y.; Clayman, G.L. Association of TERT promoter mutation, but not braf mutation, with increased mortality in PTC. J. Clin. Endocrinol. Metab. 2015, 100, E1550–E1559. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, R.; Qu, S.; Zhu, G.; Bishop, J.; Liu, X.; Sun, H.; Shan, Z.; Wang, E.; Luo, Y.; et al. Association of TERT promoter mutation 1,295,228 C>T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, E632–E637. [Google Scholar] [CrossRef]
- Bae, J.S.; Kim, Y.; Jeon, S.; Kim, S.H.; Kim, T.J.; Lee, S.; Kim, M.H.; Lim, D.J.; Lee, Y.S.; Jung, C.K. Clinical utility of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation. Diagn. Pathol. 2016, 11, 21. [Google Scholar] [CrossRef]
- Song, Y.S.; Lim, J.A.; Choi, H.; Won, J.K.; Moon, J.H.; Cho, S.W.; Lee, K.E.; Park, Y.J.; Yi, K.H.; Park, D.J.; et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016, 122, 1370–1379. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Low, K.C.; Liu, C.Y.; Yan, T.D.; Oji, A.; Ikawa, M.; Li, S.; Tergaonkar, V. Quantitative assessment of telomerase components in cancer cell lines. FEBS Lett. 2015, 589, 974–984. [Google Scholar] [CrossRef]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef]
- Chiba, K.; Johnson, J.Z.; Vogan, J.M.; Wagner, T.; Boyle, J.M.; Hockemeyer, D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife 2015, 4, e07918. [Google Scholar] [CrossRef]
- Karsy, M.; Guan, J.; Cohen, A.L.; Jensen, R.L.; Colman, H. New Molecular Considerations for Glioma: IDH, ATRX, BRAF, TERT, H3 K27M. Curr. Neurol. Neurosci. Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Nonoguchi, N.; Ohta, T.; Oh, J.E.; Kim, Y.H.; Kleihues, P.; Ohgaki, H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hosen, I.; Volz, F.; Hemminki, K.; Weyerbrock, A.; Kumar, R. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget 2015, 6, 10617–10633. [Google Scholar] [CrossRef]
- Hakin-Smith, V.; Jellinek, D.A.; Levy, D.; Carroll, T.; Teo, M.; Timperley, W.R.; McKay, M.J.; Reddel, R.R.; Royds, J.A. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet 2003, 361, 836–838. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chan, A.K.; Ding, X.J.; Qin, Z.Y.; Hong, C.S.; Chen, L.C.; Zhang, X.; Zhao, F.P.; Wang, Y.; Wang, Y.; et al. TERT promoter mutations contribute to IDH mutations in predicting differential responses to adjuvant therapies in WHO grade II and III diffuse gliomas. Oncotarget 2015, 6, 24871–24883. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, S.; Kong, H.; Srinivas, N.; Garcia-Casado, Z.; Requena, C.; Fallah, M.; Heidenreich, B.; Planelles, D.; Traves, V.; Schadendorf, D.; et al. Telomere length, telomerase reverse transcriptase promoter mutations, and melanoma risk. Genes Chromosomes Cancer 2018, 57, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Nagore, E.; Heidenreich, B.; Rachakonda, S.; Garcia-Casado, Z.; Requena, C.; Soriano, V.; Frank, C.; Traves, V.; Quecedo, E.; Sanjuan-Gimenez, J.; et al. TERT promoter mutations in melanoma survival. Int. J. Cancer 2016, 139, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Wang, K.; Liu, T.; Ge, N.; Liu, L.; Yuan, X.; Liu, J.; Kong, F.; Wang, C.; Ren, H.; Yan, K.; et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget 2014, 5, 12428–12439. [Google Scholar] [CrossRef]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef]
- Pilati, C.; Letouze, E.; Nault, J.C.; Imbeaud, S.; Boulai, A.; Calderaro, J.; Poussin, K.; Franconi, A.; Couchy, G.; Morcrette, G.; et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell 2014, 25, 428–441. [Google Scholar] [CrossRef]
- Totoki, Y.; Tatsuno, K.; Covington, K.R.; Ueda, H.; Creighton, C.J.; Kato, M.; Tsuji, S.; Donehower, L.A.; Slagle, B.L.; Nakamura, H.; et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014, 46, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Kawai-Kitahata, F.; Asahina, Y.; Tanaka, S.; Kakinuma, S.; Murakawa, M.; Nitta, S.; Watanabe, T.; Otani, S.; Taniguchi, M.; Goto, F.; et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 2016, 51, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.K.; Zheng, H.; Li, S.; Chen, R.; Liu, X.; Li, Y.; Lee, N.P.; Lee, W.H.; Ariyaratne, P.N.; Tennakoon, C.; et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 2012, 44, 765–769. [Google Scholar] [CrossRef]
- Bonilla Guerrero, R.; Roberts, L.R. The role of hepatitis B virus integrations in the pathogenesis of human hepatocellular carcinoma. J. Hepatol. 2005, 42, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, J.P.; Guetard, D.; Henry, M.; Wain-Hobson, S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science 2008, 320, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.; Chakravarthy, A.; Su, X.; Boshoff, C.; Fenton, T.R. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014, 7, 1833–1841. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Wang, H.Y.; Park, S.; Kim, S.; Lee, D.; Kim, G.; Kim, Y.; Park, K.H.; Lee, H. Use of hTERT and HPV E6/E7 mRNA RT-qPCR TaqMan assays in combination for diagnosing high-grade cervical lesions and malignant tumors. Am. J. Clin. Pathol. 2015, 143, 344–351. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Burk, R.D. Association between hTERT activation by HPV E6 proteins and oncogenic risk. Virology 2012, 433, 216–219. [Google Scholar] [CrossRef]
- Hsu, C.P.; Hsu, N.Y.; Lee, L.W.; Ko, J.L. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter--effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur. J. Cancer 2006, 42, 1466–1474. [Google Scholar] [CrossRef]
- Shen, N.; Lu, Y.; Wang, X.; Peng, J.; Zhu, Y.; Cheng, L. Association between rs2853669 in TERT gene and the risk and prognosis of human cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 50864–50872. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Ayhan, A.; Maeda, D.; Kim, K.R.; Clarke, B.A.; Shaw, P.; Chui, M.H.; Rosen, B.; Shih Ie, M.; Wang, T.L. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J. Pathol. 2014, 232, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Roberts, S.A.; Klimczak, L.J.; Sterling, J.F.; Saini, N.; Malc, E.P.; Kim, J.; Kwiatkowski, D.J.; Fargo, D.C.; Mieczkowski, P.A.; et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet. 2015, 47, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, J.P.; Henry, M.; Marchio, A.; Suspene, R.; Aynaud, M.M.; Guetard, D.; Cervantes-Gonzalez, M.; Battiston, C.; Mazzaferro, V.; Pineau, P.; et al. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010, 6, e1000928. [Google Scholar] [CrossRef]
- Singh, A.M.; Reynolds, D.; Cliff, T.; Ohtsuka, S.; Mattheyses, A.L.; Sun, Y.; Menendez, L.; Kulik, M.; Dalton, S. Signaling network crosstalk in human pluripotent cells: A Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 2012, 10, 312–326. [Google Scholar] [CrossRef]
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Li, K.; Chen, L.; Li, W.; Hou, M.; Liu, T.; Yang, J.; Lindvall, C.; Bjorkholm, M.; et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013, 32, 4203–4213. [Google Scholar] [CrossRef]
- Koh, C.M.; Khattar, E.; Leow, S.C.; Liu, C.Y.; Muller, J.; Ang, W.X.; Li, Y.; Franzoso, G.; Li, S.; Guccione, E.; et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Invest. 2015, 125, 2109–2122. [Google Scholar] [CrossRef]
- Tang, B.; Xie, R.; Qin, Y.; Xiao, Y.F.; Yong, X.; Zheng, L.; Dong, H.; Yang, S.M. Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget 2016, 7, 11364–11379. [Google Scholar] [CrossRef]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.K.; et al. Tergaonkar, Telomerase directly regulates NF-kappaB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef]
- Ding, D.; Xi, P.; Zhou, J.; Wang, M.; Cong, Y.S. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-kappaB-dependent transcription. FASEB J. 2013, 27, 4375–4383. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, X.; Sjoholm, L.; Liu, T.; Kong, F.; Ekstrom, T.J.; Bjorkholm, M.; Xu, D. Telomerase reverse transcriptase regulates DNMT3B expression/aberrant DNA methylation phenotype and AKT activation in hepatocellular carcinoma. Cancer Lett. 2018, 434, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Possemato, R.; Wong, J.M.; Currier, J.L.; Tothova, Z.; Manola, J.B.; Ganesan, S.; Lansdorp, P.M.; Collins, K.; Hahn, W.C. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. USA 2005, 102, 8222–8227. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Firpo, E.J.; Wang, Y.; Roberts, J.M. Separation of telomerase functions by reverse genetics. Proc. Natl. Acad. Sci. USA 2011, 108, E1363–E1371. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Drose, S.; Buchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Mattiussi, M.; Tilman, G.; Lenglez, S.; Decottignies, A. Human telomerase represses ROS-dependent cellular responses to Tumor Necrosis Factor-alpha without affecting NF-kappaB activation. Cell Signal 2012, 24, 708–717. [Google Scholar] [CrossRef]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef]
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar] [CrossRef]
- Zhou, J.; Mao, B.; Zhou, Q.; Ding, D.; Wang, M.; Guo, P.; Gao, Y.; Shay, J.W.; Yuan, Z.; Cong, Y.S. Endoplasmic reticulum stress activates telomerase. Aging Cell 2014, 13, 197–200. [Google Scholar] [CrossRef]
- Listerman, I.; Sun, J.; Gazzaniga, F.S.; Lukas, J.L.; Blackburn, E.H. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res. 2013, 73, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Altibi, A.M.A.; Duong, U.N.P.; Hassell, L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. 2017, 87, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, C.; Bergmann, T.K.; Henrichsen-Schnack, T.; Ladelund, S.; Nilbert, M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol. 2014, 53, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Mu, N.; Wang, N.; Straat, K.; Sofiadis, A.; Guo, Y.; Stenman, A.; Li, K.; Cheng, G.; Zhang, L.; et al. GABPA inhibits invasion/metastasis in papillary thyroid carcinoma by regulating DICER1 expression. Oncogene 2019, 38, 965–979. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, K.; Ji, P.; Zheng, X.; Jin, J.; Feng, M.; Liu, P. GABPA predicts prognosis and inhibits metastasis of hepatocellular carcinoma. BMC Cancer 2017, 17, 380. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, X.; Li, K.; Dai, M.; Zhang, L.; Wu, Y.; Sun, C.; Chen, Y.; Cheng, G.; Liu, C.; et al. GABPA is a master regulator of luminal identity and restrains aggressive diseases in bladder cancer. Cell Death Differ. 2019. [Google Scholar] [CrossRef]
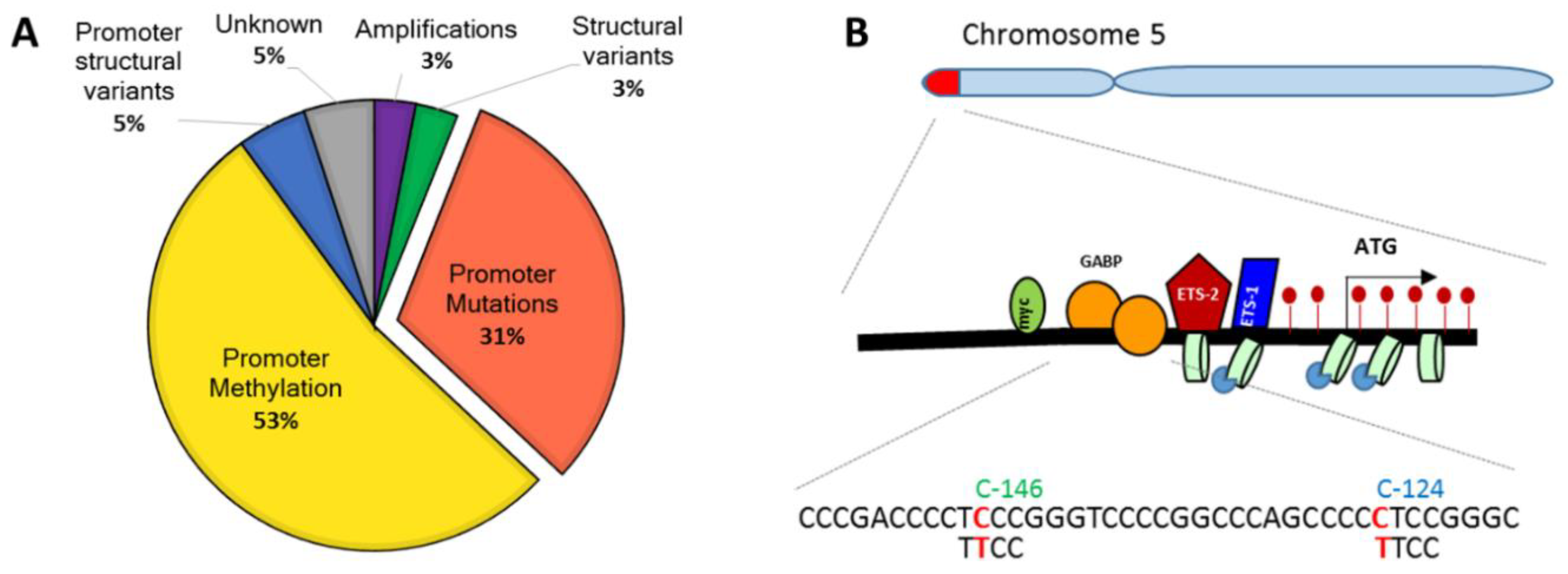
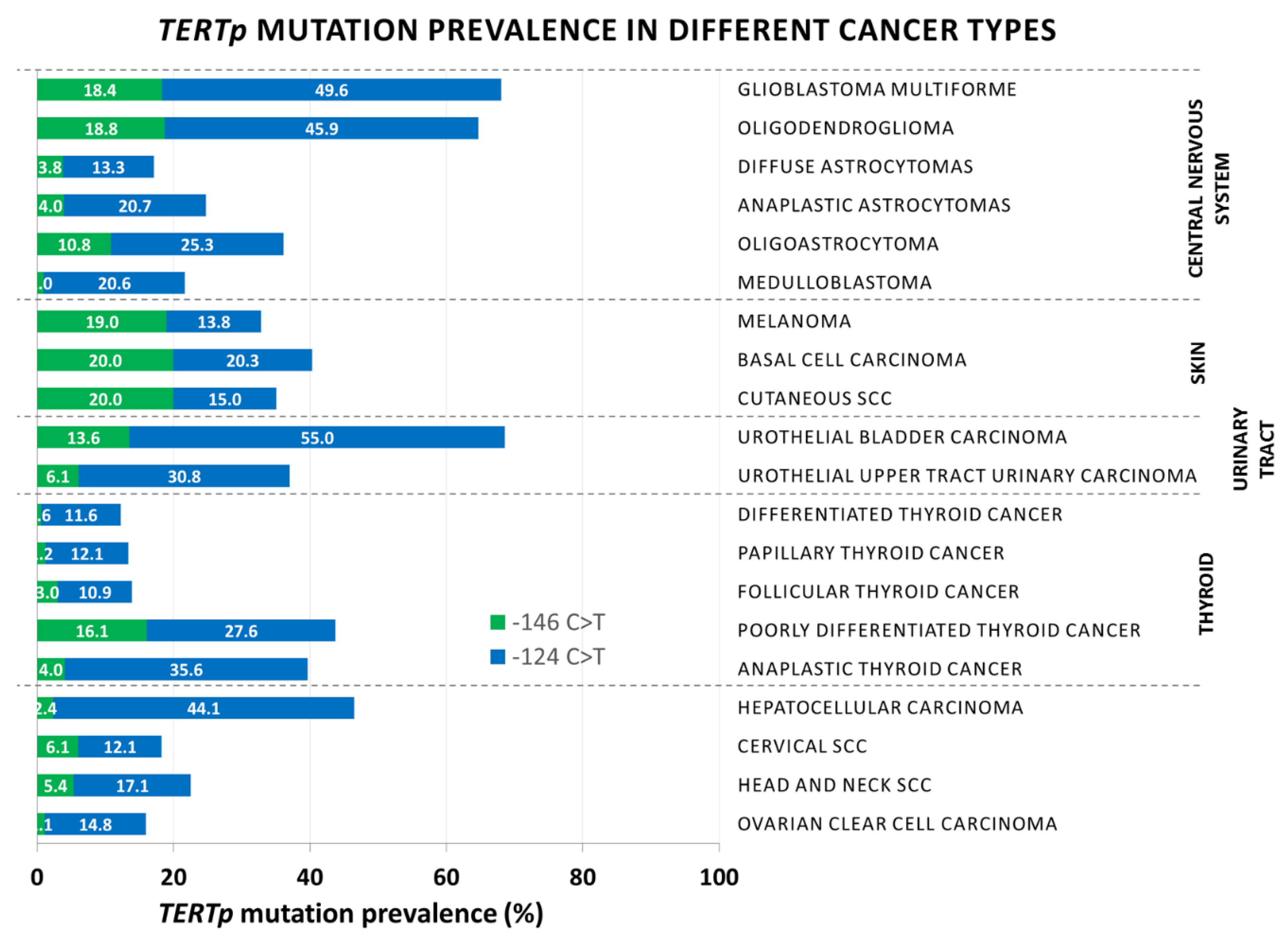
| Cancer Type | Stage | Prevalence of Mutations | −146 C>T | −124 C>T | Tert Upregulation | Methods | Sample Origin | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Central nervous system (CNS) | |||||||||
| GBM | 62% 24/39 | 25% 6/24 | 75% 18/24 | Yes | DNA sequencing, qRT-PCR, IHC, | Patients (Portugal) | Associated with older age. | [52] | |
| GBM | IV | 83.9% 47/55 | 34% 16/47 | 65.9% 31/47 | Yes | DNA sequencing, qRT-PCR, TRAP, reporter assays | Patients (China) | Associated with older age. | [57] |
| GBM (Primary) | IV | 83% 65/78 | 24.6% 16/65 | 75.4% 49/65 | N/A | DNA sequencing | Patients (US American) | Associated with shorter OS, IDH-wt, ATRX-wt, exclusively in EGFRmut samples. | [77] |
| GBM | I–IV | 44.6% 45/101 | 26.7% 12/45 | 73.3% 33/45 | Yes | DNA sequencing, qRT-PCR, reporter assays | Patients (China) | Associated with late-stage disease and patient age. Only in gliomas, not in pituitary adenocarcinomas, meningiomas or secondary metastases. | [60] |
| GBM | 55% 197/358 | 27% 54/197 | 73% 144/197 | N/A | DNA sequencing | Patients (Switzerland) | Associated with shorter OS and with EGFRmut. Negatively associated with mutant IDH and TP53. More frequent in primary (58%) than in secondary GBM (28%). One patient with both −146 C>T + −124 C>T mutation. | [111] | |
| GBM (primary & secondary) | IV | 80.3% 143/178 | * | * | N/A | DNA sequencing | Patients | Associated with shorter OS in patients without rs2853669 TERT -245 A>G polymorphism. Detected in 4/14 (28%) secondary GBM. | [81] |
| GBM | IV | 66.9% 141/211 | 25.5% 36/141 | 74.5% 105/141 | N/A | DNA sequencing | Patients (Portugal & Brazil) | Associated with older age, poor prognosis, and shorter survival. Reversed by rs2853669 TERT −245 A>G polymorphism. | [85] |
| GBM | 60.4% 29/48 | 24.1% 7/29 | 75.8% 22/29 | Yes | DNA sequencing, qRT-PCR | Patients (Korea) | Associated with older age. Not associated with OS or DFS. Associated with MGMT methylation and EGFR amplification. Associated with rs2853669 TERT −245 A>G polymorphism (21/29 patients). rs2853669 TERT −245 A>G polymorphism reversed TERT upregulation by TERTp mutations. | [64] | |
| GBM | 73% 92/126 | 28% 26/92 | 82% 66/92 | Yes | DNA sequencing, qRT-PCR, TRAP, qPCR | Mutually exclusive with IDH-1 mutations. Associated with shorter telomeres. Associated with lower OS in IDH-1wt patients. rs2853669 TERT -245 A>G polymorphism associated with improved OS in patients without TERTp mutations, and with worse OS in patients with TERTp mutations. | [65] | ||
| GBM (primary) | 86% 79/92 | 25% 20/79 | 75% 69/79 | DNA sequencing | Associated with older age and shorter OS. Homozygous rs2853669 TERT −245 A>G polymorphism associated with worse OS in patients without and with TERTp mutations. | [84] | |||
| GBM and gliomas (primary) | 100% 10/10 | 10% 1/10 | 90% 9/10 | N/A | DNA sequencing | Patients | In primary GBM, characterized by 10q deletion EFGR amplification. | [58] | |
| GBM | 94% 33/35 | 36% 12/33 | 64% 21/33 | 2.2–286-fold compared to normal astrocytes | DNA sequencing, qRT-PCR | Cell lines | [58] | ||
| Total GBM | 905/1331 (68%) | 206/762 (27%) | 567/762 (73%) | ||||||
| Oligodendroglioma | II | 45% 10/22 | 20% 2/10 | 80% 8/10 | Yes | DNA sequencing, qRT-PCR, IHC | Patients (Portugal) | [52] | |
| Oligodendroglioma | II–III | 70% 7/10 | 14.3% 1/7 | 85.7% 6/7 | Yes | DNA sequencing, qRT-PCR, TRAP, Reporter Assays | Patients (China) | Associated with older age. | [57] |
| Oligodendroglioma | II–III | 46.3% 25/54 | 24% 6/25 | 76% 19/25 | N/A | DNA sequencing | Patients (Portugal & Brazil) | Associated with older age at diagnosis. Not associated with lower survival. | [85] |
| Oligodendroglioma | 73.5% 25/34 | 20% 5/25 | 80% 20/25 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1mut if not total loss of 1p19q. | [53] | |
| Oligodendroglioma | II–IV | 66.81% 151/226 | * | * | N/A | DNA sequencing | Patients (US American) | Associated with shorter OS. Can be associated with ATRX mutations or IDHmut/1p19q loss. | [80] |
| Oligodendroglioma | II–III | 63.2% 12/19 | 41.7% 5/12 | 58.3% 7/12 | N/A | DNA sequencing | Patients (US American) | IDH-wt only. Associated with worse prognosis in IDH-wt. Associated with older age. Mutually exclusive with ATRX mutations. | [77] |
| Anaplastic oligodendroglioma | III | 54% 13/24 | 30.8% 4/13 | 69.2% 9/13 | Yes | DNA sequencing, qRT-PCR, IHC, | Patients (Portugal) | Associated with older age. | [52] |
| Anaplastic oligodendroglioma | 74.2% 23/31 | 30.4% 7/23 | 69.6% 16/23 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1 if not total loss of 1p19q. | [53] | |
| Anaplastic oligodendroglioma | III | 88.5% 23/26 | 43.5% 10/23 | 56.5% 13/23 | N/A | DNA sequencing | Patients (US American) | Associated with older age. IDH-wt only. Associated with worse prognosis in IDH-wt. Mutually exclusive with ATRX mutations. | [77] |
| Total Oligodendroglioma | 289/446 (64.7%) | 40/138 (29%) | 98/138 (71%) | ||||||
| Diffuse astrocytomas | 19.2% 10/52 | 20% 2/10 | 80% 8/10 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1 if not total loss of 1p19q. | [53] | |
| Diffuse astrocytoma | II | 15% 3/20 | 33,3% 1/3 | 66,6% 2/3 | Yes | DNA sequencing, qRT-PCR, IHC | Patients (Portugal) | Associated with older age. | [52] |
| Diffuse astrocytoma | II | 20% 8/40 | 25% 2/8 | 62.5% 5/8 | Yes | DNA sequencing, qRT-PCR, TRAP, reporter assays | Patients (China) | Associated with age. | [57] |
| Diffuse astrocytoma | II | 15.2% 7/46 | 16.7% 1/7 | 83.3% 6/7 | N/A | DNA sequencing | Patients (Portugal & Brazil) | Frequency increased with grade. | [85] |
| Total Diffuse Astrocytoma | 28/158 (17.7%) | 6/28 (21.4%) | 21/28 (75%) | ||||||
| Astocytoma | II–IV | 62.5% 416/665 | N/A | N/A | N/A | DNA sequencing | Patients (US American) | Associated with shorter OS. Can be associated with ATRX mutations or IDHmut/1p1q loss. | [80] |
| Anaplastic Astrocytomas | III | 10% 1/10 | 0% 0/1 | 100% 1/1 | N/A | DNA sequencing | Patients (Portugal & Brazil) | Frequency increased with grade. | [85] |
| Anaplastic Astrocytoma | III | 33.3% 4/12 | 0% 0/4 | 100% 4/4 | Yes | DNA sequencing, qRT-PCR, TRAP, reporter assays | Patients (China) | Correlation with age. | [57] |
| Anaplastic Astrocytomas | III | 25.3% 20/79 | 20% 4/20 | 80% 16/20 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1 if not total loss of 1p19q. | [53] |
| Total Anaplastic Astrocytomas | 25/101 (24.7%) | 4/25 (16%) | 21/25 (84%) | ||||||
| Mixed Oligoastocytoma | II–IV | 32.3% 63/195 | * | * | N/A | DNA sequencing | Patients (US American) | Associated with shorter OS. Can be associated with ATRX mutations or IDHmut/1p1q loss. | [80] |
| Oligoastrocytoma | 40% 14/35 | 28.6% 4/14 | 71.4% 10/14 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1 if not total loss of 1p19q. | [53] | |
| Oligoastrocytoma | II–III | 40.0% 4/10 | 50% 2/4 | 50% 2/4 | N/A | DNA sequencing | Patients (Portugal & Brazil) | Not associated with lower survival. | [85] |
| Anaplastic Oligoastrocytoma | 48.9% 22/45 | 27.3% 6/22 | 72.7% 16/22 | Yes | DNA sequencing, qRT-PCR | Patients (Japan) | Associated with total 1p19q loss and IDH-1/2 mutations (98%) but exclusive with IDH-1 if not total loss of 1p19q. | [53] | |
| Total Oligoastrocytoma | 103/285 (36.1%) | 12/40 (30%) | 28/40 (70%) | ||||||
| Medulloblastoma | 33.3% 2/6 | 50% 1/2 | 50% 1/2 | N/A | DNA sequencing | Patients (China) | Associated with age. | [57] | |
| Medulloblastoma | 20.9% 19/91 | 0%0/19 | 100% 19/19 | N/A | DNA sequencing | Patients (US American) | IDH-wt and ATRX-wt only. Associated with worse prognosis in IDH-1-wt. Associated with older age. Mutually exclusive with ALT. | [77] | |
| Total Medulloblastoma | 21/97 (21.6%) | 1/21 (4.7%) | 20/21 (95.3%) | ||||||
| Skin | |||||||||
| Melanoma | 71% 50/70 | 46% 23/50 | 54% 27/50 | Yes | DNA sequencing, reporter vectors | Patients & cell lines | [50] | ||
| Melanoma | 32.5% 25/77 | 20% 5/25 | 28% 7/25 | N/A | DNA sequencing | Patients | -57 C>T germline mutation in family with history of melanoma. High prevalence in metastatic cell lines (85%) compared to primary melanoma (32.5%). CC>TT −139/−138 tandem mutation in 10.4% patients. Concomitant with BRAF mutations in 47% of cases. | [49] | |
| Melanoma | 29% 16/56 | 50% 8/16 | 50% 8/16 | N/A | DNA sequencing | Patients (Portugal) | Associated with BRAF mutations. | [52] | |
| Melanoma | 34% 97/287 | 52.5% 51/97 | 36% 35/97 | Yes | DNA sequencing, qRT-PCR | Patients (Spain) | CC>TT −139/−138 tandem mutations in 4/97 (4.1%) patients. Associated with BRAF mutations in 50% cases. | [88] | |
| Melanoma | 41.6% 121/291 | * | * | N/A | DNA sequencing | Patients (Spain) | Associated with shorter telomeres in tumor and with accelerated telomere shortening rate. Associated with BRAF/NRAS mutation in 75/243 cases. Telomere shortening rate: BRAF/NRASmut+TERTpmut>TERTpmut>BRAF/NRASmut | [115] | |
| Melanoma | 22% 26/116 | 35% 9/26 | 46% 12/26 | Yes | DNA sequencing, IHC | Patients (Portugal) | Associated with reduced OS & DFS. More prevalent in sun-exposed regions. Associated with increased mitotic rates. −138/−138 CC>TT tandem mutation in 2/26 (7.7%) patients. −125/−124 CC>TT tandem mutation in 3/26 (11.5%) patients. Associated with BRAF-V600E mutation (58% of cases). | [89] | |
| Melanoma | 38.6% 116/300 | 50% 58/116 | 32.8% 32/116 | N/A | DNA sequencing | Patients (Spain) | Associated with shorter OS and DFS. −139/−138 CC>TT & −125/−124 CC>TT tandem mutations in 16/116 cases (13.8%). Associated with BRAF/NRAS mutations in 126/283 (44.5%) cases. Reversed by rs2853669 TERT -245 A>G polymorphism. | [116] | |
| Melanoma | 54.8% 63/115 | 61.9% 39/63 | 30.2% 19/63 | N/A | DNA sequencing | Patients (Austria) | −139/−138 CC>TT tandem mutations in 4/63 (6.3%) patients. −125/−124 CC>TT tandem mutation in 1/63 (1.6%) patient. Associated with BRAF/NRAS mutation in 75/243 cases. Associated with rs2853669 TERT -245 A>G polymorphism. | [91] | |
| Total Melanoma | 514/1312 (39.2%) | 193/398 (48.5%) | 140/398 (35.1%) | ||||||
| Basal cell carcinoma | 55.6% 18/32 | 55.6% 10/18 | 22.2% 4/18 | N/A | DNA sequencing | Patients (Germany) | [55] | ||
| Basal cell carcinoma (sporadic & nevoid) | 74% 31/42 | 35.5% 11/31 | 45.1% 14/31 | N/A | DNA sequencing | Patients | Mostly homozygous. −139/−138 CC>TT tandem mutation in 7/31 (22.6%) patients. −125/−124 CC–TT tandem mutation in 5/31 (16.1%) patients. 1 patient with −139/−138 CC>TT + −125/−124 CC>TT tandem mutations. Mutations more frequent in basal cell carcinoma than in squamous cell carcinoma. | [90] | |
| Basal cell carcinoma | 38.7% 76/196 | 43% 33/76 | 49% 37/76 | no | DNA sequencing, IHC | Patients (Portugal) | No correlation with clinical parameters. Higher prevalence in patients not exposed to X-irradiation: 48/94 (51%) vs. 28/102 (27%) in X-irradiated patients. −124 C>T more frequent than −146 C>T in non-X-irradiated patients; −146 C>T more frequent in X-irradiated patients. −139/138 CC>TT tandem mutation in 2/76 (2.6%) patients, 2 patients with −146 C>T + −124 C>T mutations. | [89] | |
| Total Basal cell carcinoma | 125/270 (46.2%) | 54/125 (43.2%) | 55/125 (44%) | ||||||
| Cutaneous SCC | 50% 17/34 | 29.4% 5/17 | 29.4% 5/17 | N/A | DNA sequencing | Patients (Germany) | [55] | ||
| Cutaneous SCC | 50% 13/26 | 54% 7/13 | 31% 4/13 | N/A | DNA sequencing | Patients | Mostly homozygous. −139/−138 CC>TT tandem mutation in 2/13 (15.4) patients. Mutations more frequent in basal cell carcinoma than in squamous cell carcinoma. | [90] | |
| Total Cutaneous SCC | 30/60 (50%) | 12/30 (40%) | 9/30 (30%) | ||||||
| Bladder/urinary tract cancers | |||||||||
| Bladder Cancer | 85% 44/52 | 4.5% 2/44 | 95.5% 42/44 | N/A | DNA sequencing | Patients (China) | [78] | ||
| Urothelial bladder carcinoma | III | 80% 12/15 | 17% 2/12 | 83% 10/12 | N/A | DNA sequencing | Patients (US American) | [93] | |
| Urothelial bladder carcinoma | 66.7% 14/21 | 28.6% 4/14 | 71.4% 10/14 | N/A | DNA sequencing | Patients (US American) | [77] | ||
| Urothelial bladder carcinoma | 61.7% 148/240 | 25% 37/148 | 58.8% 87/148 | N/A | DNA sequencing | Patients (China) | Not associated with age. | [57] | |
| Urothelial bladder carcinoma | 59% 48/82 | 37.5% 18/48 | 62.5% 30/48 | N/A | DNA sequencing, qRT-PCR | Patients (Portugal) | Not associated with age. Low-grade bladder cancer: 67%, high-grade bladder cancer: 56%. | [52] | |
| Urothelial bladder carcinoma | 65.4% 214/327 | 17.8% 38/214 | 81.8% 175/214 | N/A | DNA sequencing, relative telomere length | Patients (Sweden) | Associated with shorter telomeres and worse OS. Associated with FGFR3 mutation in 45% of tumors. FGFR3 mutations found in low-grade tumors, TERTp mutations in low-grade and high-grade tumors. Reversed by rs2853669 TERT −245 A>G polymorphism. | [61] | |
| Urothelial bladder carcinoma | 77.1% 361/468 | 17% 62/361 | 83% 299/361 | Not increased | DNA sequencing, qRT-PCR | Patients | Not associated with OS, DFS, or clinical outcome. Associated with FGFR3mut. | [94] | |
| Urothelial bladder carcinoma | 100% 33/33 | 12% 5/33 | 85% 28/33 | N/A | DNA sequencing | Patients | Pure micropapillary carcinoma and urothelial cancer with focal micropapillary features. | [92] | |
| Urothelial upper tract urinary carcinoma | 76.9% 40/52 | 12.5% 5/40 | 72.5% 29/40 | N/A | DNA sequencing | Patients (China) | Not associated with age. | [57] | |
| Urothelial upper tract urinary carcinoma | 47.4% 9/19 | 11.1% 1/9 | 88.9% 8/9 | N/A | DNA sequencing | Patients (US American) | [77] | ||
| Urothelial upper tract urinary carcinoma | 29.5% 65/220 | 18.5% 12/65 | 81.5% 53/65 | N/A | DNA sequencing, Detection in urine | Patients (China) | Associated with distant metastases. | [118] | |
| Total Urothelial bladder & upper tract urinary carcinoma | 988/1529 (64.6%) | 186/988 (18.8%) | 771/988 (78%) | ||||||
| Thyroid | |||||||||
| Differentiated thyroid cancer | 12.2% 41/336 | 4.9% 2/41 | 95.1% 39/41 | N/A | DNA sequencing | Patients | Only in malignant lesions. | [108] | |
| Papillary thyroid cancer | 8% 13/169 | 7.7% 1/13 | 84.6% 11/13 | Yes | DNA sequencing, qRT-PCR, IHC | Patients (Portugal) | [52] | ||
| Papillary thyroid cancer | III/IV | 11.3% 46/408 | 15.2% 7/46 | 85.8% 39/46 | N/A | DNA sequencing | Patients (China) | Associated with older age, larger tumor size, extrathyroid invasion, advanced clinical stage. Associated with BRAF-V600E mutation. | [99] |
| Papillary thyroid cancer | 27% 13/51 | 7.7% 1/13 | 92.3% 12/13 | N/A | DNA sequencing | Patients (Sweden) | Only in patients >45. Correlated with shorter telomeres and distal metastases. PTC: 27% (25/332); FTC: 22% (12/70); ATC: 50% (12/36). | [98] | |
| Papillary thyroid cancer | III/IV | 4.1% 18/432 | * | * | N/A | DNA sequencing | Patients (Korea) | Associated with BRAF/RAS mutations. Associated with tumor size, stage III-IV, recurrence, decreased OS and DFS with BRAF/RAS mutations: RAS/BRAF >TERTp > RAS/BRAF+TERTp. | [106] |
| Papillary thyroid cancer | 11.7% 30/257 | 0% 0/30 | 100% 30/30 | N/A | DNA sequencing | Patients | Only in malignant lesions. −124 C>T associated with BRAF-V600E mutation. | [108] | |
| Papillary thyroid cancer | 37.7% 10/27 | 10% 1/10 | 90% 9/10 | N/A | DNA sequencing | Patients (Korea) | No TERTp mutation found in 192 well differentiated cancers without distant metastasis. | [105] | |
| Papillary thyroid cancer | 22% 18/80 | 44% 8/18 | 66% 10/18 | N/A | DNA sequencing | Patients (US & Japan) | More frequent in BRAF-wt patients than in BRAFmut. | [100] | |
| Papillary thyroid cancer | 31.8% 77/242 | 0% 0/77 | 100% 77/77 | N/A | DNA sequencing | Patients (US) | Associated with older age (>45 years), larger tumor size, stage III–IV, distant metastases, decreased OS and DFS. rs2853669 TERT −245 A>G polymorphism (46.7% (113/242)of patients) increases OS & DFS in patients without TERTp mutations and with BRAF-V600E. | [103] | |
| Papillary thyroid cancer | 12% 22/182 | 14.6% 3/22 | 86.4% 19/22 | Yes | DNA sequencing, WB, and IHC | Patients (Italy) | Associated with older age and poor prognosis. Increased cytoplasmic localization of TERT. No impact of rs2853669 TERT -245 A>G polymorphism on outcome. | [102] | |
| Total Papillary thyroid cancer | 247/1848 (13.4%) | 21/229 (9.2%) | 207/229 (90.4%) | ||||||
| Follicular Thyroid Cancer | 13.9% 11/79 | 18.2% 2/11 | 81.8% 9/11 | N/A | DNA sequencing | Patients | Only in malignant lesions. | [108] | |
| Follicular Thyroid Cancer | 66.7% 2/3 | 50% 1/2 | 50% 1/2 | N/A | DNA sequencing | Patients (Korea) | No TERTp mutation found in 192 well-differentiated cancers without distanst metastasis. | [105] | |
| Follicular thyroid Cancer | 14% 9/64 | 22.2% 2/9 | 77.8% 7/9 | Yes | DNA sequencing, qRT-PCR, IHC | Patients (Portugal) | [52] | ||
| Follicular thyroid cancer | 22% 8/36 | 12.5% 1/8 | 87.5% 7/8 | N/A | DNA sequencing | Patients (Sweden) | Increased prevalence in ATC: PTC: 27% (25/332); FTC: 22% (12/70); ATC: 50% (12/36). | [98] | |
| Follicular thyroid cancer | 36.4% 8/22 | 12.5% 1/8 | 87.5% 7/8 | N/A | DNA sequencing | Patients (China) | Associated with older age, larger tumor size, extrathyroid invasion, advanced clinical stage. Associated with BRAF-V600E mutation. | [99] | |
| Follicular thyroid cancer | III/IV | 5.9% 7/119 | * | * | N/A | DNA sequencing | Patients (Korea) | Associated with BRAF/RAS mutations. Associated with tumor size, stage III-IV, recurrence, decreased OS and DFS with BRAF/RAS mutations: RAS/BRAF >TERTp > RAS/BRAF+TERTp. | [106] |
| Follicular thyroid cancer | 14% 8/58 | 38.5% 3/8 | 62.5% 5/8 | Yes | DNA sequencing, WB, and IHC | Patients (Italy) | Associated with older age and poor prognosis. Increased cytoplasmic TERT. No impact of rs2853669 TERT -245 A>G polymorphism on outcome. | [102] | |
| Total Follicular thyroid cancer | 53/381 (13.9%) | 10/46 (21.7%) | 36/46 (78.2%) | ||||||
| Poorly differentiated thyroid cancer | 21% 3/14 | 33.3 1/3 | 66.7 2/3 | Yes | DNA sequencing, qRT-PCR, IHC | Patients (Portugal) | [52] | ||
| Poorly differentiated thyroid cancer | 37.5% 3/8 | 0% 0/3 | 100% 3/3 | N/A | DNA sequencing | Patients | Only in malignant lesions. | [108] | |
| Poorly differentiated thyroid cancer | 29% 2/7 | 50% 1/2 | 50% 1/2 | N/A | DNA sequencing | Patients (Korea) | No TERTp mutation found in 192 well-differentiated cancers without distanst metastasis. | [105] | |
| Poorly differentiated thyroid cancer | 51.7% 30/58 | 40% 12/30 | 60% 18/30 | N/A | DNA sequencing | Patients (US & Japan) | More prevalent in advanced cancer patients with BRAF/RASmut. | [100] | |
| Total Poorly differentiated thyroid cancer | 38/87 (43.7%) | 14/38 (36.8%) | 24/38 (63.2%) | ||||||
| Anaplastic thyroid cancer | 46.3% 25/54 | 8% 2/25 | 92% 23/25 | N/A | DNA sequencing | Patients | Only in malignant lesions. | [108] | |
| Anaplastic thyroid cancer | 13% 2/16 | 50% 1/2 | 50% 1/2 | Yes | DNA sequencing, qRT-PCR | Patients (Portugal) | [52] | ||
| Anaplastic thyroid cancer | 50% 10/20 | 0% 0/10 | 100% 10/10 | N/A | DNA sequencing | Patients (US & Japan) | More prevalent in advanced cancer patients with BRAF/RASmut. | [100] | |
| Anaplastic thyroid cancer | 50% 10/20 | 20% 2/10 | 80% 8/10 | N/A | DNA sequencing | Patients (Sweden) | PTC: 27% (25/332); FTC: 22% (12/70); ATC: 50% (12/36). | [98] | |
| Anaplastic thyorid cancer | 33.3% 12/36 | * | * | N/A | DNA sequencing | Patients (Portugal & Spain) | Associated with older age, larger tumor size, distant metastases and disease-related death in FTC. PTC: 7.5% (25/332); FTC: 17.1% (12/70); PDTC: 29% (9/31); ATC: 33.4% (12/36). PTC associated with BRAF-V600E mutation in 60.3% of cases. | [101] | |
| Anaplastic thyroid cancer | 38.7% 41/106 | 10% 4/41 | 90% 37/41 | N/A | DNA sequencing | Patients (US & China) | Associated with older age and distal metastases. −124 C>T found in 56.3% of BRAF-V600E mutated cases. | [104] | |
| Total anaplastic thyroid cancer | 100/252 (39.7%) | 9/88 (10.2%) | 79/88 (89.7%) | ||||||
| Thyroid Cancer cell lines | 91.7% 11/12 | 27.3% 3/11 | 72.7% 8/11 | N/A | DNA sequencing | Cell lines | [108] | ||
| Thyroid Cancer cell lines | 75% 6/8 | 17.7% 1/6 | 83.3% 5/6 | N/A | DNA sequencing | ATC cell lines | [98] | ||
| Liver-Hepatocellular Carcinoma (HCC) | |||||||||
| HCC | 31.4% 11/35 | 18,2% 2/11 | 81,8% 9/11 | N/A | DNA sequencing | Patients (China) | [57] | ||
| HCC | 34% 15/44 | 33.3% 5/15 | 66.7% 10/15 | N/A | DNA sequencing | Patients (Africa, Asia, Europe) | Higher TERTp mutation prevalence in African (53%) compared to non-African (24%) populations. | [97] | |
| HCC | 44.3% 27/61 | 3.7% 1/27 | 96.3% 26/27 | N/A | DNA sequencing | Patients (US American) | Detected in both HBV-associated and HBV-independent HCC Frequent in HCV-associated HCC. | [77] | |
| HCC | 48.5% 65/131 | 3.1% 2/65 | 96.9% 63/65 | N/A | DNA sequencing | Patients (Italy) | 41% of mutations in HBV-associated HCC. 53.6% mutations in HCV-associated HCC. All heterozygous. No −57 A>C. | [95] | |
| HCC | 31% 85/275 | 1.1% 1/85 | 98.9% 84/85 | Yes | DNA sequencing, IHC | Patients (China) | HBV-associated HCC. Correlated with age, not with HBV status. Found in 4/7 preneoplastic lesions (HBV-associated HCC). | [63] | |
| HCC | 65.4% 68/104 | 3% 2/68 | 97% 66/68 | Yes | DNA sequencing | Patients (Japan) | Associated with older age. Associated with shorter OS and DFS. Associated with HCV infection and excluded from HBV+ HCC. | [122] | |
| HCC | 58.6% 179/305 | 6.1% 11/179 | 92.7% 166/179 | Yes 2–10-fold | DNA sequencing, qRT-PCR | Patients (French) | Detected in cirrhotic preneoplastic macronodules (25%) and cirrhotic adenomas (44%), at last step of malignant transformation into HCC. Absent from HBV-associated tumors 2/179 (1%) −146 C>T. | [62] | |
| HCC | 29.3% 57/195 | 5.3% 3/57 | 94.7% 54/57 | No | DNA sequencing, qRT-PCR | Associated with older age. No impact on overall survival. Excluded from HBV-associated HCC. Higher frequency in HCV-associated HCC. | [96] | ||
| HCC | 54% 254/469 | 4.3% 11/254 | 93% 236/254 | N/A | DNA sequencing | Patients (Japan, US-European ancestry) | −57 A>C mutation detected in 1.6%. Present in 37% HBV-associated HCC but mutually exclusive with HBV sequence integration. Mutually exclusive with TERT CNV and ATRX mutations. Associated with HCV infection (64% or TERTp mutations). Associated with Wnt pathway mutations. | [121] | |
| HCC | 60% 9/15 | 11.1% 1/9 | 88.9% 8/9 | N/A | DNA sequencing | Cell lines | [97] | ||
| Total HCC | 770/1634 (47.1%) | 39/770 (5%) | 722/770 (93.7%) | ||||||
| Cervical | |||||||||
| Cervical SCC | 21.8% 22/101 | 31.8% 7/22 | 45.5% 10/22 | Yes | qRT-PCR | Patients (Italian women) | [37] | ||
| Cervical SCC | 21.4% 30/140 | 26.7% 8/30 | 73.3% 22/30 | N/A | DNA sequencing, Association with clinical status | Patients (Indian women) | 75% TERTp mutations in HPV-negative samples. −124 C>T 6/22 were TT homozygous. −146 C>T 2/8 were TT homozygous. | [36] | |
| Cervical SCC | 4.5% 1/22 | 100% 1/1 | 0% 0/1 | N/A | DNA sequencing | Patients (US American) | 1 patient with −125 C>A mutation. | ||
| Total Cervical SCC | 53/263 (20.1%) | 16/53 (30.2%) | 32/53 (60.4%) | [77] | |||||
| Head and Neck Squamous Cell Carcinoma (HNSCC) | |||||||||
| HNSCC | 31.7% 13/41 | 30.8% 4/13 | 69.2% 9/13 | N/A | DNA sequencing | Patients (Indian women) | Association with clinical status. | [36] | |
| HNSCC | 17% 12/70 | 16.7% 2/12 | 83.3% 10/12 | N/A | DNA sequencing | Patients (US American) | 11/12 HNSCC with TERTp mutations were in the oral tongue, and 11/23 (47.8%) of HNSCC of the oral tongue harbored TERTp mutations. | [77] | |
| Total HNSCC | 25/111 (22.5%) | 6/25 (24%) | 19/25 (76%) | ||||||
| Ovarian cancer | |||||||||
| Ovarian clear cell carcinoma | 15% 3/20 | 0% 0/3 | 10% 2/3 | N/A | DNA sequencing | Patients (US American) | 1 patient with −124 C>A mutation. | [77] | |
| Ovarian clear cell carcinoma | 16.5% 37/233 | 8.1% 3/37 | 91.9% 34/37 | N/A | DNA sequencing, IHC, telomere length evaluation | Patients | No link with survival or age. TERTp mutations tended to be mutually exclusive with loss of ARID1A protein expression and PIK3CA mutation. | [132] | |
| Ovarian clear cell carcinoma | 30% 3/10 | 0% 0/3 | 100% 3/3 | Yes | qRT-PCR | Cell lines | [132] | ||
| Total ovarian clear cell carcinoma | 43/263 (16.3%) | 3/43 (6.9%) | 39/43 (90.7%) | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafezi, F.; Perez Bercoff, D. The Solo Play of TERT Promoter Mutations. Cells 2020, 9, 749. https://doi.org/10.3390/cells9030749
Hafezi F, Perez Bercoff D. The Solo Play of TERT Promoter Mutations. Cells. 2020; 9(3):749. https://doi.org/10.3390/cells9030749
Chicago/Turabian StyleHafezi, François, and Danielle Perez Bercoff. 2020. "The Solo Play of TERT Promoter Mutations" Cells 9, no. 3: 749. https://doi.org/10.3390/cells9030749
APA StyleHafezi, F., & Perez Bercoff, D. (2020). The Solo Play of TERT Promoter Mutations. Cells, 9(3), 749. https://doi.org/10.3390/cells9030749




