Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Peptides
2.2. Bacterial Culture Conditions
2.3. Fn-Functionalization of Surfaces and AFM Probes
2.4. Fn-Attachment Assay
2.5. Single-Cell Force Spectroscopy (SCFS)
2.6. Single-Molecule Force Spectroscopy (SMFS)
2.7. AFM Data Analysis
3. Results and Discussion
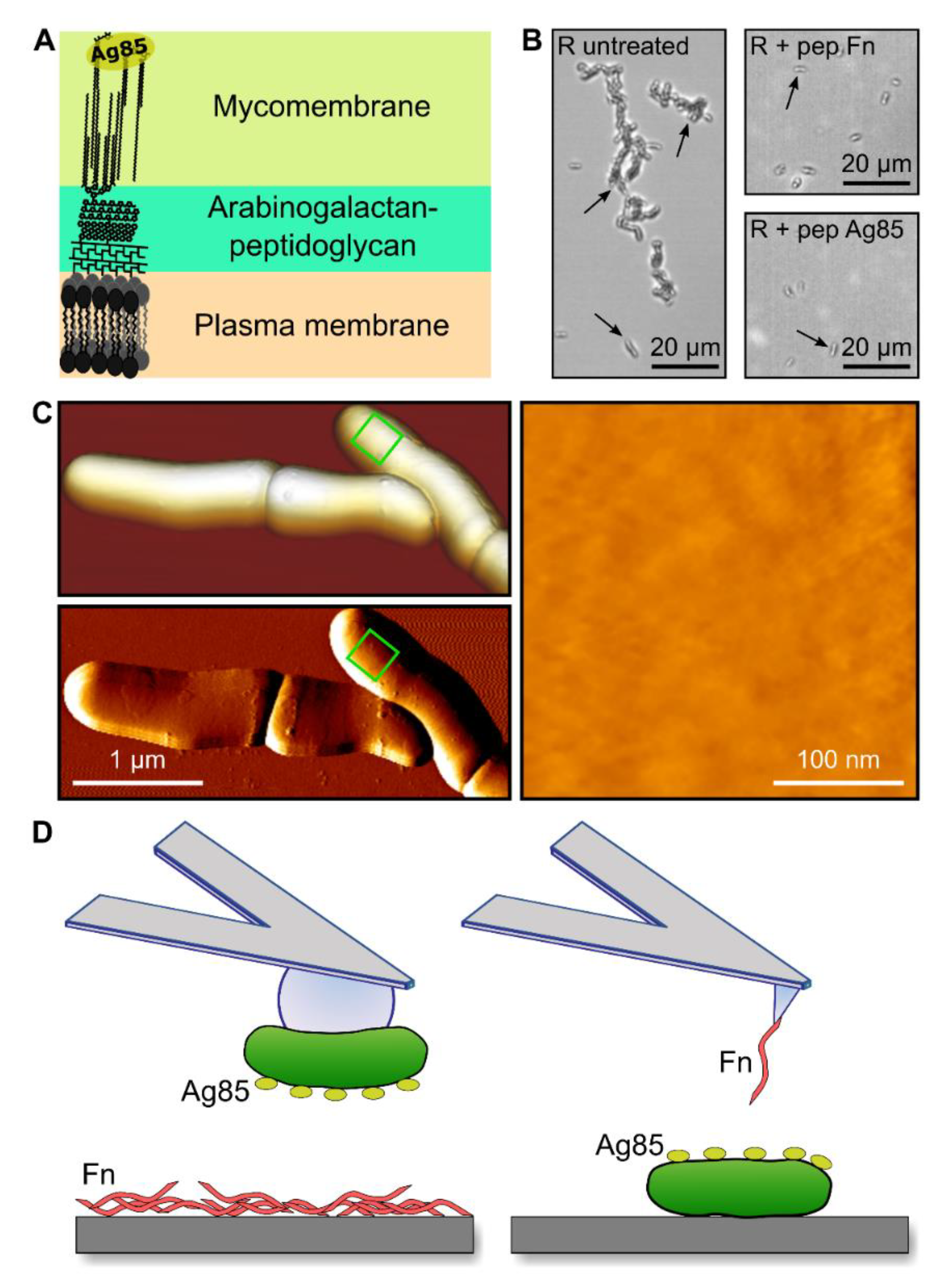
3.1. Studying Fn-Binding by M. abscessus Ag85 Down to the Single Molecule Level
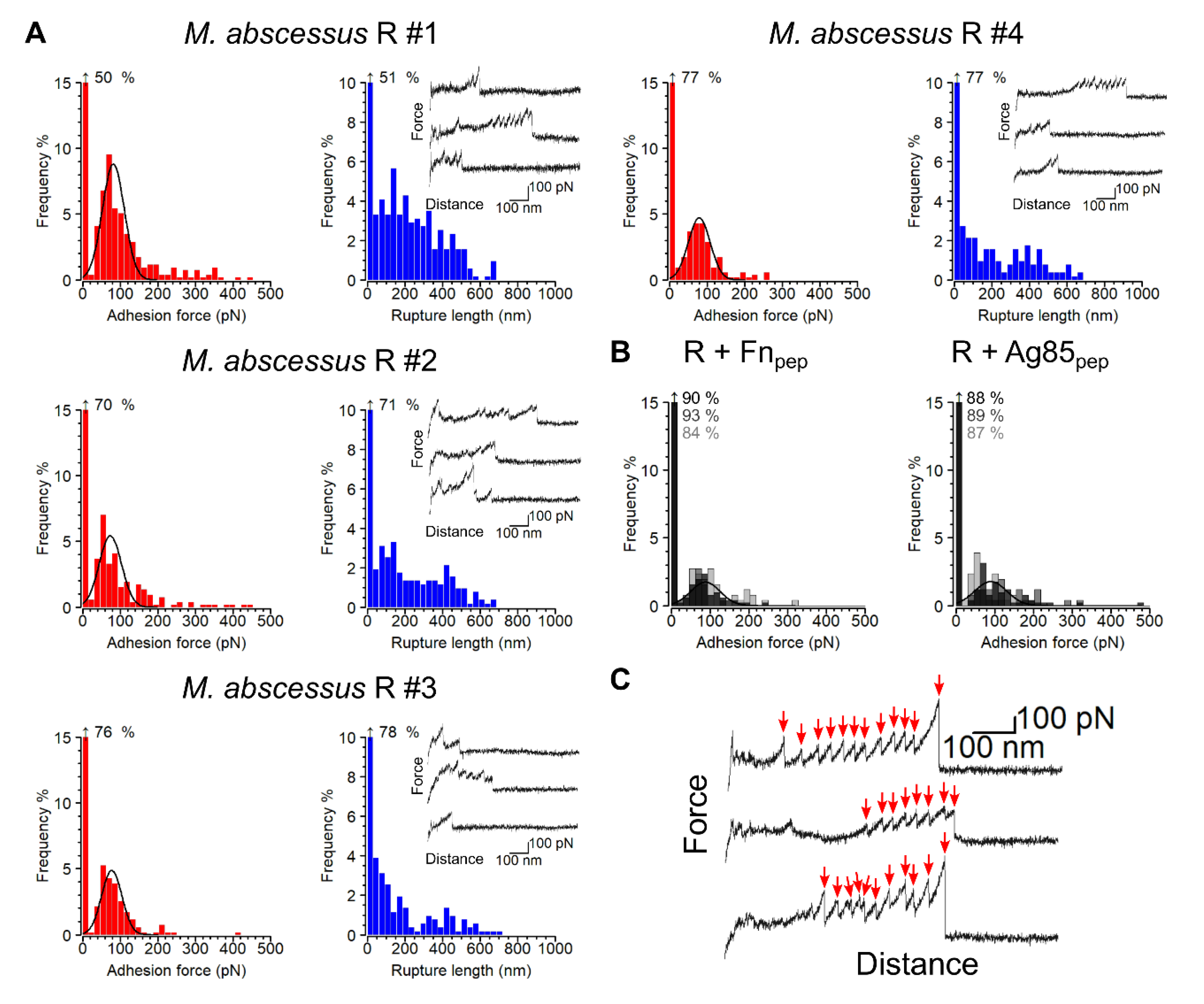
3.2. Adhesion Forces between Single Bacteria and Fn
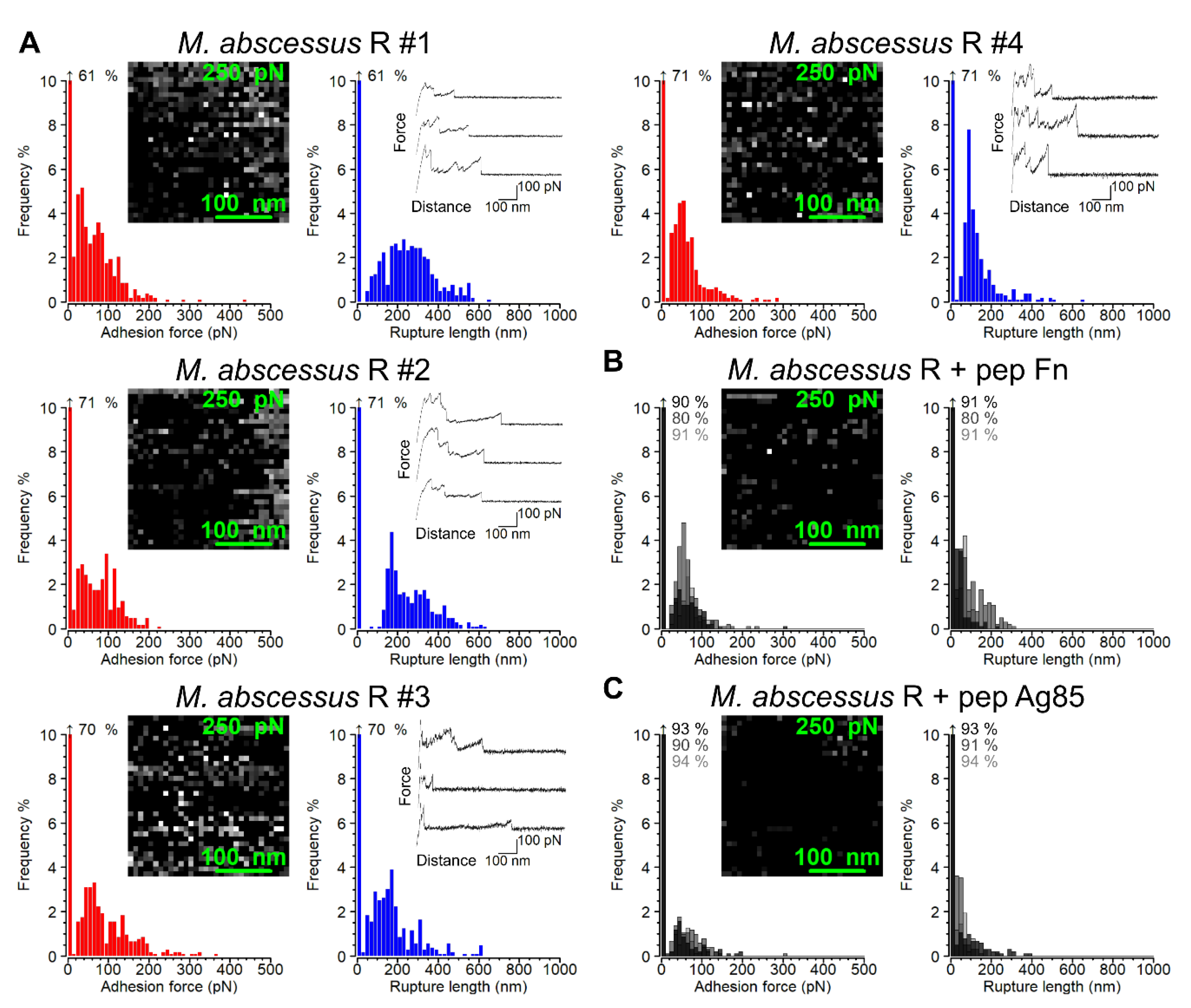
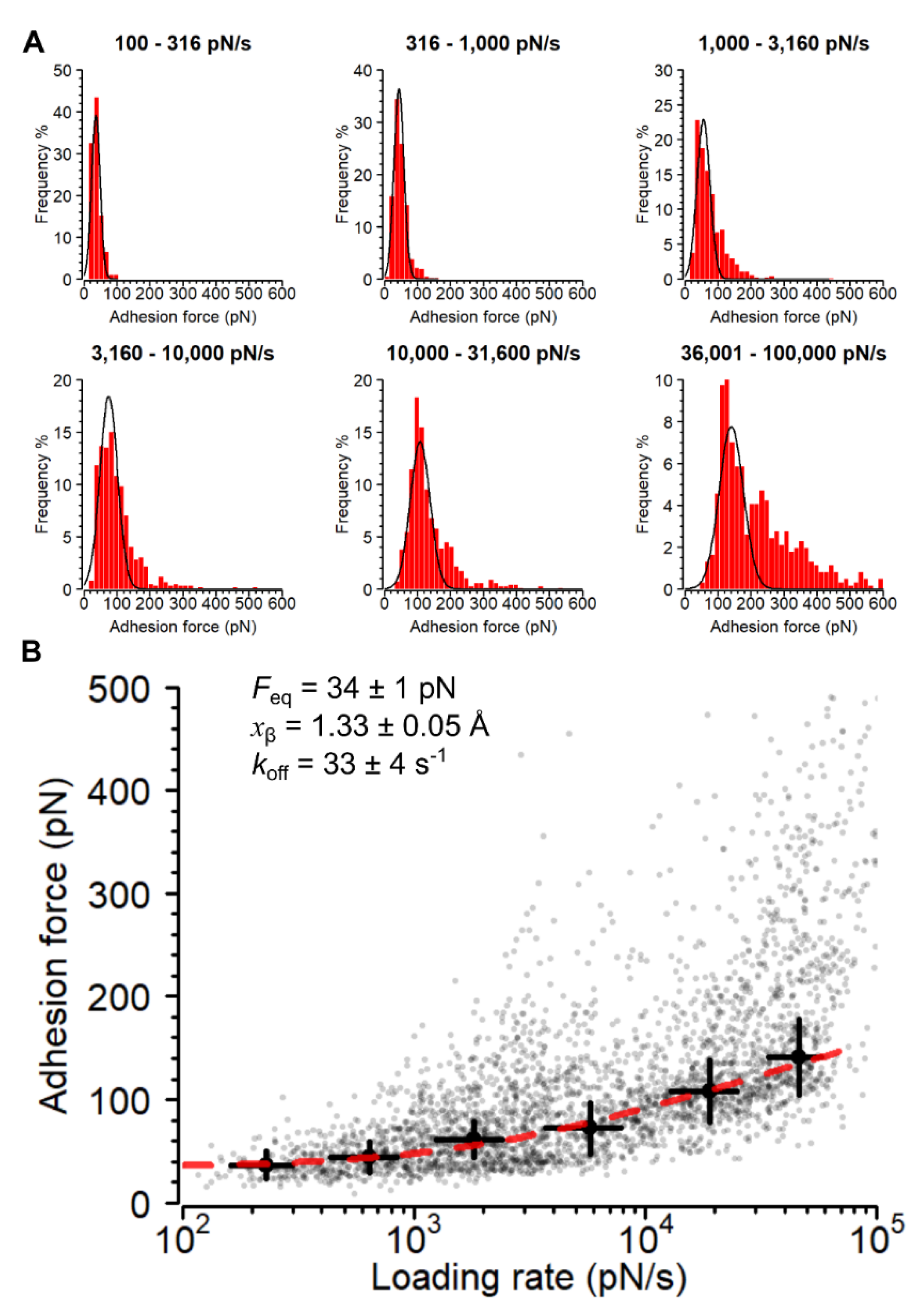
3.3. Strength and Dynamics of Single Ag85-Fn Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- van Dorn, A. Multidrug-resistant Mycobacterium abscessus threatens patients with cystic fibrosis. Lancet Respir. Med. 2017, 5, 15. [Google Scholar] [CrossRef]
- Thomas, J.E.; Taoka, C.R.; Gibbs, B.T.; Fraser, S.L. Fatal pulmonary Mycobacterium abscessus infection in a patient using etanercept. Hawaii Med. J. 2006, 65, 12–15. [Google Scholar] [PubMed]
- Mooren, V.H.J.F.; Bleeker, M.W.P.; van Ingen, J.; Hermans, M.H.A.; Wever, P.C. Disseminated Mycobacterium abscessus infection in a peritoneal dialysis patient. IDCases 2017, 9, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, R.; Espina Angulo, M.J.; Escudero Augusto, D. Cutaneous infection with Mycobacterium abscessus. Intensive Care Med. 2018, 44, 2292–2293. [Google Scholar] [CrossRef]
- Torii, R.; Noguchi, S.; Shimabukuro, I.; Inokuchi, Y.; Tabaru, A.; Yoshii, C.; Yatera, K. Pulmonary Mycobacterium abscessus Infection with Reactive AA Amyloidosis: A Case Report and Brief Review of the Literature. Int. Med. 2019, 58, 557–561. [Google Scholar] [CrossRef]
- Abou-Zeid, C.; Ratliff, T.L.; Wiker, H.G.; Harboe, M.; Bennedsen, J.; Rook, G.A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 1988, 56, 3046–3051. [Google Scholar] [CrossRef]
- Abou-Zeid, C.; Garbe, T.; Lathigra, R.; Wiker, H.G.; Harboe, M.; Rook, G.A.; Young, D.B. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect. Immun. 1991, 59, 2712–2718. [Google Scholar] [CrossRef]
- Ratliff, T.L.; McGarr, J.A.; Abou-Zeid, C.; Rook, G.A.; Stanford, J.L.; Aslanzadeh, J.; Brown, E.J. Attachment of mycobacteria to fibronectin-coated surfaces. J. Gen. Microbiol. 1988, 134, 1307–1313. [Google Scholar] [CrossRef]
- Clark, R.A.; Blakley, S.L.; Greer, D.; Smith, M.H.; Brandon, W.; Wisniewski, T.L. Hematogenous dissemination of Mycobacterium tuberculosis in patients with AIDS. Rev. Infect. Dis. 1991, 13, 1089–1092. [Google Scholar] [CrossRef]
- Wiker, H.G.; Harboe, M. The antigen 85 complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Zlotta, A.; Drowart, A.; van Vooren, J.P.; Simon, J.; Schulman, C.C.; Huygen, K. Evolution of cellular and humoral response against Tuberculin and antigen 85 complex during intravesical treatment with BCG of superficial bladder cancer. Acta Urol. Belg. 1994, 62, 63–68. [Google Scholar] [PubMed]
- Karbalaei Zadeh Babaki, M.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Schou, C.; Yuan, Z.L.; Andersen, A.B.; Bennedsen, J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol. Microbiol. Immunol. Scand. C 1985, 93, 265–272. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Palmer, J.O.; McGarr, J.A.; Brown, E.J. Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: Initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res. 1987, 47, 1762–1766. [Google Scholar]
- Rambukkana, A.; Das, P.K.; Burggraaf, J.D.; Yong, S.; Faber, W.R.; Thole, J.E.; Harboe, M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect. Immun. 1992, 60, 5172–5181. [Google Scholar] [CrossRef]
- Rambukkana, A.; Das, P.K.; Chand, A.; Baas, J.G.; Groothuis, D.G.; Kolk, A.H.J. Subcellular Distribution of Monoclonal Antibody Defined Epitopes on Immunodominant Mycobacterium tuberculosis Proteins in the 30-kDa Region: Identification and Localization of 29/33-kDa Doublet Proteins on Mycobacterial Cell Wall. Scand. J. Immunol. 1991, 33, 763–775. [Google Scholar] [CrossRef]
- Beatty, W.L.; Russell, D.G. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 2000, 68, 6997–7002. [Google Scholar] [CrossRef]
- Hodges, H.L.; Brown, R.A.; Crooks, J.A.; Weibel, D.B.; Kiessling, L.L. Imaging mycobacterial growth and division with a fluorogenic probe. Proc. Natl. Acad. Sci. USA 2018, 115, 5271–5276. [Google Scholar] [CrossRef]
- Belisle, J.T.; Vissa, V.D.; Sievert, T.; Takayama, K.; Brennan, P.J.; Besra, G.S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 1997, 276, 1420–1422. [Google Scholar] [CrossRef]
- Kuo, C.-J.; Bell, H.; Hsieh, C.-L.; Ptak, C.P.; Chang, Y.-F. Novel Mycobacteria Antigen 85 Complex Binding Motif on Fibronectin. J. Biol. Chem. 2012, 287, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Hibbert, S.I.; Quan, X.; Newman, T.; Huygen, K.; Godfrey, H.P. Pathophysiology of antigen 85 in patients with active tuberculosis: Antigen 85 circulates as complexes with fibronectin and immunoglobulin G. Infect. Immun. 1999, 67, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Ohara, N.; Matsumoto, S.; Yamada, T. The novel fibronectin-binding motif and key residues of mycobacteria. J. Biol. Chem. 1998, 273, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Hymes, J.P.; Klaenhammer, T.R. Stuck in the Middle: Fibronectin-Binding Proteins in Gram-Positive Bacteria. Front. Microbiol. 2016, 7, 1504. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Raynaud, C.; Lanéelle, M.A.; Guilhot, C.; Laurent-Winter, C.; Ensergueix, D.; Gicquel, B.; Daffé, M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol. Microbiol. 1999, 31, 1573–1587. [Google Scholar] [CrossRef]
- Puech, V.; Bayan, N.; Salim, K.; Leblon, G.; Daffé, M. Characterization of the in vivo acceptors of the mycoloyl residues transferred by the corynebacterial PS1 and the related mycobacterial antigens 85. Mol. Microbiol. 2000, 35, 1026–1041. [Google Scholar] [CrossRef]
- Bryant, J.M.; Grogono, D.M.; Rodriguez-Rincon, D.; Everall, I.; Brown, K.P.; Moreno, P.; Verma, D.; Hill, E.; Drijkoningen, J.; Gilligan, P.; et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 2016, 354, 751–757. [Google Scholar] [CrossRef]
- Richards, C.J.; Olivier, K.N. Nontuberculous Mycobacteria in Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 8, 259–274. [Google Scholar] [CrossRef]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.-L.; Kremer, L. Glycopeptidolipids, a Double-Edged Sword of the Mycobacterium abscessus Complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Catherinot, E.; Clarissou, J.; Etienne, G.; Ripoll, F.; Emile, J.-F.; Daffé, M.; Perronne, C.; Soudais, C.; Gaillard, J.-L.; Rottman, M. Hypervirulence of a Rough Variant of the Mycobacterium abscessus Type Strain. Infect. Immun. 2007, 75, 1055–1058. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.-L.; Kissa, K.; Dubremetz, J.-F.; Gaillard, J.-L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef] [PubMed]
- Ronning, D.R.; Klabunde, T.; Besra, G.S.; Vissa, V.D.; Belisle, J.T.; Sacchettini, J.C. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat. Struct. Biol. 2000, 7, 141–146. [Google Scholar] [PubMed]
- Anderson, D.H.; Harth, G.; Horwitz, M.A.; Eisenberg, D. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kda major secretory protein (antigen 85B), a mycolyl transferase. J. Mol. Biol. 2001, 307, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Visai, L.; Rindi, S.; Di Poto, A. Purification of human plasma fibronectin using immobilized gelatin and Arg affinity chromatography. Nat. Protoc. 2008, 3, 525–533. [Google Scholar] [CrossRef]
- Beaussart, A.; El-Kirat-Chatel, S.; Herman, P.; Alsteens, D.; Mahillon, J.; Hols, P.; Dufrêne, Y.F. Single-cell force spectroscopy of probiotic bacteria. Biophys. J. 2013, 104, 1886–1892. [Google Scholar] [CrossRef]
- Beaussart, A.; El-Kirat-Chatel, S.; Sullan, R.M.A.; Alsteens, D.; Herman, P.; Derclaye, S.; Dufrêne, Y.F. Quantifying the forces guiding microbial cell adhesion using single-cell force spectroscopy. Nat. Protoc. 2014, 9, 1049–1055. [Google Scholar] [CrossRef]
- Dupres, V.; Menozzi, F.D.; Locht, C.; Clare, B.H.; Abbott, N.L.; Cuenot, S.; Bompard, C.; Raze, D.; Dufrêne, Y.F. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods 2005, 2, 515–520. [Google Scholar] [CrossRef]
- Alsteens, D.; Verbelen, C.; Dague, E.; Raze, D.; Baulard, A.R.; Dufrêne, Y.F. Organization of the mycobacterial cell wall: A nanoscale view. Pflugers Arch. 2008, 456, 117–125. [Google Scholar] [CrossRef]
- Oberhauser, A.F.; Badilla-Fernandez, C.; Carrion-Vazquez, M.; Fernandez, J.M. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J. Mol. Biol. 2002, 319, 433–447. [Google Scholar] [CrossRef]
- Deshayes, C.; Bach, H.; Euphrasie, D.; Attarian, R.; Coureuil, M.; Sougakoff, W.; Laval, F.; Av-Gay, Y.; Daffé, M.; Etienne, G.; et al. MmpS4 promotes glycopeptidolipids biosynthesis and export in Mycobacterium smegmatis. Mol. Microbiol. 2010, 78, 989–1003. [Google Scholar] [CrossRef]
- Verbelen, C.; Dufrêne, Y.F. Direct measurement of Mycobacterium-fibronectin interactions. Integr. Biol. 2009, 1, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Bustanji, Y.; Arciola, C.R.; Conti, M.; Mandello, E.; Montanaro, L.; Samorí, B. Dynamics of the interaction between a fibronectin molecule and a living bacterium under mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 13292–13297. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ituarte, N.N.; Lower, B.H.; Lamlertthon, S.; Fowler, V.G.; Lower, S.K. Dissociation Rate Constants of Human Fibronectin Binding to Fibronectin-binding Proteins on Living Staphylococcus aureus Isolated from Clinical Patients. J. Biol. Chem. 2012, 287, 6693–6701. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ituarte, N.N.; Cruz, C.H.B.; Lins, R.D.; DiBartola, A.C.; Howard, J.; Liang, X.; Höök, M.; Viana, I.F.T.; Sierra-Hernández, M.R.; Lower, S.K. Amino acid polymorphisms in the fibronectin-binding repeats of fibronectin-binding protein A affect bond strength and fibronectin conformation. J. Biol. Chem. 2017, 292, 8797–8810. [Google Scholar] [CrossRef]
- Merkel, R.; Nassoy, P.; Leung, A.; Ritchie, K.; Evans, E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 1999, 397, 50–53. [Google Scholar] [CrossRef]
- Friddle, R.W.; Noy, A.; Yoreo, J.J.D. Interpreting the widespread nonlinear force spectra of intermolecular bonds. Proc. Natl. Acad. Sci. USA 2012, 109, 13573–13578. [Google Scholar] [CrossRef]
- Alsteens, D.; Pfreundschuh, M.; Zhang, C.; Spoerri, P.M.; Coughlin, S.R.; Kobilka, B.K.; Müller, D.J. Imaging G protein-coupled receptors while quantifying their ligand-binding free-energy landscape. Nat. Methods 2015, 12, 845–851. [Google Scholar] [CrossRef]
- Casillas-Ituarte, N.N.; DiBartola, A.C.; Broughton, M.J.; Pérez-Guzmán, L.; Wheeler, R.M.; Ibaraki, M.; Lower, B.A.; Dunn, J.A.; Lower, B.H.; Fowler, V.G.; et al. Fibrinogen binding is affected by amino acid substitutions in C-terminal repeat region of fibronectin binding protein A. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Li, F.; Redick, S.D.; Erickson, H.P.; Moy, V.T. Force measurements of the alpha5beta1 integrin-fibronectin interaction. Biophys. J. 2003, 84, 1252–1262. [Google Scholar] [CrossRef]
- Stahl, S.W.; Nash, M.A.; Fried, D.B.; Slutzki, M.; Barak, Y.; Bayer, E.A.; Gaub, H.E. Single-molecule dissection of the high-affinity cohesin–dockerin complex. Proc. Natl. Acad. Sci. USA 2012, 109, 20431–20436. [Google Scholar] [CrossRef]
- Schoeler, C.; Malinowska, K.H.; Bernardi, R.C.; Milles, L.F.; Jobst, M.A.; Durner, E.; Ott, W.; Fried, D.B.; Bayer, E.A.; Schulten, K.; et al. Ultrastable cellulosome-adhesion complex tightens under load. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, C.; Bernardi, R.C.; Malinowska, K.H.; Durner, E.; Ott, W.; Bayer, E.A.; Schulten, K.; Nash, M.A.; Gaub, H.E. Mapping Mechanical Force Propagation through Biomolecular Complexes. Nano Lett. 2015, 15, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Jobst, M.A.; Milles, L.F.; Schoeler, C.; Ott, W.; Fried, D.B.; Bayer, E.A.; Gaub, H.E.; Nash, M.A. Resolving dual binding conformations of cellulosome cohesin-dockerin complexes using single-molecule force spectroscopy. eLife 2015, 4, e10319. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.C.; Durner, E.; Schoeler, C.; Malinowska, K.H.; Carvalho, B.G.; Bayer, E.A.; Luthey-Schulten, Z.; Gaub, H.E.; Nash, M.A. Mechanisms of Nanonewton Mechanostability in a Protein Complex Revealed by Molecular Dynamics Simulations and Single-Molecule Force Spectroscopy. J. Am. Chem. Soc. 2019, 141, 14752–14763. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, F.; Ruggiero, A.; De Simone, A.; Berisio, R. A structural overview of mycobacterial adhesins: Key biomarkers for diagnostics and therapeutics. Protein Sci. 2018, 27, 369–380. [Google Scholar] [CrossRef]
- Govender, V.S.; Ramsugit, S.; Pillay, M. Mycobacterium tuberculosis adhesins: Potential biomarkers as anti-tuberculosis therapeutic and diagnostic targets. Microbiology 2014, 160, 1821–1831. [Google Scholar] [CrossRef]
- Vinod, V.; Vijayrajratnam, S.; Vasudevan, A.K.; Biswas, R. The cell surface adhesins of Mycobacterium tuberculosis. Microbiol. Res. 2020, 232, 126392. [Google Scholar] [CrossRef]
- Schorey, J.S.; Li, Q.; McCourt, D.W.; Bong-Mastek, M.; Clark-Curtiss, J.E.; Ratliff, T.L.; Brown, E.J. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect Immun. 1995, 63, 2652–2657. [Google Scholar] [CrossRef]
- Becich, M.J.; Carroll, S.; Ratliff, T.L. Internalization of Bacille Calmette-Guerin by Bladder Tumor Cells. J. Urol. 1991, 145, 1316–1324. [Google Scholar] [CrossRef]
- Rao, S.P.; Gehlsen, K.R.; Catanzaro, A. Identification of a beta 1 integrin on Mycobacterium avium-Mycobacterium intracellulare. Infect. Immun. 1992, 60, 3652–3657. [Google Scholar] [CrossRef]
- Kuroda, K.; Brown, E.J.; Telle, W.B.; Russell, D.G.; Ratliff, T.L. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J. Clin. Invest. 1993, 91, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, T.L.; McCarthy, R.; Telle, W.B.; Brown, E.J. Purification of a mycobacterial adhesin for fibronectin. Infect. Immun. 1993, 61, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Shu, W.-P.; Choi Jimmy, C.S.; Margolis Eric, J.; Droller Michael, J.; Liu Brian, C.-S. Bacillus Calmette-Guérin Interacts with the Carboxyl-Terminal Heparin Binding Domain of Fibronectin: Implications for BCG-Mediated Antitumor Activity. J. Urol. 1994, 152, 1275–1280. [Google Scholar] [CrossRef]
- Schorey, J.S.; Holsti, M.A.; Ratliff, T.L.; Allen, P.M.; Brown, E.J. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol. Microbiol. 1996, 21, 321–329. [Google Scholar] [CrossRef]
- Zhao, W.; Schorey, J.S.; Groger, R.; Allen, P.M.; Brown, E.J.; Ratliff, T.L. Characterization of the Fibronectin Binding Motif for a Unique Mycobacterial Fibronectin Attachment Protein, FAP. J. Biol. Chem. 1999, 274, 4521–4526. [Google Scholar] [CrossRef]
- Middleton, A.M.; Chadwick, M.V.; Nicholson, A.G.; Dewar, A.; Groger, R.K.; Brown, E.J.; Wilson, R. The role of Mycobacterium avium complex fibronectin attachment protein in adherence to the human respiratory mucosa. Mol. Microbiol. 2000, 38, 381–391. [Google Scholar] [CrossRef]
- Persat, A.; Nadell, C.D.; Kim, M.K.; Ingremeau, F.; Siryaporn, A.; Drescher, K.; Wingreen, N.S.; Bassler, B.L.; Gitai, Z.; Stone, H.A. The mechanical world of bacteria. Cell 2015, 161, 988–997. [Google Scholar] [CrossRef]
- Shenkman, B.; Rubinstein, E.; Cheung, A.L.; Brill, G.E.; Dardik, R.; Tamarin, I.; Savion, N.; Varon, D. Adherence Properties of Staphylococcus aureus under Static and Flow Conditions: Roles of agrand sar Loci, Platelets, and Plasma Ligands. Infect. Immun. 2001, 69, 4473–4478. [Google Scholar] [CrossRef]
- Claes, J.; Ditkowski, B.; Liesenborghs, L.; Veloso, T.R.; Entenza, J.M.; Moreillon, P.; Vanassche, T.; Verhamme, P.; Hoylaerts, M.F.; Heying, R. Assessment of the Dual Role of Clumping Factor A in S. aureus Adhesion to Endothelium in Absence and Presence of Plasma. Thromb. Haemost. 2018, 118, 1230–1241. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Crosby, H.A.; Valotteau, C.; Hippensteel, J.A.; Nayak, M.K.; Chauhan, A.K.; Schmidt, E.P.; Dufrêne, Y.F.; Horswill, A.R. Staphylococcus aureus adhesion in endovascular infections is controlled by the ArlRS–MgrA signaling cascade. PLoS Pathog. 2019, 15, e1007800. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Watts, G.; Crowther, J.E.; Balagopal, A.; Torrelles, J.B.; Robison-Cox, J.; Bargatze, R.F.; Harmsen, A.G.; Crouch, E.C.; Schlesinger, L.S. Mycobacterium tuberculosis binding to human surfactant proteins A and D, fibronectin, and small airway epithelial cells under shear conditions. Infect. Immun. 2006, 74, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Tarran, R.; Button, B.; Picher, M.; Paradiso, A.M.; Ribeiro, C.M.; Lazarowski, E.R.; Zhang, L.; Collins, P.L.; Pickles, R.J.; Fredberg, J.J.; et al. Normal and Cystic Fibrosis Airway Surface Liquid Homeostasis. J. Biol. Chem. 2005, 280, 35751–35759. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; Backus, K.M.; Barry, C.E.; Davis, B.G. ESI-MS assay of M. tuberculosis cell wall antigen 85 enzymes permits substrate profiling and design of a mechanism-based inhibitor. J. Am. Chem. Soc. 2011, 133, 13232–13235. [Google Scholar] [CrossRef] [PubMed]
- Warrier, T.; Tropis, M.; Werngren, J.; Diehl, A.; Gengenbacher, M.; Schlegel, B.; Schade, M.; Oschkinat, H.; Daffe, M.; Hoffner, S.; et al. Antigen 85C inhibition restricts Mycobacterium tuberculosis growth through disruption of cord factor biosynthesis. Antimicrob. Agents Chemother. 2012, 56, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Favrot, L.; Grzegorzewicz, A.E.; Lajiness, D.H.; Marvin, R.K.; Boucau, J.; Isailovic, D.; Jackson, M.; Ronning, D.R. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nat. Commun. 2013, 4, 2748. [Google Scholar] [CrossRef] [PubMed]
- Favrot, L.; Lajiness, D.H.; Ronning, D.R. Inactivation of the Mycobacterium tuberculosis antigen 85 complex by covalent, allosteric inhibitors. J. Biol. Chem. 2014, 289, 25031–25040. [Google Scholar] [CrossRef]
- Gahoi, S.; Mandal, R.S.; Ivanisenko, N.; Shrivastava, P.; Jain, S.; Singh, A.K.; Raghunandanan, M.V.; Kanchan, S.; Taneja, B.; Mandal, C.; et al. Computational screening for new inhibitors of M. tuberculosis mycolyltransferases antigen 85 group of proteins as potential drug targets. J. Biomol. Struct. Dyn. 2013, 31, 30–43. [Google Scholar] [CrossRef]
- Goins, C.M.; Dajnowicz, S.; Smith, M.D.; Parks, J.M.; Ronning, D.R. Mycolyltransferase from Mycobacterium tuberculosis in covalent complex with tetrahydrolipstatin provides insights into antigen 85 catalysis. J. Biol. Chem. 2018, 293, 3651–3662. [Google Scholar] [CrossRef]
- Goins, C.M.; Dajnowicz, S.; Thanna, S.; Sucheck, S.J.; Parks, J.M.; Ronning, D.R. Exploring Covalent Allosteric Inhibition of Antigen 85C from Mycobacterium tuberculosis by Ebselen Derivatives. ACS Infect. Dis. 2017, 3, 378–387. [Google Scholar] [CrossRef]
- Viljoen, A.; Richard, M.; Nguyen, P.C.; Fourquet, P.; Camoin, L.; Paudal, R.R.; Gnawali, G.R.; Spilling, C.D.; Cavalier, J.-F.; Canaan, S.; et al. Cyclipostins and Cyclophostin analogs inhibit the antigen 85C from Mycobacterium tuberculosis both in vitro and in vivo. J. Biol. Chem. 2018, 293, 2755–2769. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viljoen, A.; Alsteens, D.; Dufrêne, Y. Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin. Cells 2020, 9, 716. https://doi.org/10.3390/cells9030716
Viljoen A, Alsteens D, Dufrêne Y. Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin. Cells. 2020; 9(3):716. https://doi.org/10.3390/cells9030716
Chicago/Turabian StyleViljoen, Albertus, David Alsteens, and Yves Dufrêne. 2020. "Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin" Cells 9, no. 3: 716. https://doi.org/10.3390/cells9030716
APA StyleViljoen, A., Alsteens, D., & Dufrêne, Y. (2020). Mechanical Forces between Mycobacterial Antigen 85 Complex and Fibronectin. Cells, 9(3), 716. https://doi.org/10.3390/cells9030716




