MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Small Extracellular Vesicles (sEVs) Isolation
2.3. Immunohistochemistry
2.4. Small Extracellular Vesicles (sEVs) Tracking Analysis
2.5. Transmission Electron Microscopy (TEM)
2.6. Scanning Electron Microscopy (SEM)
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. Western Blot Analysis
2.9. mRNA Datasets Analysis
2.10. miRNA Agomir Injection
2.11. RNA Extraction and qRT-PCR
2.12. Statistical Analysis
3. Results
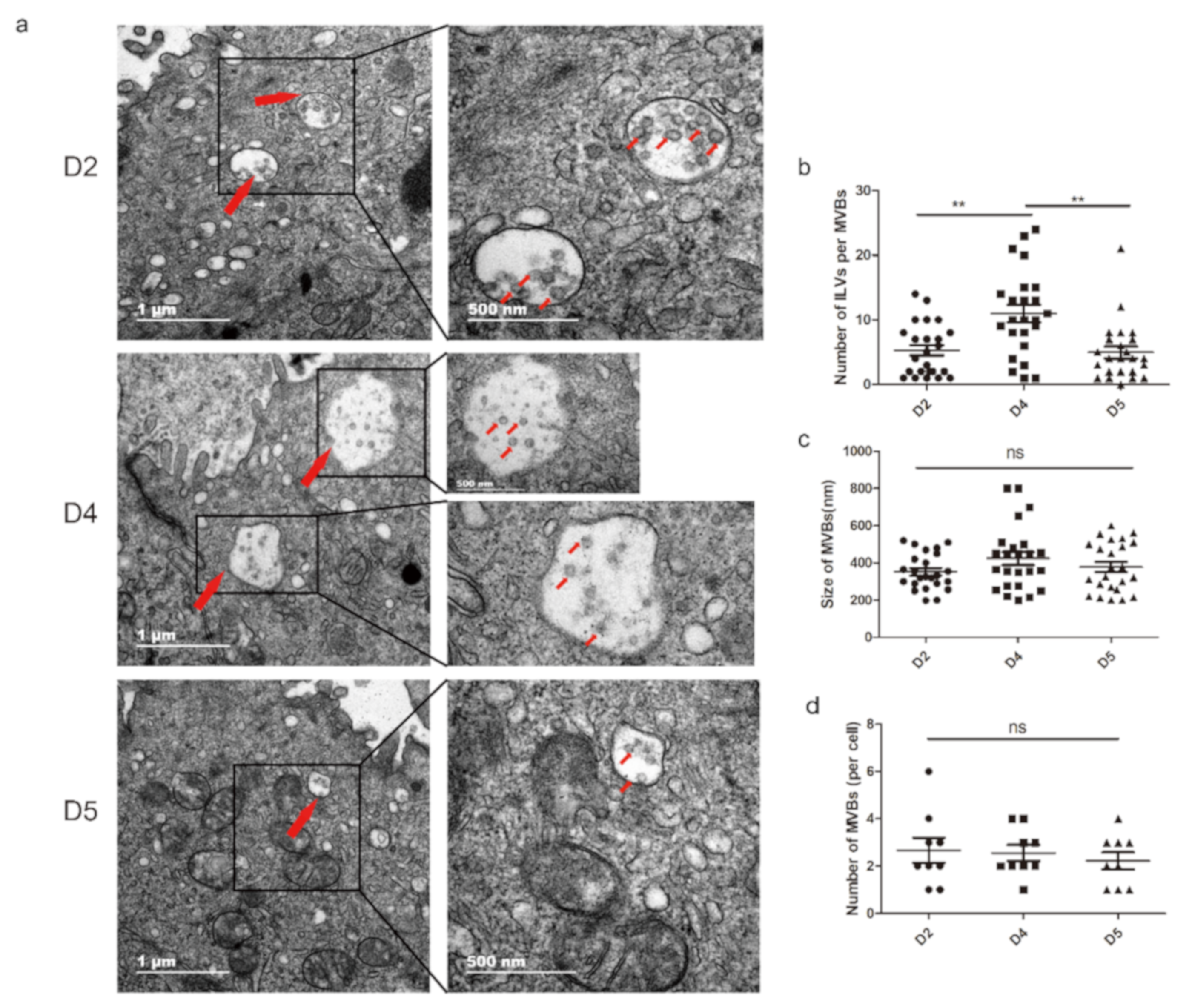
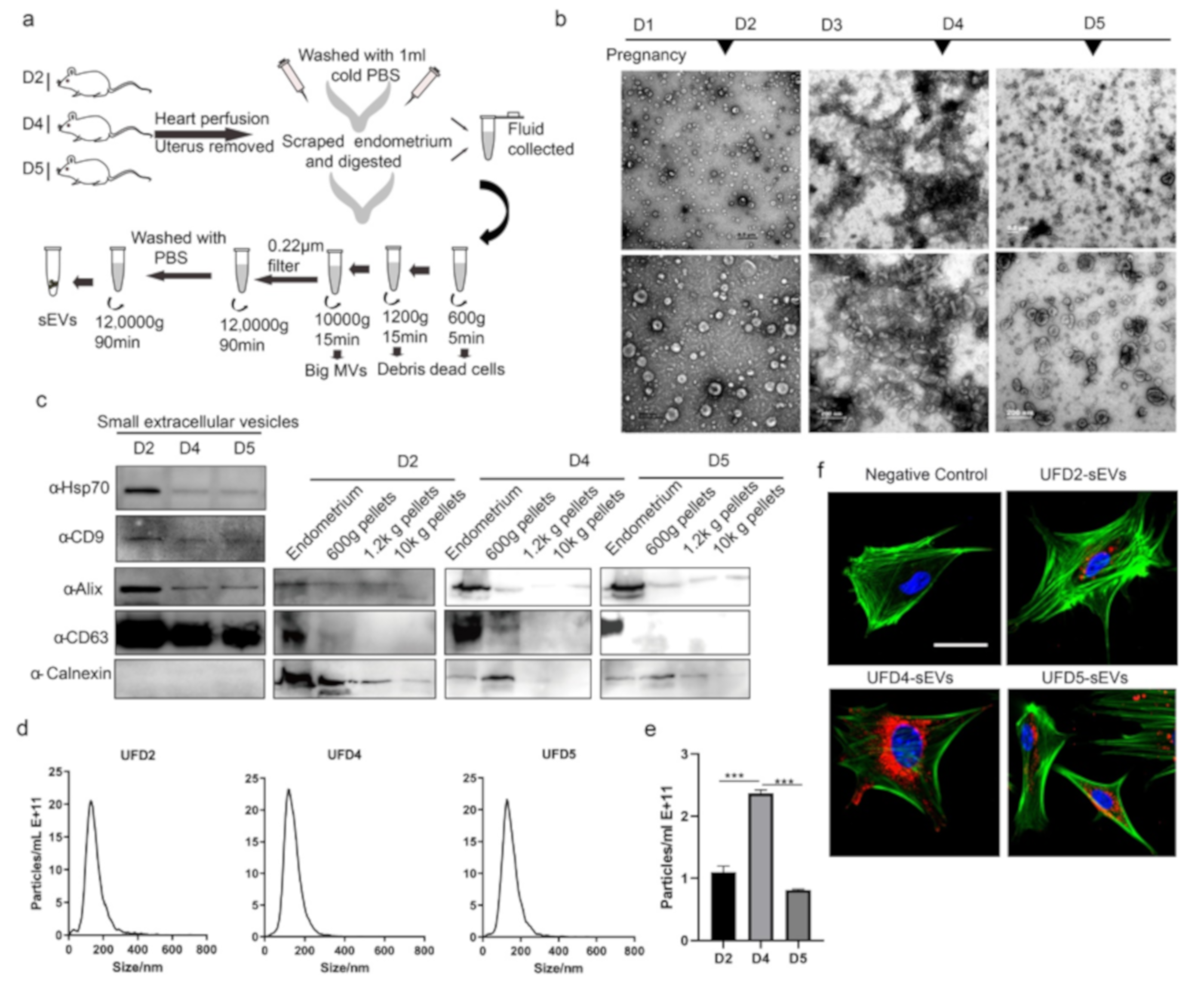
3.1. Extracellular Vesicles are Present and Secreted by the Endometrium
3.2. Characterization of the Endometrium Derived Small Extracellular Vesicles (sEVs)
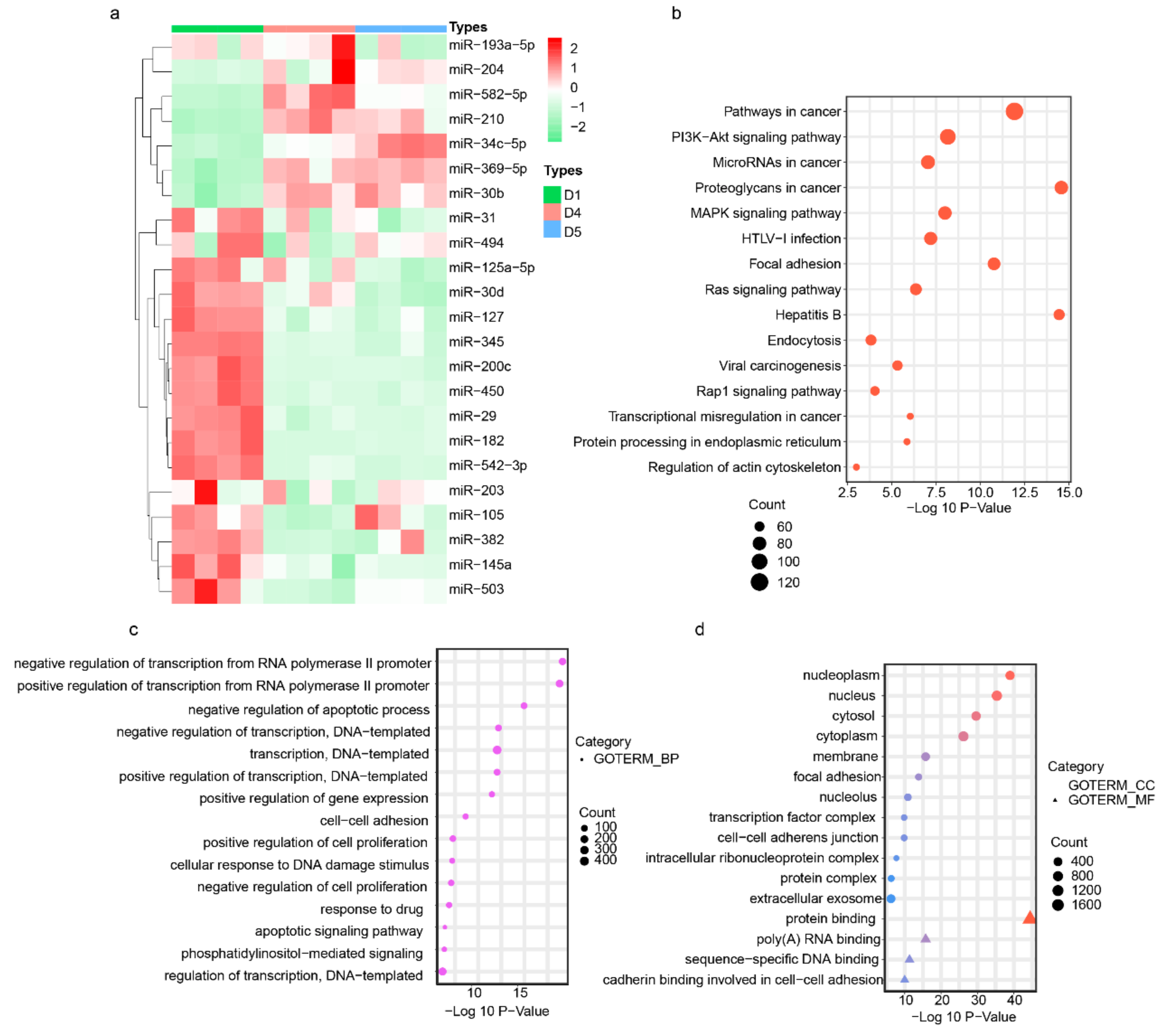
3.3. Endometrium-Derived sEVs Carry Receptive miRNAs
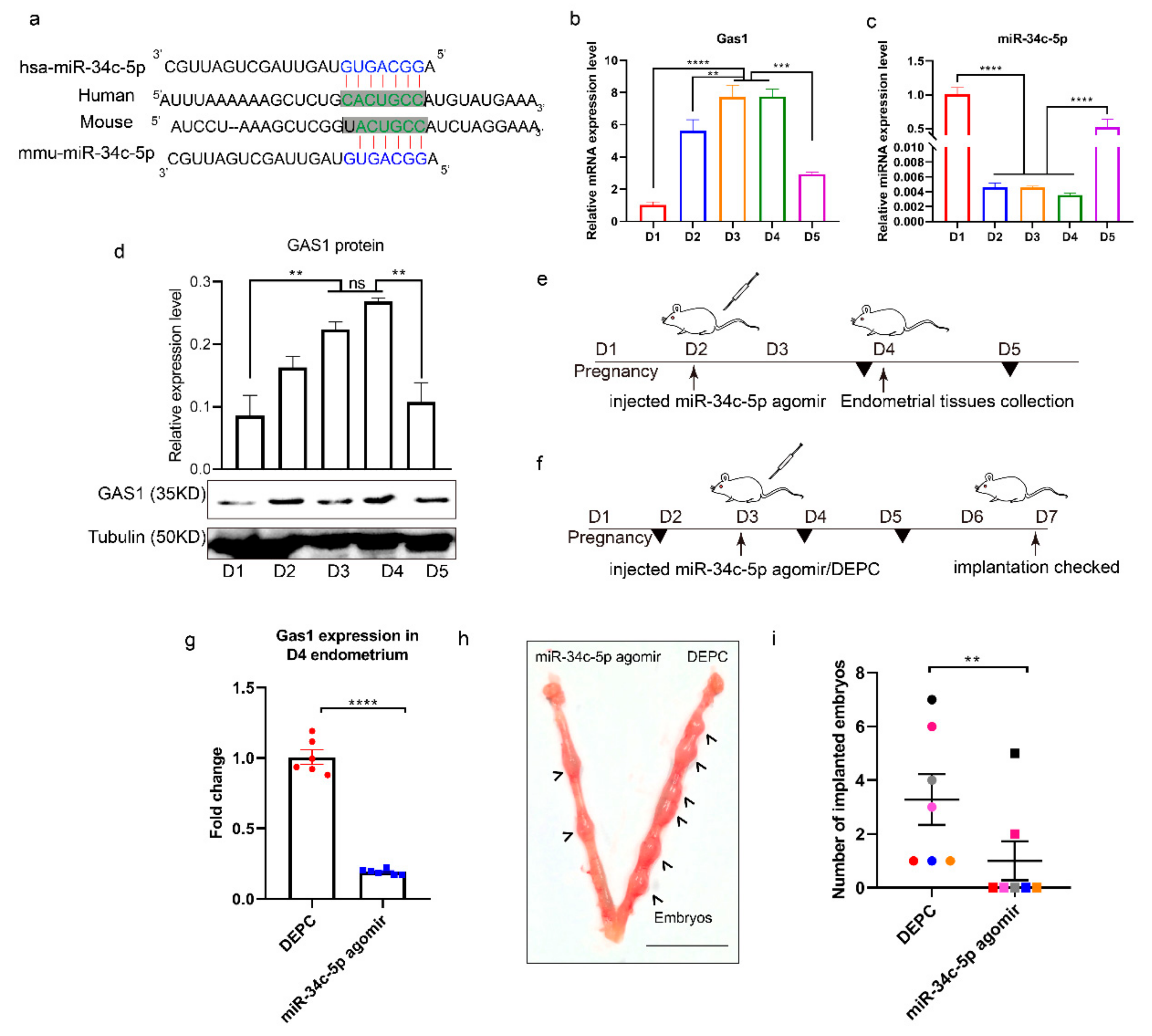
3.4. miR-34c-5p Targets GAS1 (Growth Arrest Specific 1) in RIF (Recurrent Implantation Failure) Endometrium
3.5. miR-34c-5p is Poorly Expressed during Embryo Implantation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Baird, D.D.; Weinberg, C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999, 340, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, P. Understanding implantation window, a crucial phenomenon. J. Hum. Reprod. Sci. 2012, 5, 2–6. [Google Scholar] [PubMed]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, W.; Zhang, L.; Yin, G.; Wang, X.; Wang, J.; Zhao, H.; Han, Y.; Yao, Y.Q. Determination of microRNAs in mouse preimplantation embryos by microarray. Dev. Dyn. 2008, 237, 2315–2327. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, D.; Yang, Y.U.; Cui, X.; Sun, J.; Liang, C.; Qin, H.; Yang, X.; Liu, S.; Yan, Q. MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and alpha1,3-fucosylation. Cell Death Differ. 2017, 24, 2161–2172. [Google Scholar] [CrossRef]
- Altmäe, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod. Sci. 2013, 20, 308–317. [Google Scholar] [CrossRef]
- Von Grothusen, C.; Lalitkumar, S.; Boggavarapu, N.R.; Gemzell-Danielsson, K.; Lalitkumar, P.G. Recent advances in understanding endometrial receptivity: Molecular basis and clinical applications. Am. J. Reprod. Immunol. 2014, 72, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhu, X.X.; Li, Z.F.; Zhu, Y.P.; Lang, J.H. MicroRNA Dysregulation and Steroid Hormone Receptor Expression in Uterine Tissues of Rats with Endometriosis during the Implantation Window. Chin. Med. J. (Engl.) 2018, 131, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar]
- Rosenbluth, E.M.; Shelton, D.N.; Wells, L.M.; Sparks, A.E.T.; Van Voorhis, B.J. Human embryos secrete microRNAs into culture media-a potential biomarker for implantation. Fertil. Steril. 2014, 101, 1493–1500. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial Exosomes/Microvesicles in the Uterine Microenvironment: A New Paradigm for Embryo-Endometrial Cross Talk at Implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef]
- Tannetta, D.; Dragovic, R.; Alyahyaei, Z.; Southcombe, J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell. Mol. Immunol. 2014, 11, 548–563. [Google Scholar] [CrossRef]
- Latifi, Z.; Fattahi, A.; Ranjbaran, A.; Nejabati, H.R.; Imakawa, K. Potential roles of metalloproteinases of endometrium-derived exosomes in embryo-maternal crosstalk during implantation. J. Cell. Physiol. 2018, 233, 4530–4545. [Google Scholar] [CrossRef]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martínez, S.; Marcilla, A.; Simón, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3232. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Evans, J.; Nguyen, H.P.T.; Edgell, T.A. The Microenvironment of Human Implantation: Determinant of Reproductive Success. Am. J. Reprod. Immunol. 2016, 75, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Oh, H.J.; Lee, B.C. Embryonic-maternal cross-talk via exosomes: Potential implications. Stem Cells Cloning. 2015, 8, 103–107. [Google Scholar] [PubMed]
- Thery, C. Cancer: Diagnosis by extracellular vesicles. Nature 2015, 523, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Bhagwat, N.; Black, T.; Yee, S.S.; Na, Y.J.; Fisher, S.; Kim, J.; Carpenter, E.L.; Stanger, B.Z.; Issadore, D. miRNA Profiling of Magnetic Nanopore-Isolated Extracellular Vesicles for the Diagnosis of Pancreatic Cancer. Cancer Res. 2018, 78, 3688–3697. [Google Scholar] [CrossRef]
- Greening, D.W.; Nguyen, H.P.T.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef]
- Nikas, G. Endometrial receptivity: Changes in cell-surface morphology. Semin. Reprod. Med. 2000, 18, 229–235. [Google Scholar] [CrossRef]
- Salehnia, M. Different pattern of pinopodes expression in stimulated mouse endometrium. Exp. Anim. 2005, 54, 349–352. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Thouas, G.A.; Dominguez, F.; Green, M.P.; Vilella, F.; Simon, C.; Gardner, D.K. Soluble Ligands and Their Receptors in Human Embryo Development and Implantation. Endocr. Rev. 2015, 36, 92–130. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Ma, X.H.; Hu, S.J.; Ni, H.; Zhao, Y.C.; Tian, Z.; Liu, J.L.; Ren, G.; Liang, X.H.; Yu, H.; Wan, P.; et al. Serial analysis of gene expression in mouse uterus at the implantation site. J. Biol. Chem. 2006, 281, 9351–9360. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.Y.; Wang, H.F.; Li, L.; Ma, X.; Chen, Y.; Zhou, H.; Luo, Y.; Xiao, Y.; Liu, L. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018, 32, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotech. 2019, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, S.; Vafaee, F.; Young, P.E.; Hur, S.S.J.; Hawke, S.; Devenney, E.; Beadnall, H.; Barnett, M.H.; Suter, C.M.; Buckland, M.E. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci. Rep. 2017, 7, 14293. [Google Scholar] [CrossRef]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Min, L.; Zhu, S.; Chen, L.; Liu, X.; Wei, R.; Zhao, L.; Yang, Y.; Zhang, Z.; Kong, G.; Li, P.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Nie, G.Y.; Hannan, N.J.; Dimitriadis, E. Society for Reproductive Biology Founders’ Lecture 2009 Preparing fertile soil: The importance of endometrial receptivity. Reprod. Fertil. Dev. 2009, 21, 923–934. [Google Scholar] [CrossRef]
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganò, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210. [Google Scholar] [CrossRef]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Pissios, P.; Out, H.; Roberson, R.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.F.; Kahn, B.B.; et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, E1724–E1739. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment Of Selective miRNAs In Exosomes And Delivery Of Exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Ramanathan, S.; Shenoda, B.B.; Lin, Z.; Alexander, G.M.; Huppert, A.; Sacan, A.; Ajita, S.K. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1650595. [Google Scholar] [CrossRef]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 2016, 5, e19276. [Google Scholar] [CrossRef]
- Valdes, C.T.; Schutt, A.; Simon, C. Implantation failure of endometrial origin: It is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil. Steril. 2017, 108, 15–18. [Google Scholar] [CrossRef]
- Oeyen, E.; Willems, H.; Kindt, R.’T.; Sandra, K.; Boonen, K.; Hoekx, L.; De Wachter, S.; Ameye, F.; Mertens, I. Determination of variability due to biological and technical variation in urinary extracellular vesicles as a crucial step in biomarker discovery studies. J. Extracell. Vesicles 2019, 8, 1676035. [Google Scholar] [CrossRef] [PubMed]
- Thind, A.; Wilson, C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 2016, 5, 31292. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.C.; Fan, C.M. The role of Gas1 in embryonic development and its implications for human disease. Cell Cycle 2007, 6, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; Su, Y.; Zhang, A.L.; Xu, J.W.; Fu, Q.; Yan, L. MiR-34c-5p plays a protective role in chronic obstructive pulmonary disease via targeting CCL22. Exp. Lung Res. 2019, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells 2020, 9, 645. https://doi.org/10.3390/cells9030645
Tan Q, Shi S, Liang J, Zhang X, Cao D, Wang Z. MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells. 2020; 9(3):645. https://doi.org/10.3390/cells9030645
Chicago/Turabian StyleTan, Qiang, Shuang Shi, Jingjie Liang, Xiaowei Zhang, Dingren Cao, and Zhengguang Wang. 2020. "MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy" Cells 9, no. 3: 645. https://doi.org/10.3390/cells9030645
APA StyleTan, Q., Shi, S., Liang, J., Zhang, X., Cao, D., & Wang, Z. (2020). MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells, 9(3), 645. https://doi.org/10.3390/cells9030645



