Loss of Cx43 in Murine Sertoli Cells Leads to Altered Prepubertal Sertoli Cell Maturation and Impairment of the Mitosis-Meiosis Switch
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of SCCx43KO Mice
2.2. Tissue Sampling and Treatment
2.3. Histochemical Techniques and ‘Cell Counting’
2.4. Immunohistochemistry
2.5. Next-Generation Sequencing and Real-Time Reverse Transcription-PCR
2.5.1. RNA Extraction
2.5.2. mRNA Sequencing
2.5.3. cDNA Synthesis and Quantitative Real-Time Reverse Transcription-PCR
2.5.4. Statistical and Bioinformatics Analysis
2.5.5. Further Analysis of Differentially Expressed Genes
3. Results
3.1. Confirmation of Cx43 Gene Loss on Protein Level
3.1.1. β-galactosidase Immunohistochemistry
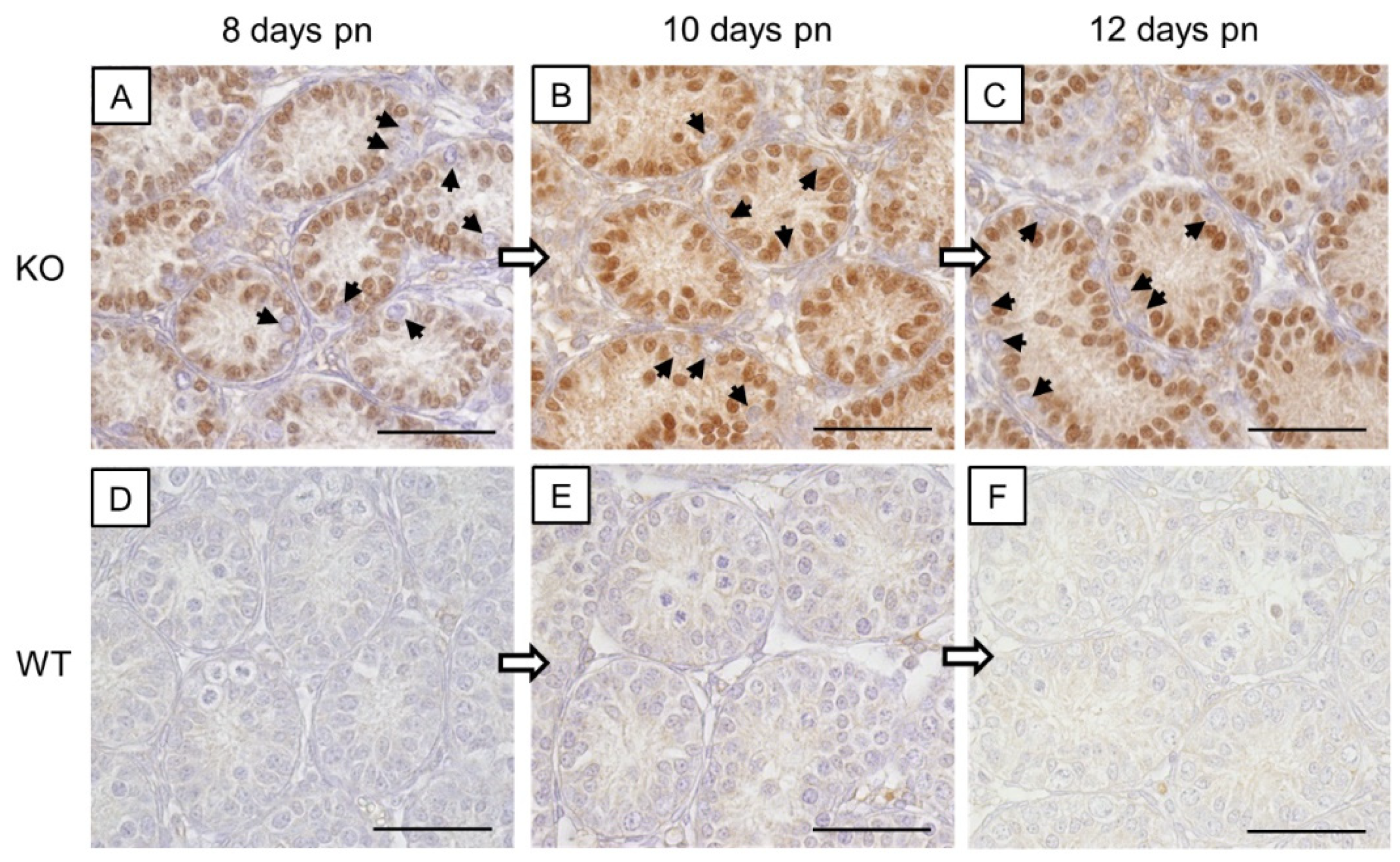
3.1.2. Cx43 Immunohistochemistry
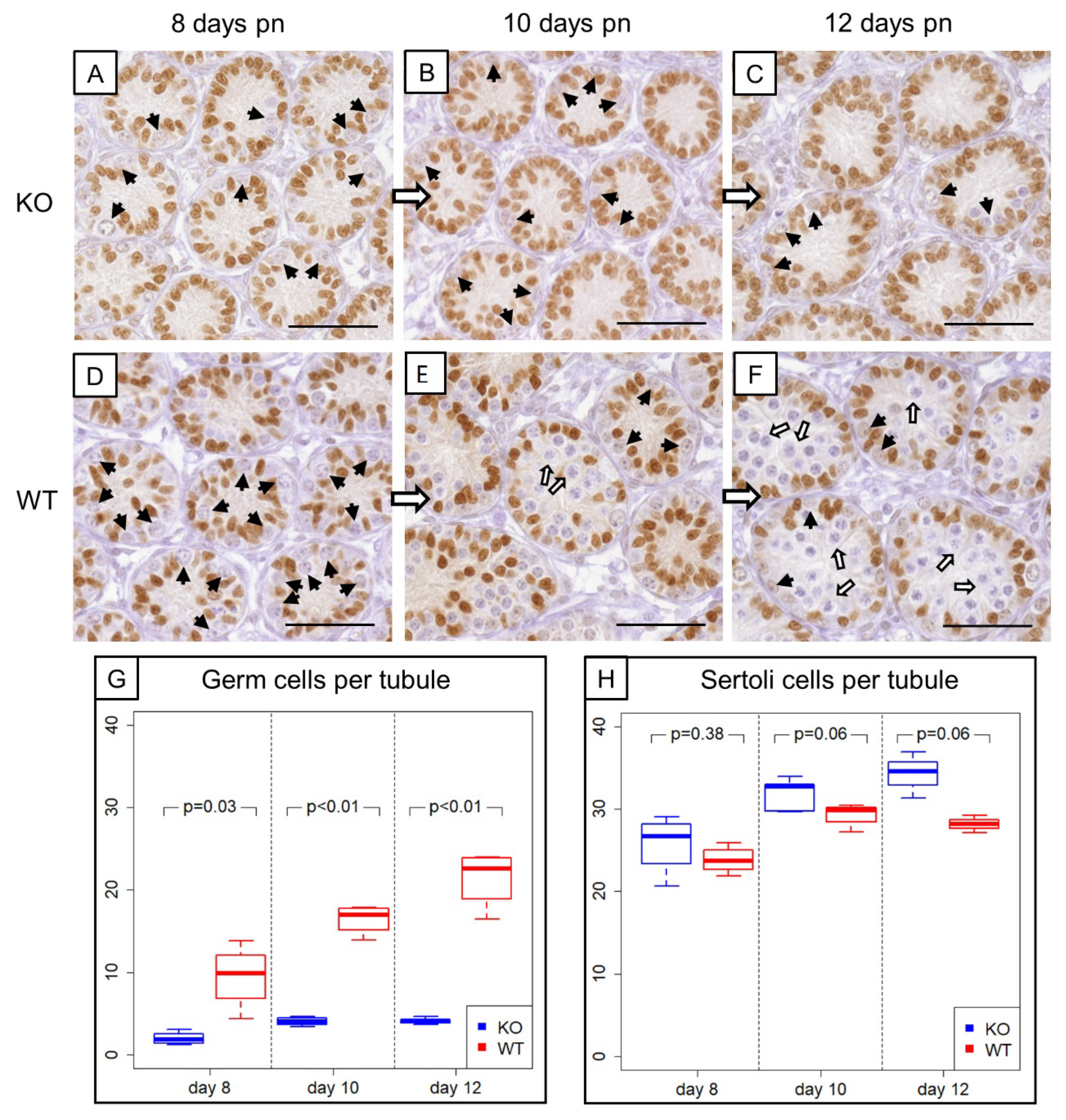
3.2. Prepubertal SCCx43KO Mice Show Evident Differences in the Composition of Intratubular Cells
3.3. Next-Generation Sequencing
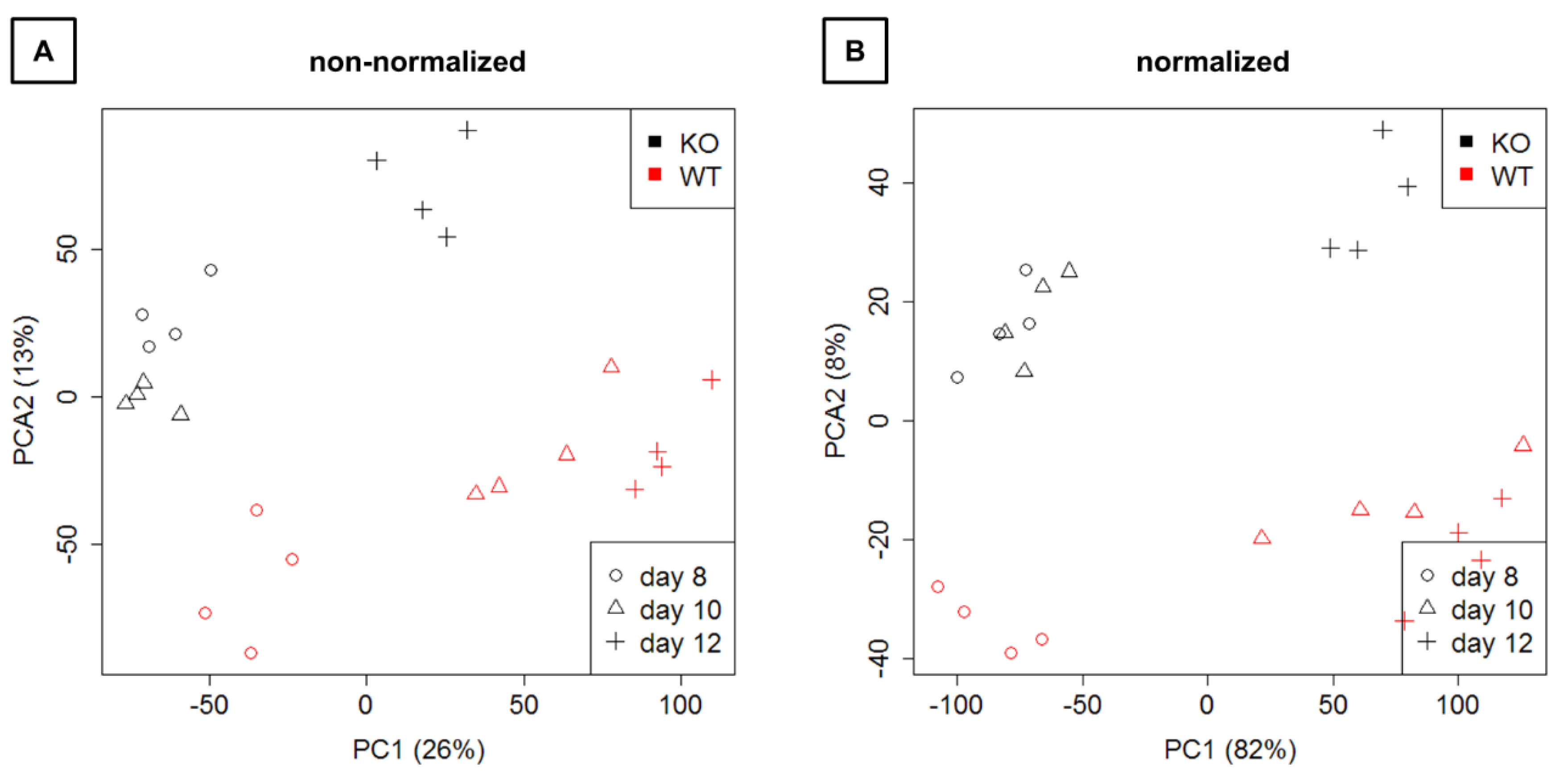
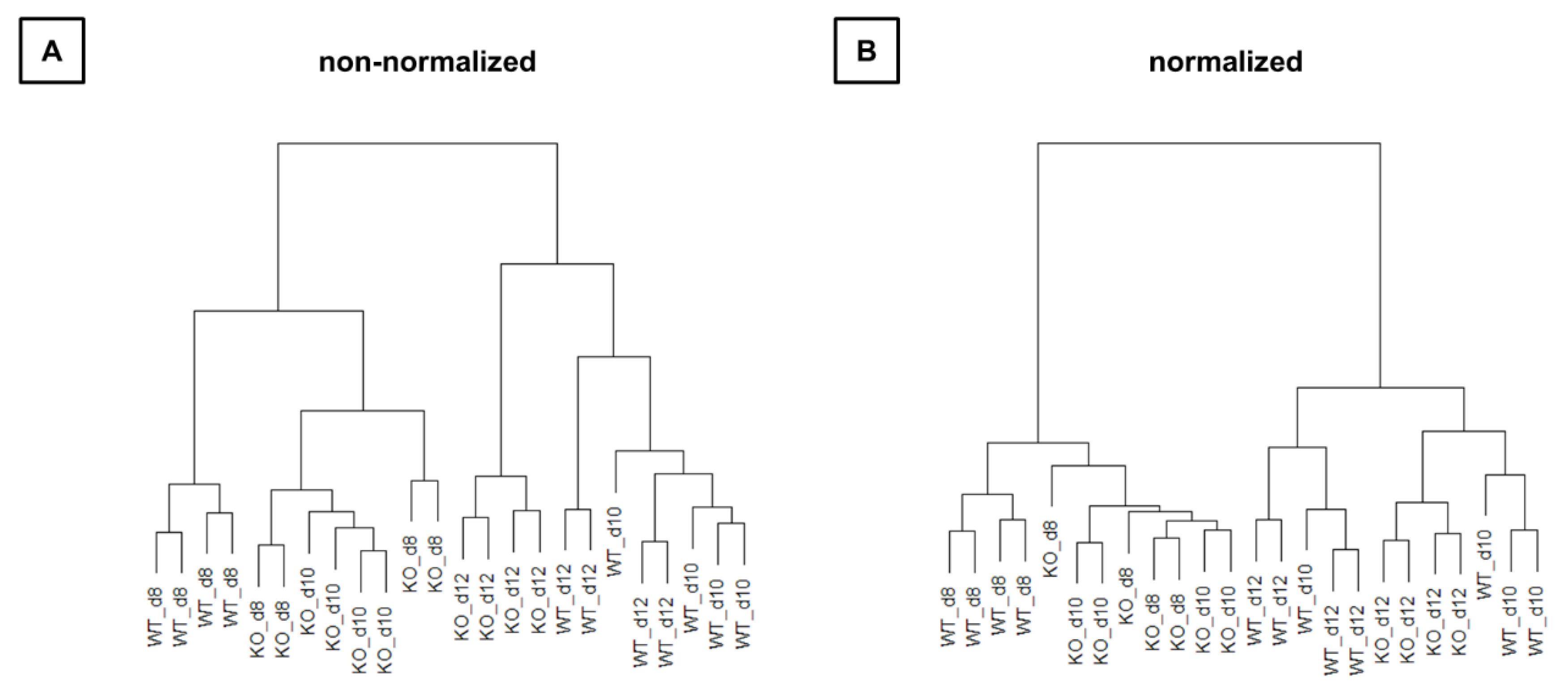
3.3.1. Preprocessing and Data Exploration
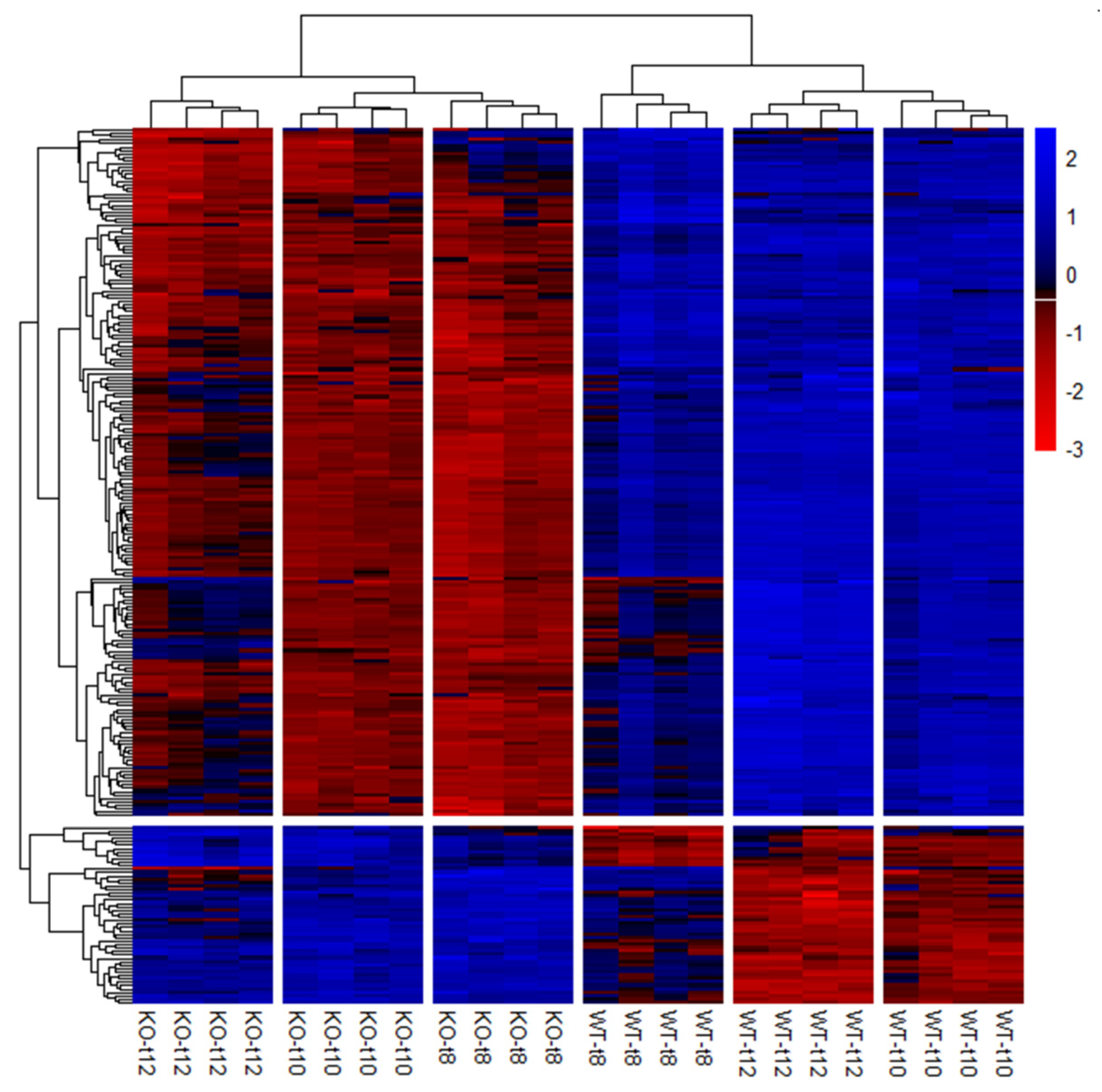
3.3.2. Differential Expression Analysis
3.3.3. Gene Ontology Analysis by GSEA
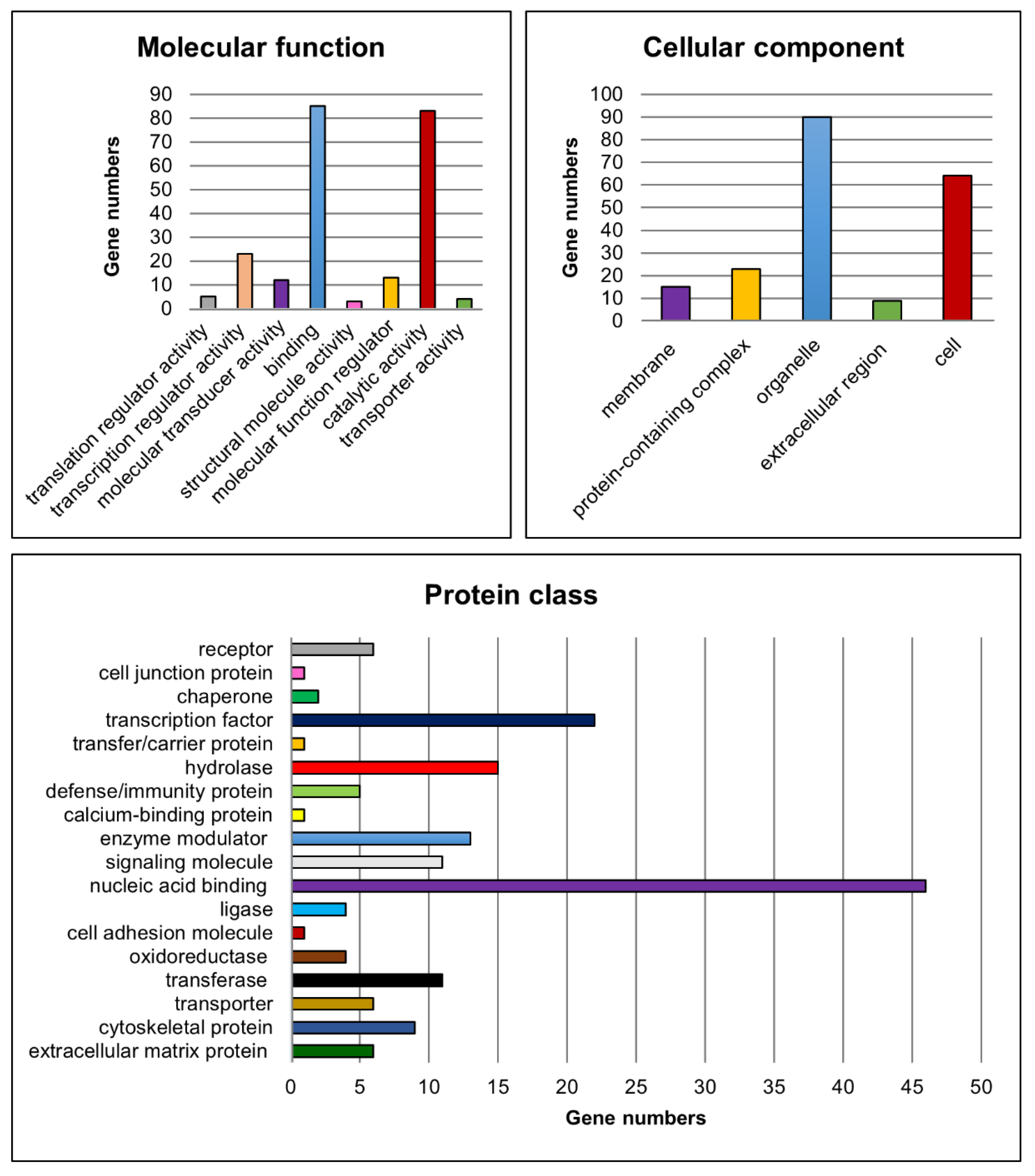
3.3.4. PANTHER Pathway Analysis of Significantly differentially Expressed Genes
3.3.5. Further Characterization of Differentially Expressed Genes
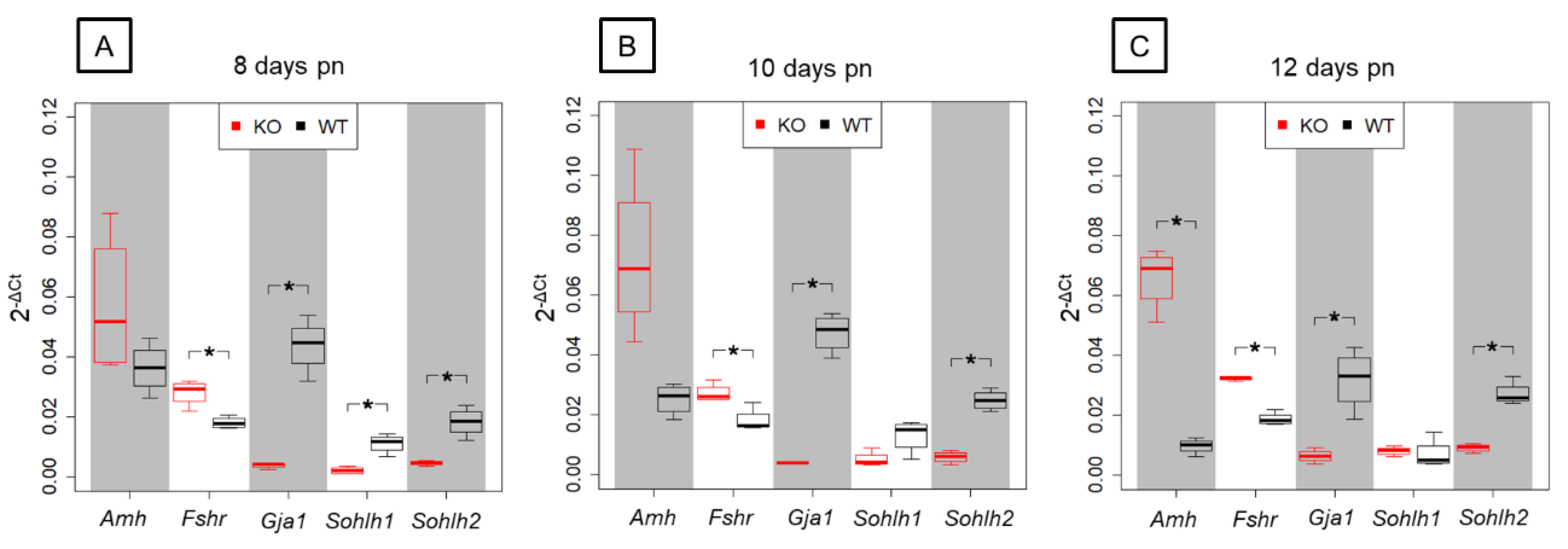
3.4. Confirmation of NGS Candidate Genes by qRT-PCR
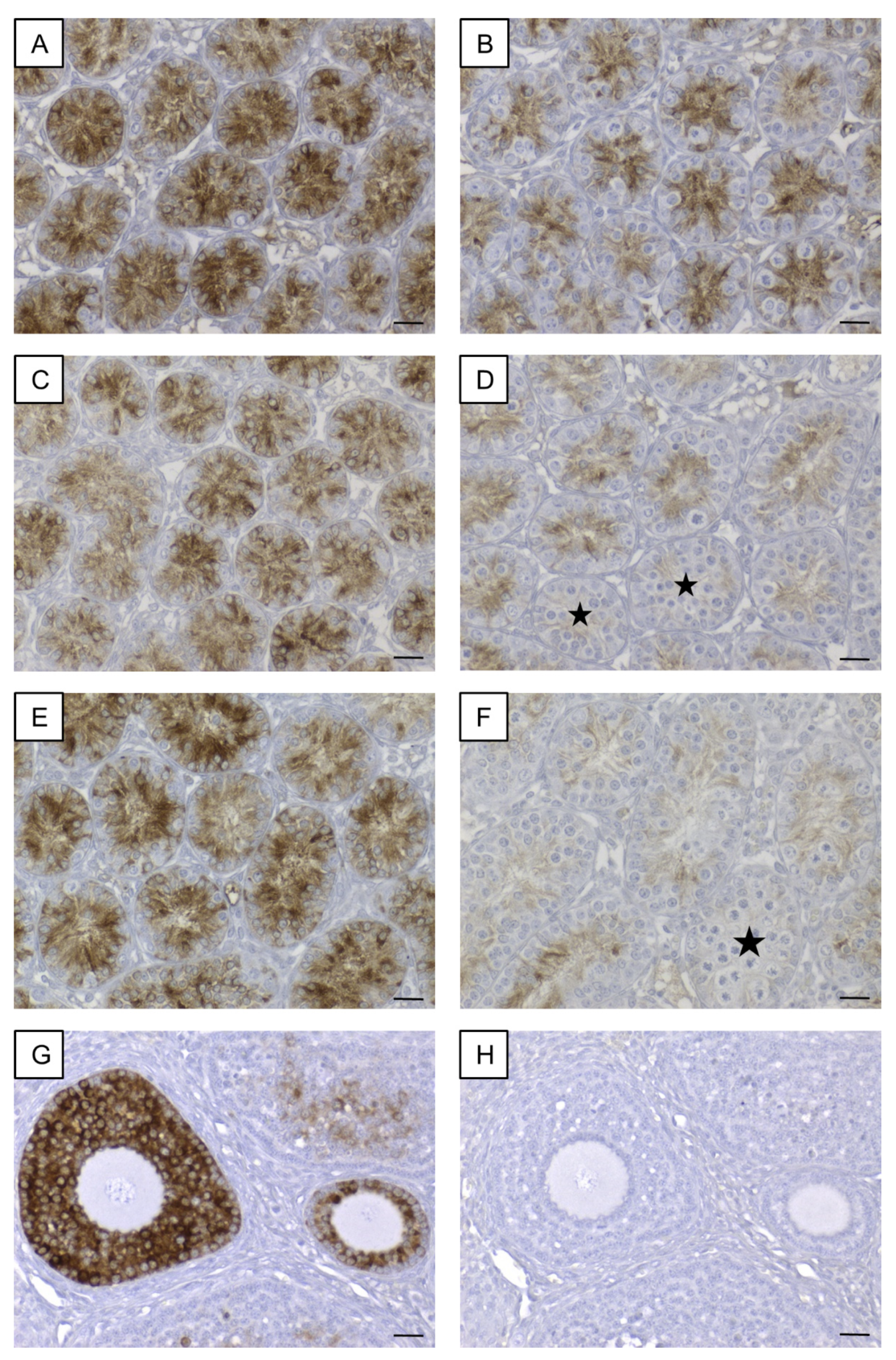
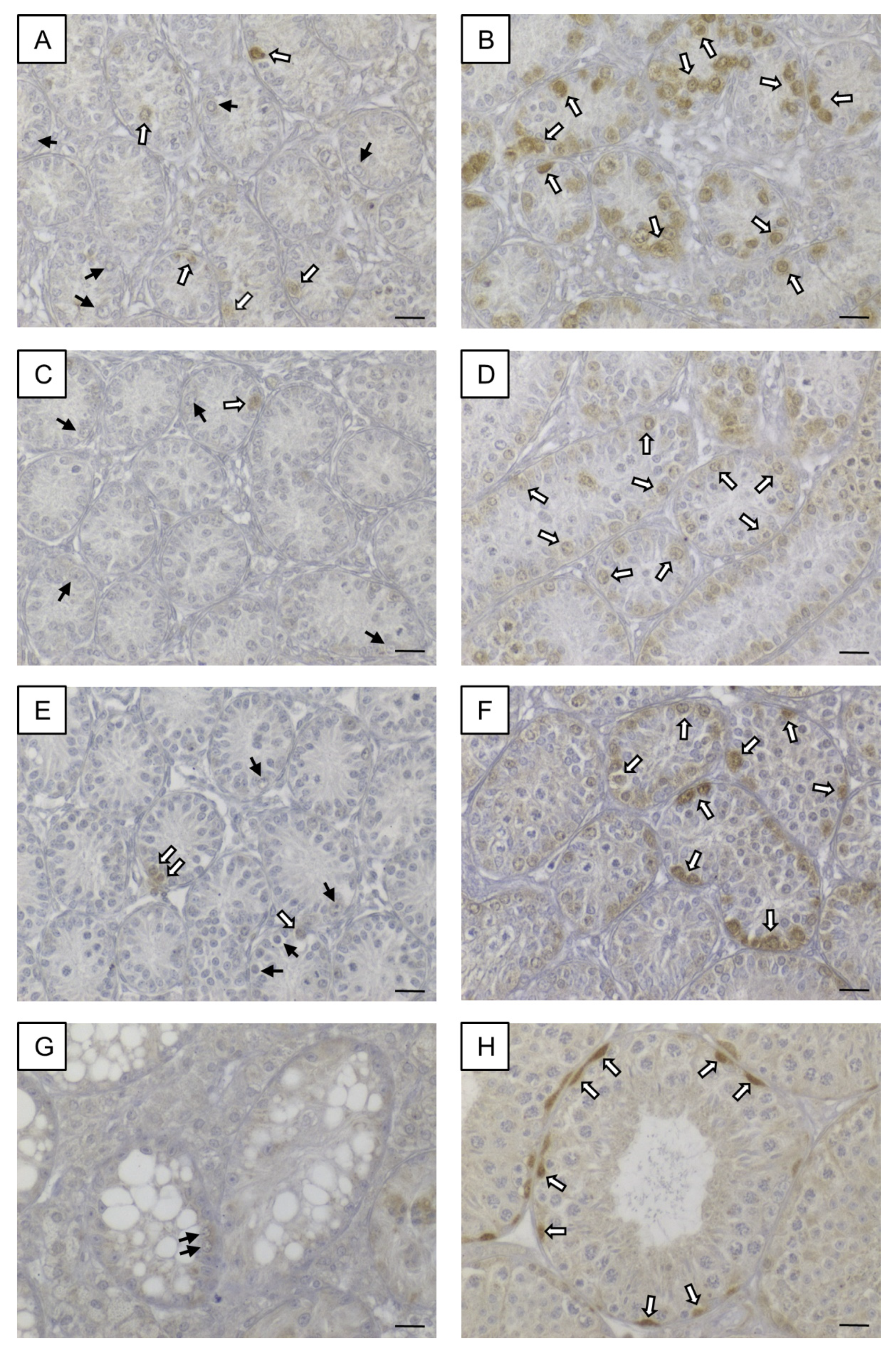
3.5. Confirmation of Candidate Genes at Protein Level by Immunohistochemistry
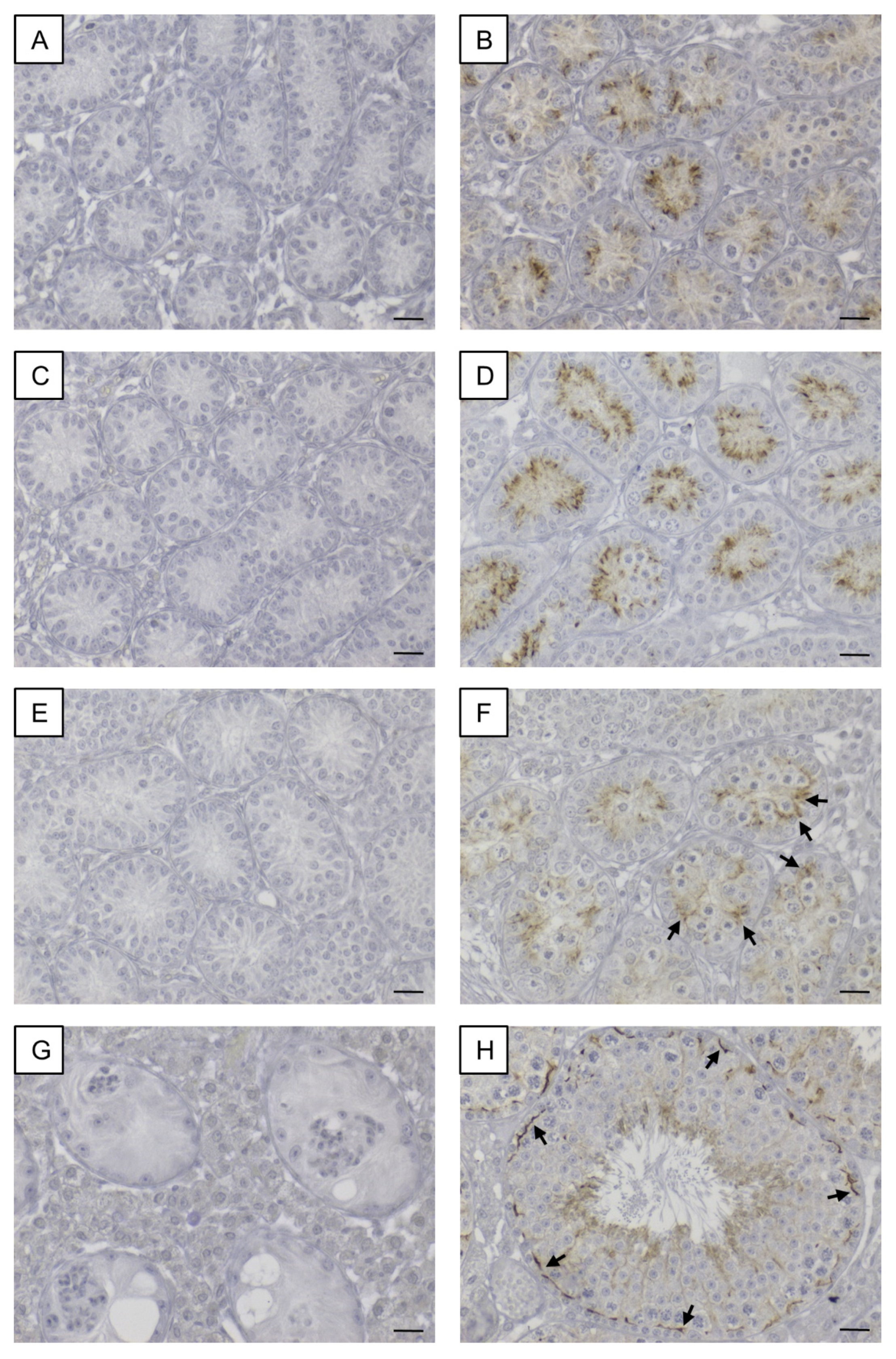
3.5.1. AMH
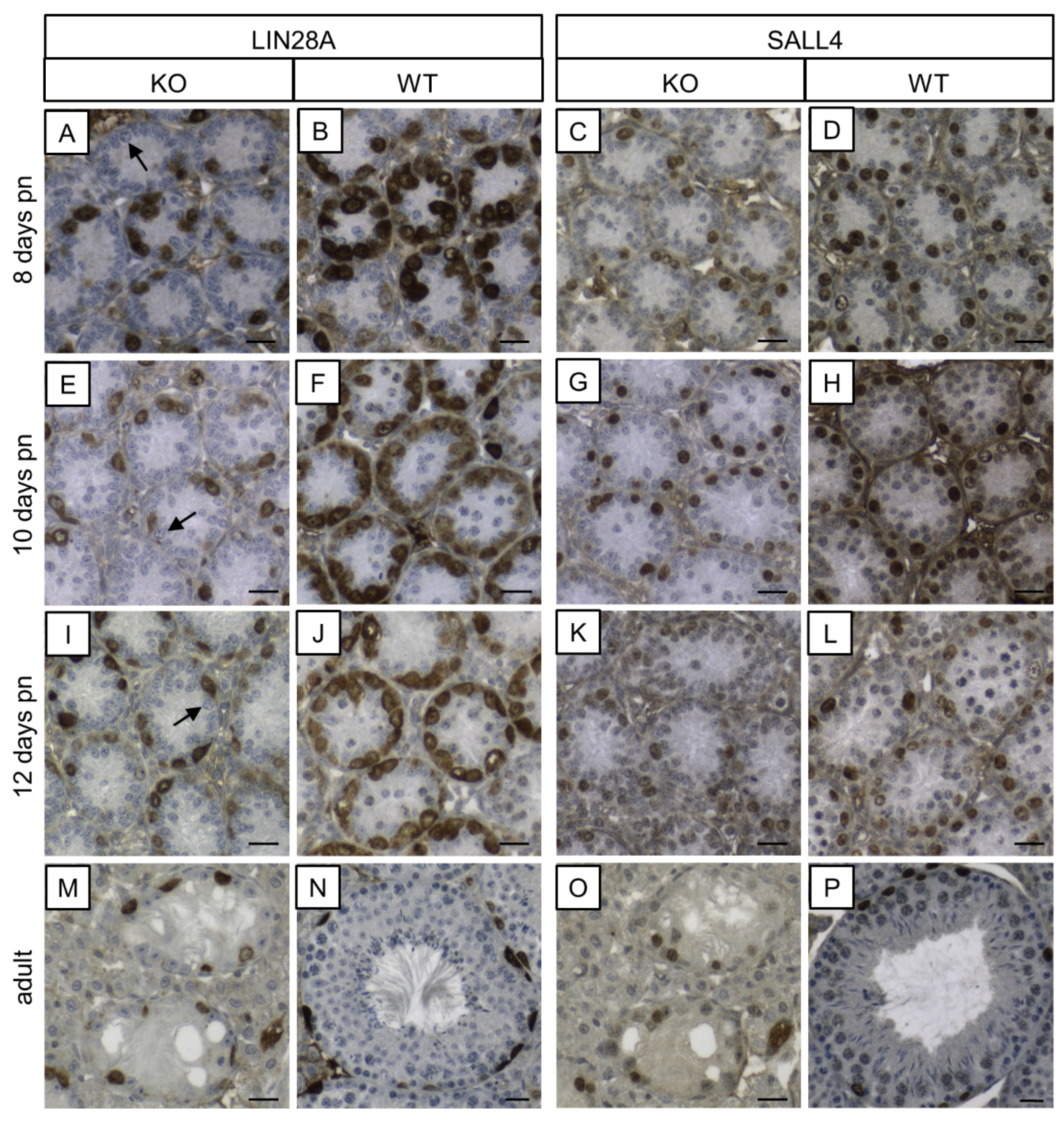
3.5.2. LIN28A and SALL4
3.5.3. SOHLH1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyamoto, T.; Tsujimura, A.; Miyagawa, Y.; Koh, E.; Namiki, M.; Sengoku, K. Male infertility and its causes in human. Adv. Urol. 2012, 2012, 384520. [Google Scholar] [CrossRef]
- Povey, A.C.; Stocks, S.J. Epidemiology and trends in male subfertility. Hum. Fertil. (Camb.) 2010, 13, 182–188. [Google Scholar] [CrossRef]
- Pointis, G.; Gilleron, J.; Carette, D.; Segretain, D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis 2011, 1, 303–317. [Google Scholar] [CrossRef]
- Asklund, C.; Jorgensen, N.; Kold Jensen, T.; Skakkebaek, N.E. Biology and epidemiology of testicular dysgenesis syndrome. BJU Int. 2004, 93, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Steger, K.; Tetens, F.; Bergmann, M. Expression of connexin 43 in human testis. Histochem. Cell Biol. 1999, 112, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Brehm, R.; Marks, A.; Rey, R.; Kliesch, S.; Bergmann, M.; Steger, K. Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J. Pathol. 2002, 197, 647–653. [Google Scholar] [CrossRef]
- Defamie, N.; Berthaut, I.; Mograbi, B.; Chevallier, D.; Dadoune, J.P.; Fenichel, P.; Segretain, D.; Pointis, G. Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: A marker of undifferentiated Sertoli cells. Lab. Investig. 2003, 83, 449–456. [Google Scholar] [CrossRef]
- Giese, S.; Hossain, H.; Markmann, M.; Chakraborty, T.; Tchatalbachev, S.; Guillou, F.; Bergmann, M.; Failing, K.; Weider, K.; Brehm, R. Sertoli-cell-specific knockout of connexin 43 leads to multiple alterations in testicular gene expression in prepubertal mice. Dis. Model. Mech. 2012, 5, 895–913. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Paul, D.L. Connections with connexins: The molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996, 238, 1–27. [Google Scholar] [CrossRef]
- Thevenin, A.F.; Kowal, T.J.; Fong, J.T.; Kells, R.M.; Fisher, C.G.; Falk, M.M. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology (Bethesda) 2013, 28, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Guldenagel, M.; Deutsch, U.; Sohl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Goodenough, D.A. The cellular Internet: On-line with connexins. Bioessays 1996, 18, 709–718. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Batias, C.; Siffroi, J.P.; Fenichel, P.; Pointis, G.; Segretain, D. Connexin43 gene expression and regulation in the rodent seminiferous epithelium. J. Histochem. Cytochem. 2000, 48, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Risley, M.S.; Tan, I.P.; Roy, C.; Saez, J.C. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J. Cell Sci. 1992, 103, 81–96. [Google Scholar]
- Batias, C.; Defamie, N.; Lablack, A.; Thepot, D.; Fenichel, P.; Segretain, D.; Pointis, G. Modified expression of testicular gap-junction connexin 43 during normal spermatogenic cycle and in altered spermatogenesis. Cell Tissue Res. 1999, 298, 113–121. [Google Scholar] [CrossRef]
- Bravo-Moreno, J.F.; Diaz-Sanchez, V.; Montoya-Flores, J.G.; Lamoyi, E.; Saez, J.C.; Perez-Armendariz, E.M. Expression of connexin43 in mouse Leydig, Sertoli, and germinal cells at different stages of postnatal development. Anat. Rec 2001, 264, 13–24. [Google Scholar] [CrossRef]
- Juneja, S.C.; Barr, K.J.; Enders, G.C.; Kidder, G.M. Defects in the germ line and gonads of mice lacking connexin43. Biol. Reprod. 1999, 60, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Juneja, S.C. mRNA expression pattern of multiple members of connexin gene family in normal and abnormal fetal gonads in mouse. Indian J. Physiol. Pharmacol. 2003, 47, 147–156. [Google Scholar] [PubMed]
- Perez-Armendariz, E.M.; Lamoyi, E.; Mason, J.I.; Cisneros-Armas, D.; Luu-The, V.; Bravo Moreno, J.F. Developmental regulation of connexin 43 expression in fetal mouse testicular cells. Anat. Rec. 2001, 264, 237–246. [Google Scholar] [CrossRef]
- Roscoe, W.A.; Barr, K.J.; Mhawi, A.A.; Pomerantz, D.K.; Kidder, G.M. Failure of spermatogenesis in mice lacking connexin43. Biol. Reprod. 2001, 65, 829–838. [Google Scholar] [CrossRef]
- Sridharan, S.; Simon, L.; Meling, D.D.; Cyr, D.G.; Gutstein, D.E.; Fishman, G.I.; Guillou, F.; Cooke, P.S. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 2007, 76, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Brehm, R.; Zeiler, M.; Ruttinger, C.; Herde, K.; Kibschull, M.; Winterhager, E.; Willecke, K.; Guillou, F.; Lecureuil, C.; Steger, K.; et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 2007, 171, 19–31. [Google Scholar] [CrossRef]
- Brehm, R.; Rey, R.; Kliesch, S.; Steger, K.; Marks, A.; Bergmann, M. Mitotic activity of Sertoli cells in adult human testis: An immunohistochemical study to characterize Sertoli cells in testicular cords from patients showing testicular dysgenesis syndrome. Anat. Embryol. (Berl.) 2006, 211, 223–236. [Google Scholar] [CrossRef]
- Roger, C.; Mograbi, B.; Chevallier, D.; Michiels, J.F.; Tanaka, H.; Segretain, D.; Pointis, G.; Fenichel, P. Disrupted traffic of connexin 43 in human testicular seminoma cells: Overexpression of Cx43 induces membrane location and cell proliferation decrease. J. Pathol. 2004, 202, 241–246. [Google Scholar] [CrossRef]
- Steiner, M.; Weipoltshammer, K.; Viehberger, G.; Meixner, E.M.; Lunglmayr, G.; Schofer, C. Immunohistochemical expression analysis of Cx43, Cx26, c-KIT and PlAP in contralateral testis biopsies of patients with non-seminomatous testicular germ cell tumor. Histochem. Cell Biol. 2011, 135, 73–81. [Google Scholar] [CrossRef]
- De Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar]
- Drumond, A.L.; Meistrich, M.L.; Chiarini-Garcia, H. Spermatogonial morphology and kinetics during testis development in mice: A high-resolution light microscopy approach. Reproduction 2011, 142, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bellve, A.R.; Cavicchia, J.C.; Millette, C.F.; O’Brien, D.A.; Bhatnagar, Y.M.; Dym, M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977, 74, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Murphy, M.W.; Gearhart, M.D.; Bardwell, V.J.; Zarkower, D. The mammalian Doublesex homolog DMRT6 coordinates the transition between mitotic and meiotic developmental programs during spermatogenesis. Development 2014, 141, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Hilbold, E.; Bergmann, M.; Fietz, D.; Kliesch, S.; Weidner, W.; Langeheine, M.; Rode, K.; Brehm, R. Immunolocalization of DMRTB1 in human testis with normal and impaired spermatogenesis. Andrology 2019, 7, 428–440. [Google Scholar] [CrossRef]
- Weider, K.; Bergmann, M.; Giese, S.; Guillou, F.; Failing, K.; Brehm, R. Altered differentiation and clustering of Sertoli cells in transgenic mice showing a Sertoli cell specific knockout of the connexin 43 gene. Differentiation 2011, 82, 38–49. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef]
- Hollenbach, J.; Jung, K.; Noelke, J.; Gasse, H.; Pfarrer, C.; Koy, M.; Brehm, R. Loss of connexin43 in murine Sertoli cells and its effect on blood-testis barrier formation and dynamics. PLoS ONE 2018, 13, e0198100. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Consortium, T.G.O. Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mi, H.; Thomas, P. PANTHER pathway: An ontology-based pathway database coupled with data analysis tools. Methods Mol. Biol. 2009, 563, 123–140. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Cappallo-Obermann, H.; Feig, C.; Schulze, W.; Spiess, A.N. Fold-change correction values for testicular somatic transcripts in gene expression studies of human spermatogenesis. Hum. Reprod. 2013, 28, 590–598. [Google Scholar] [CrossRef]
- Ballow, D.; Meistrich, M.L.; Matzuk, M.; Rajkovic, A. Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 2006, 294, 161–167. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.; Weider, K.; Hambruch, N.; Brehm, R. Loss of connexin43 (Cx43) in Sertoli cells leads to spatio-temporal alterations in occludin expression. Histol. Histopathol. 2014, 29, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.L.; Bucco, R.A.; Schmitt, M.C.; Wardlaw, S.A.; Ong, D.E. Localization of cellular retinoic acid-binding protein (CRABP) II and CRABP in developing rat testis. Endocrinology 1996, 137, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. 8—The initiation of spermatogenesis and the cycle of the seminiferous epithelium. In Sertoli Cell Biology, 2nd ed.; Griswold, M.D., Ed.; Academic Press: Oxford, UK, 2015; pp. 233–245. [Google Scholar] [CrossRef]
- Wang, P.J.; Page, D.C.; McCarrey, J.R. Differential expression of sex-linked and autosomal germ-cell-specific genes during spermatogenesis in the mouse. Hum. Mol. Genet. 2005, 14, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Hsu, T.H.; Yen, P.H. Localization of ubiquitin specific protease 26 at blood-testis barrier and near Sertoli cell-germ cell interface in mouse testes. Int. J. Androl. 2011, 34, e368–e377. [Google Scholar] [CrossRef] [PubMed]
- Wosnitzer, M.S.; Mielnik, A.; Dabaja, A.; Robinson, B.; Schlegel, P.N.; Paduch, D.A. Ubiquitin Specific Protease 26 (USP26) expression analysis in human testicular and extragonadal tissues indicates diverse action of USP26 in cell differentiation and tumorigenesis. PLoS ONE 2014, 9, e98638. [Google Scholar] [CrossRef]
- Felipe-Medina, N.; Gomez, H.L.; Condezo, Y.B.; Sanchez-Martin, M.; Barbero, J.L.; Ramos, I.; Llano, E.; Pendas, A.M. Ubiquitin-specific protease 26 (USP26) is not essential for mouse gametogenesis and fertility. Chromosoma 2019, 128, 237–247. [Google Scholar] [CrossRef]
- Gray, P.A.; Fu, H.; Luo, P.; Zhao, Q.; Yu, J.; Ferrari, A.; Tenzen, T.; Yuk, D.I.; Tsung, E.F.; Cai, Z.; et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 2004, 306, 2255–2257. [Google Scholar] [CrossRef]
- Harding, S.D.; Armit, C.; Armstrong, J.; Brennan, J.; Cheng, Y.; Haggarty, B.; Houghton, D.; Lloyd-MacGilp, S.; Pi, X.; Roochun, Y.; et al. The GUDMAP database--an online resource for genitourinary research. Development 2011, 138, 2845–2853. [Google Scholar] [CrossRef]
- Mlacki, M.; Kikulska, A.; Krzywinska, E.; Pawlak, M.; Wilanowski, T. Recent discoveries concerning the involvement of transcription factors from the Grainyhead-like family in cancer. Exp. Biol. Med. (Maywood) 2015, 240, 1396–1401. [Google Scholar] [CrossRef]
- Zhou, Q.; Nie, R.; Li, Y.; Friel, P.; Mitchell, D.; Hess, R.A.; Small, C.; Griswold, M.D. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: An in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 2008, 79, 35–42. [Google Scholar] [CrossRef]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; de Rooij, D.G.; van Pelt, A.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef]
- Yang, F.; Eckardt, S.; Leu, N.A.; McLaughlin, K.J.; Wang, P.J. Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J. Cell Biol. 2008, 180, 673–679. [Google Scholar] [CrossRef]
- Libby, B.J.; Reinholdt, L.G.; Schimenti, J.C. Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 15706–15711. [Google Scholar] [CrossRef]
- Libby, B.J.; De La Fuente, R.; O’Brien, M.J.; Wigglesworth, K.; Cobb, J.; Inselman, A.; Eaker, S.; Handel, M.A.; Eppig, J.J.; Schimenti, J.C. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev. Biol. 2002, 242, 174–187. [Google Scholar] [CrossRef]
- Reinholdt, L.G.; Schimenti, J.C. Mei1 is epistatic to Dmc1 during mouse meiosis. Chromosoma 2005, 114, 127–134. [Google Scholar] [CrossRef]
- Dai, X.; Schonbaum, C.; Degenstein, L.; Bai, W.; Mahowald, A.; Fuchs, E. The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice. Genes Dev. 1998, 12, 3452–3463. [Google Scholar] [CrossRef]
- Li, B.; Nair, M.; Mackay, D.R.; Bilanchone, V.; Hu, M.; Fallahi, M.; Song, H.; Dai, Q.; Cohen, P.E.; Dai, X. Ovol1 regulates meiotic pachytene progression during spermatogenesis by repressing Id2 expression. Development 2005, 132, 1463–1473. [Google Scholar] [CrossRef]
- Seo, E.K.; Choi, J.Y.; Jeong, J.H.; Kim, Y.G.; Park, H.H. Crystal Structure of C-Terminal Coiled-Coil Domain of SYCP1 Reveals Non-Canonical Anti-Parallel Dimeric Structure of Transverse Filament at the Synaptonemal Complex. PLoS ONE 2016, 11, e0161379. [Google Scholar] [CrossRef]
- Acampora, D.; Mazan, S.; Tuorto, F.; Avantaggiato, V.; Tremblay, J.J.; Lazzaro, D.; di Carlo, A.; Mariano, A.; Macchia, P.E.; Corte, G.; et al. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development 1998, 125, 1229–1239. [Google Scholar]
- Jorgez, C.J.; Rosenfeld, J.A.; Wilken, N.R.; Vangapandu, H.V.; Sahin, A.; Pham, D.; Carvalho, C.M.; Bandholz, A.; Miller, A.; Weaver, D.D.; et al. Genitourinary defects associated with genomic deletions in 2p15 encompassing OTX1. PLoS ONE 2014, 9, e107028. [Google Scholar] [CrossRef]
- Toyoda, S.; Miyazaki, T.; Miyazaki, S.; Yoshimura, T.; Yamamoto, M.; Tashiro, F.; Yamato, E.; Miyazaki, J. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev. Biol. 2009, 325, 238–248. [Google Scholar] [CrossRef]
- Hao, J.; Yamamoto, M.; Richardson, T.E.; Chapman, K.M.; Denard, B.S.; Hammer, R.E.; Zhao, G.Q.; Hamra, F.K. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells 2008, 26, 1587–1597. [Google Scholar] [CrossRef]
- Barrios, F.; Filipponi, D.; Campolo, F.; Gori, M.; Bramucci, F.; Pellegrini, M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Dolci, S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J. Cell Sci. 2012, 125, 1455–1464. [Google Scholar] [CrossRef]
- Park, M.; Lee, Y.; Jang, H.; Lee, O.H.; Park, S.W.; Kim, J.H.; Hong, K.; Song, H.; Park, S.P.; Park, Y.Y.; et al. SOHLH2 is essential for synaptonemal complex formation during spermatogenesis in early postnatal mouse testes. Sci. Rep. 2016, 6, 20980. [Google Scholar] [CrossRef]
- Cheng, Y.; Buffone, M.G.; Kouadio, M.; Goodheart, M.; Page, D.C.; Gerton, G.L.; Davidson, I.; Wang, P.J. Abnormal sperm in mice lacking the Taf7l gene. Mol. Cell Biol. 2007, 27, 2582–2589. [Google Scholar] [CrossRef]
- Zeng, W.; Baumann, C.; Schmidtmann, A.; Honaramooz, A.; Tang, L.; Bondareva, A.; Dores, C.; Fan, T.; Xi, S.; Geiman, T.; et al. Lymphoid-specific helicase (HELLS) is essential for meiotic progression in mouse spermatocytes. Biol. Reprod. 2011, 84, 1235–1241. [Google Scholar] [CrossRef]
- Pangas, S.A.; Choi, Y.; Ballow, D.J.; Zhao, Y.; Westphal, H.; Matzuk, M.M.; Rajkovic, A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. USA 2006, 103, 8090–8095. [Google Scholar] [CrossRef]
- Choi, Y.; Ballow, D.J.; Xin, Y.; Rajkovic, A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol. Reprod. 2008, 79, 442–449. [Google Scholar] [CrossRef]
- Soper, S.F.; van der Heijden, G.W.; Hardiman, T.C.; Goodheart, M.; Martin, S.L.; de Boer, P.; Bortvin, A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell 2008, 15, 285–297. [Google Scholar] [CrossRef]
- Costa, Y.; Speed, R.M.; Gautier, P.; Semple, C.A.; Maratou, K.; Turner, J.M.; Cooke, H.J. Mouse MAELSTROM: The link between meiotic silencing of unsynapsed chromatin and microRNA pathway? Hum. Mol. Genet. 2006, 15, 2324–2334. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Rey, R.A. Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis. Cells 2019, 8, 861. [Google Scholar] [CrossRef]
- Brehm, R.; Steger, K. Regulation of Sertoli cell and germ cell differentation. Adv. Anat. Embryol. Cell Biol. 2005, 181, 1–93. [Google Scholar]
- Chen, S.R.; Liu, Y.X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef]
- Bergmann, M. [Spermatogenesis--physiology and pathophysiology]. Urologe A 2005, 44, 1131–1132. [Google Scholar] [CrossRef]
- Weinbauer, G.F.; Luetjens, C.M.; Simoni, M.; Nieschlag, E. Physiology of Testicular Function. In Andrology: Male Reproductive Health and Dysfunction, 3rd ed.; Nieschlag, E., Behre, H.M., Nieschlag, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 11–59. [Google Scholar] [CrossRef]
- Franca, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girao, H. Role of connexin 43 in different forms of intercellular communication—Gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef]
- Haverfield, J.T.; Meachem, S.J.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Claudin-11 and connexin-43 display altered spatial patterns of organization in men with primary seminiferous tubule failure compared with controls. Fertil. Steril. 2013, 100, 658–666. [Google Scholar] [CrossRef]
- Theis, M.; Magin, T.M.; Plum, A.; Willecke, K. General or cell type-specific deletion and replacement of connexin-coding DNA in the mouse. Methods 2000, 20, 205–218. [Google Scholar] [CrossRef][Green Version]
- Theis, M.; de Wit, C.; Schlaeger, T.M.; Eckardt, D.; Kruger, O.; Doring, B.; Risau, W.; Deutsch, U.; Pohl, U.; Willecke, K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 2001, 29, 1–13. [Google Scholar] [CrossRef]
- Rode, K.; Weider, K.; Damm, O.S.; Wistuba, J.; Langeheine, M.; Brehm, R. Loss of connexin 43 in Sertoli cells provokes postnatal spermatogonial arrest, reduced germ cell numbers and impaired spermatogenesis. Reprod. Biol. 2018, 18, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.M.; Pouchnik, D.; Banasik, B.; McCarrey, J.R.; Small, C.; et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Raverdeau, M.; Gely-Pernot, A.; Feret, B.; Dennefeld, C.; Benoit, G.; Davidson, I.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16582–16587. [Google Scholar] [CrossRef]
- Tong, M.H.; Yang, Q.E.; Davis, J.C.; Griswold, M.D. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc. Natl. Acad. Sci. USA 2013, 110, 543–548. [Google Scholar] [CrossRef]
- Pellegrini, M.; Filipponi, D.; Gori, M.; Barrios, F.; Lolicato, F.; Grimaldi, P.; Rossi, P.; Jannini, E.A.; Geremia, R.; Dolci, S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008, 7, 3878–3888. [Google Scholar] [CrossRef]
- Gely-Pernot, A.; Raverdeau, M.; Celebi, C.; Dennefeld, C.; Feret, B.; Klopfenstein, M.; Yoshida, S.; Ghyselinck, N.B.; Mark, M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology 2012, 153, 438–449. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, Q.; Chen, X.L.; Yang, S.J.; Gao, L.; Gao, L.; Zhang, C.; Li, J.L.; Xiang, X.X.; Wan, K.; et al. All-trans retinoic acid arrests cell cycle in leukemic bone marrow stromal cells by increasing intercellular communication through connexin 43-mediated gap junction. J. Hematol. Oncol. 2015, 8, 110. [Google Scholar] [CrossRef]
- Ruttenstock, E.M.; Doi, T.; Dingemann, J.; Puri, P. Prenatal retinoic acid upregulates connexin 43 (Cx43) gene expression in pulmonary hypoplasia in the nitrofen-induced congenital diaphragmatic hernia rat model. J. Pediatr. Surg. 2012, 47, 336–340. [Google Scholar] [CrossRef]
- Wu, J.; Taylor, R.N.; Sidell, N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J. Cell Physiol. 2013, 228, 903–910. [Google Scholar] [CrossRef]
- Han, X.; Tong, X.H.; Dong, S.Y.; Zheng, C.; Yu, B.B. [Effects of retinoic acid on the expression of Cx43 and its gap juncion intercellular communication function in testicular cancer cell]. Sichuan Da Xue Xue Bao Yi Xue Ban 2013, 44, 924–927. [Google Scholar]
- Vine, A.L.; Bertram, J.S. Upregulation of connexin 43 by retinoids but not by non-provitamin A carotenoids requires RARs. Nutr. Cancer 2005, 52, 105–113. [Google Scholar] [CrossRef]
- Chung, S.S.; Choi, C.; Wang, X.; Hallock, L.; Wolgemuth, D.J. Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc Res. Tech. 2010, 73, 583–596. [Google Scholar] [CrossRef]
- Qian, X.; Mruk, D.D.; Cheng, C.Y. Rai14 (retinoic acid induced protein 14) is involved in regulating f-actin dynamics at the ectoplasmic specialization in the rat testis. PLoS ONE 2013, 8, e60656. [Google Scholar] [CrossRef]
- Hasegawa, K.; Saga, Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development 2012, 139, 4347–4355. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Stojilkovic, S.S.; Naor, Z. Editorial: Gonadotropin-Releasing Hormone Receptor Signaling and Functions. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- De Kretser, D.M.; Loveland, K.; O’Bryan, M. Chapter 136—Spermatogenesis. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., et al., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 2325–2353. [Google Scholar] [CrossRef]
- Steger, K.; Rey, R.; Louis, F.; Kliesch, S.; Behre, H.M.; Nieschlag, E.; Hoepffner, W.; Bailey, D.; Marks, A.; Bergmann, M. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathological human testis. Hum. Reprod. 1999, 14, 136–143. [Google Scholar] [CrossRef]
- Rajpert-De Meyts, E.; Jorgensen, N.; Graem, N.; Muller, J.; Cate, R.L.; Skakkebaek, N.E. Expression of anti-Mullerian hormone during normal and pathological gonadal development: Association with differentiation of Sertoli and granulosa cells. J. Clin. Endocrinol. Metab. 1999, 84, 3836–3844. [Google Scholar] [CrossRef]
- Regadera, J.; Martinez-Garcia, F.; Gonzalez-Peramato, P.; Serrano, A.; Nistal, M.; Suarez-Quian, C. Androgen receptor expression in sertoli cells as a function of seminiferous tubule maturation in the human cryptorchid testis. J. Clin. Endocrinol. Metab. 2001, 86, 413–421. [Google Scholar] [CrossRef]
- Steger, K.; Rey, R.; Kliesch, S.; Louis, F.; Schleicher, G.; Bergmann, M. Immunohistochemical detection of immature Sertoli cell markers in testicular tissue of infertile adult men: A preliminary study. Int. J. Androl. 1996, 19, 122–128. [Google Scholar] [CrossRef]
- Kim, S.; Bardwell, V.J.; Zarkower, D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev. Biol. 2007, 307, 314–327. [Google Scholar] [CrossRef]
- Kato, T.; Esaki, M.; Matsuzawa, A.; Ikeda, Y. NR5A1 is required for functional maturation of Sertoli cells during postnatal development. Reproduction 2012, 143, 663–672. [Google Scholar] [CrossRef]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. (Lausanne) 2019, 10, 224. [Google Scholar] [CrossRef]
- Barakat, B.; O’Connor, A.E.; Gold, E.; de Kretser, D.M.; Loveland, K.L. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction 2008, 136, 345–359. [Google Scholar] [CrossRef]
- Tran, D.; Josso, N. Localization of anti-Mullerian hormone in the rough endoplasmic reticulum of the developing bovine sertoli cell using immunocytochemistry with a monoclonal antibody. Endocrinology 1982, 111, 1562–1567. [Google Scholar] [CrossRef]
- Josso, N.; di Clemente, N.; Gouedard, L. Anti-Mullerian hormone and its receptors. Mol. Cell Endocrinol. 2001, 179, 25–32. [Google Scholar] [CrossRef]
- Rey, R.; Lordereau-Richard, I.; Carel, J.C.; Barbet, P.; Cate, R.L.; Roger, M.; Chaussain, J.L.; Josso, N. Anti-mullerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 1993, 77, 1220–1226. [Google Scholar] [CrossRef]
- Al-Attar, L.; Noel, K.; Dutertre, M.; Belville, C.; Forest, M.G.; Burgoyne, P.S.; Josso, N.; Rey, R. Hormonal and cellular regulation of Sertoli cell anti-Mullerian hormone production in the postnatal mouse. J. Clin. Investig. 1997, 100, 1335–1343. [Google Scholar] [CrossRef]
- Rotgers, E.; Cisneros-Montalvo, S.; Nurmio, M.; Toppari, J. Retinoblastoma protein represses E2F3 to maintain Sertoli cell quiescence in mouse testis. J. Cell Sci. 2019, 132, jcs229849. [Google Scholar] [CrossRef]
- Rossi, P.; Dolci, S. Paracrine mechanisms involved in the control of early stages of Mammalian spermatogenesis. Front. Endocrinol. (Lausanne) 2013, 4, 181. [Google Scholar] [CrossRef]
- Viger, R.S.; Guittot, S.M.; Anttonen, M.; Wilson, D.B.; Heikinheimo, M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 2008, 22, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.A.; Tao, S.; Lei, N.; Heckert, L.L. A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1(-/-) testes: A novel model of DMRT1-deficient germ cells. Biol. Reprod. 2013, 88, 51. [Google Scholar] [CrossRef]
- Heckert, L.L.; Agbor, V.A. 5—DMRT1 and the road to masculinity. In Sertoli Cell Biology, 2nd ed.; Griswold, M.D., Ed.; Academic Press: Oxford, UK, 2015; pp. 123–174. [Google Scholar] [CrossRef]
- Yan, W.; West, A.; Toppari, J.; Lahdetie, J. Stage-specific expression and phosphorylation of retinoblastoma protein (pRb) in the rat seminiferous epithelium. Mol. Cell Endocrinol. 1997, 132, 137–148. [Google Scholar] [CrossRef]
- Nalam, R.L.; Andreu-Vieyra, C.; Braun, R.E.; Akiyama, H.; Matzuk, M.M. Retinoblastoma protein plays multiple essential roles in the terminal differentiation of Sertoli cells. Mol. Endocrinol. 2009, 23, 1900–1913. [Google Scholar] [CrossRef]
- Tarulli, G.A.; Stanton, P.G.; Meachem, S.J. Is the adult Sertoli cell terminally differentiated? Biol. Reprod. 2012, 87, 11. [Google Scholar] [CrossRef]
- Rotgers, E.; Rivero-Muller, A.; Nurmio, M.; Parvinen, M.; Guillou, F.; Huhtaniemi, I.; Kotaja, N.; Bourguiba-Hachemi, S.; Toppari, J. Retinoblastoma protein (RB) interacts with E2F3 to control terminal differentiation of Sertoli cells. Cell Death Dis. 2014, 5, e1274. [Google Scholar] [CrossRef]
- Sin, W.C.; Crespin, S.; Mesnil, M. Opposing roles of connexin43 in glioma progression. Biochim. Biophys. Acta 2012, 1818, 2058–2067. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Morita, I.; Ikeda, M.; Ma, K.W.; Murota, S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p27. Oncogene 2001, 20, 4138–4149. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, S.; Gangoso, E.; Giaume, C.; Naus, C.C.; Medina, J.M.; Tabernero, A. Connexin43 inhibits the oncogenic activity of c-Src in C6 glioma cells. Oncogene 2010, 29, 5712–5723. [Google Scholar] [CrossRef]
- Sanchez-Alvarez, R.; Paino, T.; Herrero-Gonzalez, S.; Medina, J.M.; Tabernero, A. Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia 2006, 54, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Lukas, C.; Sorensen, C.S.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Lukas, J.; Bartek, J. Deregulation of the RB pathway in human testicular germ cell tumours. J. Pathol. 2003, 200, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Lukas, J.; Bartek, J. Deregulation of the G1/S-phase control in human testicular germ cell tumours. APMIS 2003, 111, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Strohmeyer, T.; Reissmann, P.; Cordon-Cardo, C.; Hartmann, M.; Ackermann, R.; Slamon, D. Correlation between retinoblastoma gene expression and differentiation in human testicular tumors. Proc. Natl. Acad. Sci. USA 1991, 88, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lindahl, M.; Hyvonen, M.E.; Parvinen, M.; de Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wein, F.; Roderburg, C.; Saffrich, R.; Faber, A.; Krause, U.; Schubert, M.; Benes, V.; Eckstein, V.; Maul, H.; et al. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp. Hematol. 2007, 35, 314–325. [Google Scholar] [CrossRef]
- Genet, N.; Bhatt, N.; Bourdieu, A.; Hirschi, K.K. Multifaceted Roles of Connexin 43 in Stem Cell Niches. Curr. Stem Cell Rep. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- González-Nieto, D.; Chang, K.-H.; Fasciani, I.; Nayak, R.; Fernandez-García, L.; Barrio, L.C.; Cancelas, J.A. Chapter Two—Connexins: Intercellular Signal Transmitters in Lymphohematopoietic Tissues. In International Review of Cell and Molecular Biology, 1st ed.; Jeon, K.W., Ed.; Academic Press: Oxford, UK, 2015; Volume 318, pp. 27–62. [Google Scholar]
- Suzuki, H.; Ahn, H.W.; Chu, T.; Bowden, W.; Gassei, K.; Orwig, K.; Rajkovic, A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev. Biol. 2012, 361, 301–312. [Google Scholar] [CrossRef]
- Desimio, M.G.; Campolo, F.; Dolci, S.; De Felici, M.; Farini, D. SOHLH1 and SOHLH2 directly down-regulate STIMULATED BY RETINOIC ACID 8 (STRA8) expression. Cell Cycle 2015, 14, 1036–1045. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef]
- Krentz, A.D.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev. Biol. 2011, 356, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.W.; Sarver, A.L.; Rice, D.; Hatzi, K.; Ye, K.; Melnick, A.; Heckert, L.L.; Zarkower, D.; Bardwell, V.J. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc. Natl. Acad. Sci. USA 2010, 107, 13360–13365. [Google Scholar] [CrossRef] [PubMed]
- L’Hote, D.; Vatin, M.; Auer, J.; Castille, J.; Passet, B.; Montagutelli, X.; Serres, C.; Vaiman, D. Fidgetin-like1 is a strong candidate for a dynamic impairment of male meiosis leading to reduced testis weight in mice. PLoS ONE 2011, 6, e27582. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Razack, B.S.; Roslund, R.M.; Suzuki, H.; Marshall, G.R.; Rajkovic, A.; Plant, T.M. Spermatogonial SOHLH1 nucleocytoplasmic shuttling associates with initiation of spermatogenesis in the rhesus monkey (Macaca mulatta). Mol. Hum. Reprod. 2014, 20, 350–357. [Google Scholar] [CrossRef]
- Nakamura, S.; Miyado, M.; Saito, K.; Katsumi, M.; Nakamura, A.; Kobori, Y.; Tanaka, Y.; Ishikawa, H.; Yoshida, A.; Okada, H.; et al. Next-generation sequencing for patients with non-obstructive azoospermia: Implications for significant roles of monogenic/oligogenic mutations. Andrology 2017, 5, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jeon, S.; Choi, M.; Lee, M.H.; Park, M.; Lee, D.R.; Jun, K.Y.; Kwon, Y.; Lee, O.H.; Song, S.H.; et al. Mutations in SOHLH1 gene associate with nonobstructive azoospermia. Hum. Mutat. 2010, 31, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Y.; He, X.J.; Du, W.D.; Ruan, J.; Zhou, F.S.; Wu, H.; Zha, X.; Xie, X.S.; Ye, L.; et al. Association of genetic variants in SOHLH1 and SOHLH2 with non-obstructive azoospermia risk in the Chinese population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Ji, S.; Hao, C.; Mu, Y.; Sun, J.; Hao, J. Sohlh2 inhibits ovarian cancer cell proliferation by upregulation of p21 and downregulation of cyclin D1. Carcinogenesis 2014, 35, 1863–1871. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, C.; Wang, Y.; Ji, S.; Zhang, X.; Zhang, W.; Zhao, Q.; Sun, J.; Hao, J. Sohlh2 inhibits human ovarian cancer cell invasion and metastasis by transcriptional inactivation of MMP9. Mol. Carcinog. 2016, 55, 1127–1137. [Google Scholar] [CrossRef]
- Qin, Y.; Jiao, X.; Dalgleish, R.; Vujovic, S.; Li, J.; Simpson, J.L.; Al-Azzawi, F.; Chen, Z.J. Novel variants in the SOHLH2 gene are implicated in human premature ovarian failure. Fertil. Steril. 2014, 101, 1104–1109. [Google Scholar] [CrossRef]
- Koyanagi, M.; Brandes, R.P.; Haendeler, J.; Zeiher, A.M.; Dimmeler, S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: A novel mechanism for cell fate changes? Circ. Res. 2005, 96, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, L.; Pantazis, P.; Dempsey, W.; Fraser, S.E. Intercellular bridges in vertebrate gastrulation. PLoS ONE 2011, 6, e20230. [Google Scholar] [CrossRef] [PubMed]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef]
- Pyrgaki, C.; Trainor, P.; Hadjantonakis, A.K.; Niswander, L. Dynamic imaging of mammalian neural tube closure. Dev. Biol. 2010, 344, 941–947. [Google Scholar] [CrossRef]
- Salas-Vidal, E.; Lomeli, H. Imaging filopodia dynamics in the mouse blastocyst. Dev. Biol. 2004, 265, 75–89. [Google Scholar] [CrossRef]
- Wang, X.; Veruki, M.L.; Bukoreshtliev, N.V.; Hartveit, E.; Gerdes, H.H. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA 2010, 107, 17194–17199. [Google Scholar] [CrossRef]
- Lock, J.T.; Parker, I.; Smith, I.F. Communication of Ca(2+) signals via tunneling membrane nanotubes is mediated by transmission of inositol trisphosphate through gap junctions. Cell Calcium 2016, 60, 266–272. [Google Scholar] [CrossRef]
- Li, X. Gap junction protein connexin43 and tunneling nanotubes in human trabecular meshwork cells. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 212–219. [Google Scholar]
- Vidulescu, C.; Clejan, S.; O’Connor K, C. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J. Cell. Mol. Med. 2004, 8, 388–396. [Google Scholar] [CrossRef]
- Gungor-Ordueri, N.E.; Celik-Ozenci, C.; Cheng, C.Y. Ezrin: A regulator of actin microfilaments in cell junctions of the rat testis. Asian J. Androl. 2015, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Verlaan, I.; Moolenaar, W.H. Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun. Adhes 2001, 8, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Verlaan, I.; Hengeveld, T.; Janssen, H.; Calafat, J.; Falk, M.M.; Moolenaar, W.H. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001, 11, 1364–1368. [Google Scholar] [CrossRef]
- Gungor-Ordueri, N.E.; Tang, E.I.; Celik-Ozenci, C.; Cheng, C.Y. Ezrin is an actin binding protein that regulates sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology 2014, 155, 3981–3995. [Google Scholar] [CrossRef]
- Inaba, M.; Buszczak, M.; Yamashita, Y.M. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature 2015, 523, 329–332. [Google Scholar] [CrossRef]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 2004, 1662, 96–101. [Google Scholar] [CrossRef]
- Decrouy, X.; Gasc, J.M.; Pointis, G.; Segretain, D. Functional characterization of Cx43 based gap junctions during spermatogenesis. J. Cell Physiol. 2004, 200, 146–154. [Google Scholar] [CrossRef]
- Risley, M.S.; Tan, I.P.; Farrell, J. Gap Junctions with Varied Permeability Properties Establish Cell-Type Specific Communication Pathways in the Rat Seminiferous Epithelium1. Biol. Reprod. 2002, 67, 945–952. [Google Scholar] [CrossRef][Green Version]
- Kidder, G.M.; Cyr, D.G. Roles of connexins in testis development and spermatogenesis. Semin. Cell Dev. Biol. 2016, 50, 22–30. [Google Scholar] [CrossRef]
- Günther, S.; Fietz, D.; Weider, K.; Bergmann, M.; Brehm, R. Effects of a murine germ cell-specific knockout of Connexin 43 on Connexin expression in testis and fertility. Transgenic Res. 2013, 22, 631–641. [Google Scholar] [CrossRef]
- Gregory, M.; Kahiri, C.N.; Barr, K.J.; Smith, C.E.; Hermo, L.; Cyr, D.G.; Kidder, G.M. Male reproductive system defects and subfertility in a mutant mouse model of oculodentodigital dysplasia. Int. J. Androl. 2011, 34, e630–e641. [Google Scholar] [CrossRef] [PubMed]
- Esseltine, J.L.; Laird, D.W. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016, 26, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Leo-Macias, A.; Agullo-Pascual, E.; Delmar, M. The cardiac connexome: Non-canonical functions of connexin43 and their role in cardiac arrhythmias. Semin. Cell Dev. Biol. 2016, 50, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Anjo, S.I.; Pereira, P.; Manadas, B.; Girao, H. Interacting Network of the Gap Junction (GJ) Protein Connexin43 (Cx43) is Modulated by Ischemia and Reperfusion in the Heart. Mol. Cell. Proteom. 2015, 14, 3040–3055. [Google Scholar] [CrossRef]
| Protein | Primary Antibody | Host, Mono-/Polyclonal | Dilution | Secondary Antibody |
|---|---|---|---|---|
| AMH | Anti-AMH antibody [5/6] (Abcam, ab24542) | Mouse, monoclonal | 1:50 | Labelled Polymer-HRP Anti-mouse, ready to use |
| β-galactosidase | Anti-beta Galactosidase antibody (Abcam, ab616) | E. coli, polyclonal | 1:1000 | Labelled Polymer-HRP Anti-rabbit, ready to use |
| Cx43 | Connexin 43 antibody (Cell signaling, #3512) | Rabbit, polyclonal | 1:250 | Biotinylated Goat Anti-Rabbit, 1:200 |
| LIN28A | LIN28A (A177) antibody (Cell signaling, #3978S) | Rabbit, polyclonal | 1:70 | Labelled Polymer-HRP Anti-rabbit, ready to use |
| SALL4 | Anti-Sall4 antibody (Abcam, ab57577) | Mouse, monoclonal | 1:200 | Labelled Polymer-HRP Anti-mouse, ready to use |
| SOHLH1 | Kindly provided by Dr. Aleksandar Rajkovic (University of California, San Francisco) | Rabbit, polyclonal | 1:500 | Biotinylated Goat Anti-Rabbit, 1:200 |
| SOX9 | Anti-Sox9 (EMD Millipore, #AB5535) | Rabbit, polyclonal | 1:1500 | Labelled Polymer-HRP Anti-rabbit, ready to use |
| Gene Name | Primer | Primer Sequence (5′->3′) | Accession Number/Reference | Amplicon Length (bp) |
|---|---|---|---|---|
| Amh | Forward | CCA ACG ACT CCC GCA GCT C | [9] | 93 |
| Reverse | CTT CCC GCC CAT GCC ACT C | |||
| Fshr | Forward | CTC TGG GCC AGT CGT TTT AG | NM_013523.3 | 150 |
| Reverse | GCC TCA ATG AGC ATG ACA AA | |||
| Gja1 | Forward | ACA GCG GTT GAG TCA GCT TG | [9] | 106 |
| Reverse | GAG AGA TGG GGA AGG ACT TGT | |||
| Sohlh1 | Forward | ATG TGG CAG GGT GAT GTT CT | NM_001001714.1 | 146 |
| Reverse | GCC TGG CTC TGG TCT GTA TC | |||
| Sohlh2 | Forward | GCC GCT GAC CTT GGA AAA AG | NM_028937.3 | 121 |
| Reverse | GCG GGA CGT CTG AAA GTA CA | |||
| β-Actin | Forward | CAC TGT CGA GTC GCG TCC | [38] | 102 |
| Reverse | CGC AGC GAT ATC GTC ATC CA |
| Cells | Factor | p-Value |
|---|---|---|
| Germ cells | Group | < 0.01 |
| Time | < 0.01 | |
| (Group x Time) Interaction | < 0.01 | |
| Sertoli cells | Group | < 0.01 |
| Time | < 0.01 | |
| (Group x Time) Interaction | 0.11 |
| Samples | Comparison | Cell Count Specific Normalization | ||
|---|---|---|---|---|
| None | Germ Cells | Sertoli Cells | ||
| Number of Genes with p (FDR) < 0.05 | Number of Genes with p (FDR) < 0.05 | Number of Genes with p (FDR) < 0.05 | ||
| All | KO vs. WT | 11,635 | 33,614 | 10,994 |
| day10 vs. day8 | 2565 | 30,170 | 29,691 | |
| day12 vs. day8 | 24,558 | 35,062 | 34,937 | |
| WT | day10 vs. day8 | 20,115 | 32,042 | 31,723 |
| day12 vs. day8 | 23,334 | 34,026 | 33,741 | |
| KO | day10 vs. day8 | 16039 | 532 | 532 |
| day12 vs. day8 | 21882 | 33,614 | 33,614 | |
| Day8 | KO vs. WT | 20,429 | 4253 | 4253 |
| Day10 | KO vs. WT | 24,793 | 34,325 | 34,183 |
| Day12 | KO vs. WT | 7126 | 9517 | 7783 |
| Gene Ontology (GO) Term | Genes |
|---|---|
| DNA methylation involved in gamete generation [GO:0043046] | Tdrd1, Tdrd9, Tdrkh, Tdrd12, Kdm1b, Fkbp6, Mov10l1, Mael, Piwil2, Pld6, Piwil4, Ddx4, Ctcfl, Prmt7, Dnmt3a, Dnmt3c, Dnmt3l, Asz1, Morc1, Tdrd5 |
| male meiotic nuclear division [GO:0007140] | Sycp2, Rspo1, Suv39h2, Spo11, Spdya, Rec8, Tex14, Tdrd9, Tdrkh, Tdrd12, Tex11, Siah1a, Sgo2, Tex15, Kif18al Ing2, Hspa2, Slc2a8, Mov10l1, Mael, Mlh1, Meiob, Rad51c, Trip13, Meioc, Mybl1, Mei1, Fanca, Fignl1, Tex19.2, Ubb, Tex19.1, Ubr2, Ddx4, Catsperz, Cyp26b1, Chtf18, Brca2, Btbd18, Brdt, Ago4, Dmc1, Dmrtc2, Dnmt3c, Dnmt3l, Dpep3, Atm, Asz1 |
| meiotic DNA repair synthesis [GO:0000711] | Sycp3, Sycp1, Spata22, Tex12 |
| synaptonemal complex [GO:0000795] | Sycp3, Sycp1, Sycp2, Stag3, Rec8, Syn1, Smc1b, Incenp, Fkbp6, Hspa2, Hormad1, Mlh1, Rad51, Msh4, Plk1, Wapl, Polb, Lig3, P3h4, Plk1, Hormad2, Mlh3, Syce2, Mlh3, Syce1, Syce1l, Tex12, Rnf212b, Msh5 |
| lateral element [GO:0000800] | Sycp3, Sycp1, Sycp2, Stag3, Rec8, Rpa1, Smc1b, Smc3, Ccdc155, Incenp, Rad51, Rad21l1, Blm, Brca2, Brca1, Mei4, Sycp2l, Xlr3b, Xlr3c, Xlr, Xlr3a |
| transverse filament [GO:0000802] | Sycp3, Sycp1, Stag3 |
| female meiosis sister chromatid cohesion [GO:0007066] | Sycp3, Stag3, Rad51c |
| central element [GO:0000801] | Syce3, Sycp1, Tex11, Incenp, Six6os1, Syce2, Syce1, Tex12 |
| positive regulation of cell proliferation in bone marrow [GO:0071864] | Shc1, Il6, Fgfr3, Hmga2, Mef2c, Map3k3, Lef1, Flt3lg |
| nuclear meiotic cohesin complex [GO:0034991] | Stag3, Rec8, Smc1b, Smc3, Rad21l1 |
| Gene | p(FDR) d8 | p(FDR) d10 | p(FDR) d12 | Localization (Cell Type) | Functions in Male Spermatogenesis |
|---|---|---|---|---|---|
| Crabp1 | 3.84 × 10−05 | 8.26 × 10−08 | 1.82 × 10−05 | Spg [53] | Promotion of cytoplasmic degeneration of retinoic acid via the cytochrome P450 family 26 (CYP26) enzymes [54] |
| Dmrtb1 | 9.96 × 10−05 | 2.08 × 10−07 | 3.15 × 10−05 | Spg, Spc (preleptotene up to pachytene stage) [33,34] | Coordination of the transition between mitosis and meiosis [33,34] |
| Usp26 | 2.48 × 10−05 | 3.78 × 10−07 | 6.30 × 10−06 | Spg, decreases in Spc at leptotene/zygotene stage [55]; round and elongated Spd, localized at the blood-testis-barrier and near SC-GC interface [56]; and in human SCs and Leydig cells [57] | Does not play a decisive role in murine gametogenesis [58] Possible role in GC movement along the seminiferous epithelium [56] |
| Grhl1 | 3.4 × 10−04 | 5.4 × 10−07 | 2.90 × 10−05 | Testis [59,60] | Regulating expression of genes implicated in cellular proliferation, differentiation, adhesion, and polarity [61] |
| Stra8 | 4.6 × 10−04 | 6.03 × 10−07 | 5.06 × 10−06 | Spg type A and B, preleptotene and early leptotene Spc [62] | Pivotal for transition into meiotic prophase [63] |
| Tex15 | 1.7 × 10−04 | 6.03 × 10−07 | 1.56 × 10−06 | Spg, early Spc, round Spd (postmeiotic reactivation) [55] | Crucial for meiotic recombination [64] |
| Mei1 | 3.6 × 10−04 | 6.45 × 10−07 | 1.56 × 10−06 | Gonads; gene KO leads to arrested Spc at zygotene/pachytene stage [65,66] | Possible role for the initiation of meiotic recombination [67] |
| Ovol1 | 2.0 × 10−04 | 6.47 × 10−07 | 4.00 × 10−05 | Spc, round Spd [68,69] | Regulation of meiotic pachytene progression of GCs [69] |
| Sycp1 | 1.4 × 10−04 | 6.47 × 10−07 | 6.56 × 10−06 | Spc (meiosis-marker) [70] | Represents the main structural element of transverse filaments of the synaptonemal complex (a complex structure formed during meiosis) [70] |
| Otx1 | 4.6 × 10−04 | 8.36 × 10−07 | 1.53 × 10−04 | Testis [59,60] | Otx1 KO leads to a selective loss of differentiating GCs but not of spermatogonial precursors [71] Otx1 seems to be involved in genitourinary tract development [72] |
| Sohlh2 | 9.52 × 10−06 | 8.36 × 10−07 | 2.87 × 10−06 | Spg [50,73] | Required for spermatogonial differentiation [74] Promotion of spermatogonial differentiation by controlling Kit expression [73,75] Crucial for synaptonemal complex formation by regulating Sycp1 expression [76] |
| Taf7l | 3.81 × 10−05 | 8.36 × 10−07 | 1.50 × 10−06 | Spg, Spc, round Spd [77] | Essential for normal sperm count and motility [77] |
| Hells | 9.96 × 10−05 | 8.73 × 10−07 | 1.38 × 10−06 | Spg, Spc (up to zygotene) [78] | Crucial for meiotic progression [78] |
| Lhx8 | 1.3 × 10−04 | 8.73 × 10−07 | 1.12 × 10−04 | GCs [79] | Possibly involved in the regulation of spermatogonial differentiation [50,79] Primarily a female-specific transcriptional regulator [80] |
| Mael | 4.64 × 10−05 | 8.73 × 10−07 | 1.16 × 10−05 | Spc, round Spd [81,82] | Pivotal for spermatogenesis and transposon repression in meiosis [81] |
| PANTHER Biological Process | Genes |
|---|---|
| cellular component organization or biogenesis (GO:0071840) | Patl2 |
| cellular process (GO:0009987) | Spry4, Naa11, Mmp2, Birc5, Ascl2, Igf1r, Nos1, Dgkz, Mtr, Uba6, Inhbb, Zbtb42, Clgn, Parp1, Fshr, Rad51, Dpy1912, Blm, Sat2, Bcl2l2, Dab1, Kit, Usp26, Sox3, Cdc20, Rictor, Atm, Bub1b, Fignl1, Lef1, Tle3, Serpine1, Fgfr3, Wapl, Mtor, Kif18a, Xpc, Stag3, Tex19, Gdnf, Rec8, Ccne1, Ccnd1, Cib1, Smc1b, Axl, Ctcfl, Mdm2, Casp3, Foxs1, Ccnb1, Chrna7, Sohlh2, Rpl10l, Bub1, Gamt, Brca1, Sox11, Trip13, Kif17, Serpina5, Tert, Plk1, Plk4, Inha, Adgrg1, Insr, Lig4, Tssk6, Brca2, Adamts5, Rnf212, Kif2c, Il6ra, Rb1, Trf, Tyro3, Kif3a, Nek1, Sohlh1, Adamts1, Gabpa, Lhcgr, Asb9, Trdrd9, Psma8, Fmr1, Spag4 |
| biological phase (GO:0044848) | Rad51, Rad51c, Meioc, Ccne1, Trip13, Rnf212 |
| localization (GO:0051179) | Clgn, Sdc1, Kit, Wapl, Mtor, Axl, Trf, Tyro3, Nxf2, Fmr1, Spag4 |
| reproduction (GO:0000003) | Mael, Sycp3, Rad51, Dpy1912, Piwil2, Rad51c, Piwil1, Meioc, Bub1b, Espl1, Wapl, Tex19, Rec8, Ccne1, Piwil4, Bub1, Trip13, Rnf212, Tcf15 |
| biological regulation (GO:0065007) | Spry4, Mov10l1, Ascl2, Igf1r, Nos1, Dgkz, Mael, Inhbb, Fshr, Kdm1b, Neurog3, Boll, Bcl2l2, Kit, Rictor, Esr2, Meioc, Atm, Bub1b, Taf4b, Fignl1, Esr1, Lef1, Apbb1, Tle3, Fgfr3, Cpeb1, Wapl, Mtor, Mt2, Dusp6, Tex19, Ccne1, Lhx6, Ccnd1, Rffl, Axl, Ctffl, Taf1, Rbm38, Sall4, Dicer1, Ccnb1, Chrna7, Bub1, Prok2, Brca1, Trip13, Taf7l, Plk1, Plk4, Inha, Adgrg1, Insr, Lhx8, Ahr, Grhl1, Dmxl2, Rbmxl2, Il6ra, Rb1, Tcfl5, Trf, Nek1, Lhcgr, Dazl, Asb9, Tdrd9, Fmr1, Spag4, Lifr |
| response to stimulus (GO:0050896) | Spry4, Mmp4, Igf1r, Nos1, Fshr, Kit, Fgfr3, Mt2, Axl, Insr, Ahr, Lhcgr |
| developmental process (GO:0032502) | Igf1r, Gata4, Lhx6, Foxs1, Insr, Lhx8, Ovol1, Sox8 |
| rhythmic process (GO:0048511) | Prok2 |
| multicellular organismal process (GO:0032501) | Otx1, Mmp2, Ascl2, Nos1, Gata4, Tex19, Lhx6, Axl, Glis1, Chrna7, Hspb1, Prok2, Lhx8, Il6ra, Rb1, Cnn1, Tyro3, Fmr1 |
| biological adhesion (GO:0022610) | Il6ra |
| metabolic process (GO:0008152) | Naa11, Mmp2, Mov10l1, Hsd17b1, Ascl2, Pld6, Nos1, Mtr, Mael, Clgn, Il4i1, Etnk2, Kdm1b, Neurog3, Rad51, Dpy19l2, Blm, Boll, Sat2, Gata4, Ocln, Usp26, Wbp2nl, Sox3, Cdc20, Esr2, Egr4, Meioc, Ttll5, Taf4b, Tbpl1, Esr1, Lef1, Apbb1, Tle3, Hs6st1, Uchl3, Cpeb1, Snai3, Cyp26b1, Adad1, Lhx6, Ctcfl, Taf1, Rbm38, Mdm2, Sall4, Foxs1, Gtpbp4, Dicer1, Patl2, Gamt, Brca1, Sox11, Tert, Taf7l, Lhx8, Ctsl, Ahr, Grhl1, Rbmxl2, Drosha, Gstt1, Tcfl5, Trf, Uchl1, Gabpa, Dazl, Asb9, Psma8, Fmr1 |
| cell proliferation (GO:0008283) | Ccne1, Ccnd1, Ccnb1, Prok2, Lifr |
| immune system process (GO:0002376) | Dab1, Kit, Axl, Hspb1, Il6ra |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilbold, E.; Distl, O.; Hoedemaker, M.; Wilkening, S.; Behr, R.; Rajkovic, A.; Langeheine, M.; Rode, K.; Jung, K.; Metzger, J.; et al. Loss of Cx43 in Murine Sertoli Cells Leads to Altered Prepubertal Sertoli Cell Maturation and Impairment of the Mitosis-Meiosis Switch. Cells 2020, 9, 676. https://doi.org/10.3390/cells9030676
Hilbold E, Distl O, Hoedemaker M, Wilkening S, Behr R, Rajkovic A, Langeheine M, Rode K, Jung K, Metzger J, et al. Loss of Cx43 in Murine Sertoli Cells Leads to Altered Prepubertal Sertoli Cell Maturation and Impairment of the Mitosis-Meiosis Switch. Cells. 2020; 9(3):676. https://doi.org/10.3390/cells9030676
Chicago/Turabian StyleHilbold, Erika, Ottmar Distl, Martina Hoedemaker, Sandra Wilkening, Rüdiger Behr, Aleksandar Rajkovic, Marion Langeheine, Kristina Rode, Klaus Jung, Julia Metzger, and et al. 2020. "Loss of Cx43 in Murine Sertoli Cells Leads to Altered Prepubertal Sertoli Cell Maturation and Impairment of the Mitosis-Meiosis Switch" Cells 9, no. 3: 676. https://doi.org/10.3390/cells9030676
APA StyleHilbold, E., Distl, O., Hoedemaker, M., Wilkening, S., Behr, R., Rajkovic, A., Langeheine, M., Rode, K., Jung, K., Metzger, J., & Brehm, R. H. J. (2020). Loss of Cx43 in Murine Sertoli Cells Leads to Altered Prepubertal Sertoli Cell Maturation and Impairment of the Mitosis-Meiosis Switch. Cells, 9(3), 676. https://doi.org/10.3390/cells9030676






