3,4-Dihydroxybenzalacetone (DBL) Prevents Aging-Induced Myocardial Changes in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Acute Oral Toxicity
2.3. Drugs and Chemicals
2.4. Experimental Design
2.5. Oxygen Radical Absorbing Capacity (ORAC)
2.6. Alkaline Single-Cell Gel Electrophoresis Method or Comet Assay—DNA Damage Assay
2.7. Histopathological Examination
2.8. Gene Expression Analysis by Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.9. Immunofluorescence
2.10. Analysis of Myocardial Apoptosis by Terminal-Transferase-Mediated Dutp Nick-End Labeling (TUNEL) Assays
2.11. Statistical Analysis
3. Results
3.1. Antioxidant Potential of the Treatment with DBL as Measured by the ORAC Assay
3.2. Treatment with DBL Protects the Cardiomyocytes from Aging-Induced DNA Damage as Measured by Comet Assay
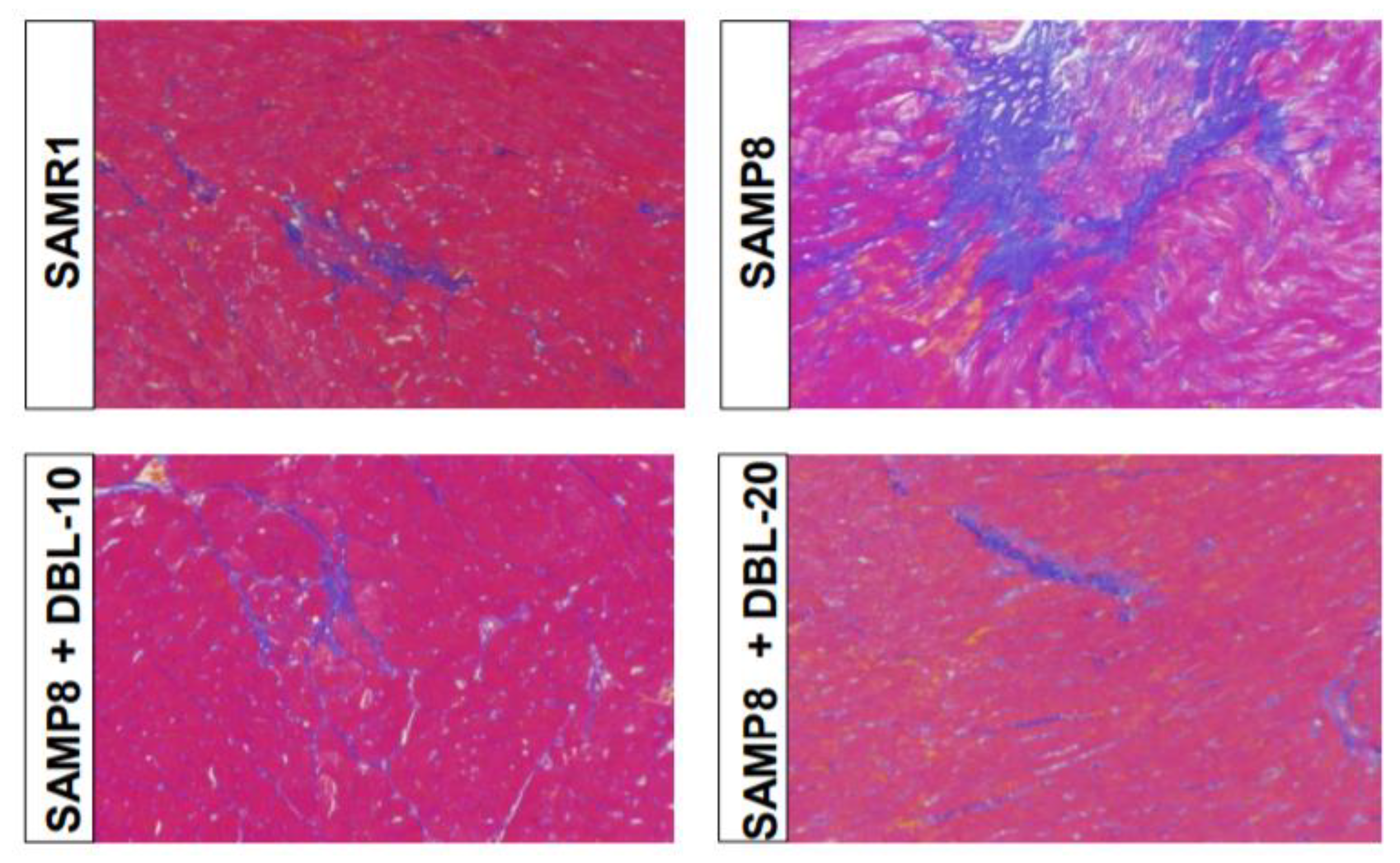
3.3. DBL Prevents Aging-Induced Fibrotic Changes in the SAMP8 Mice Hearts
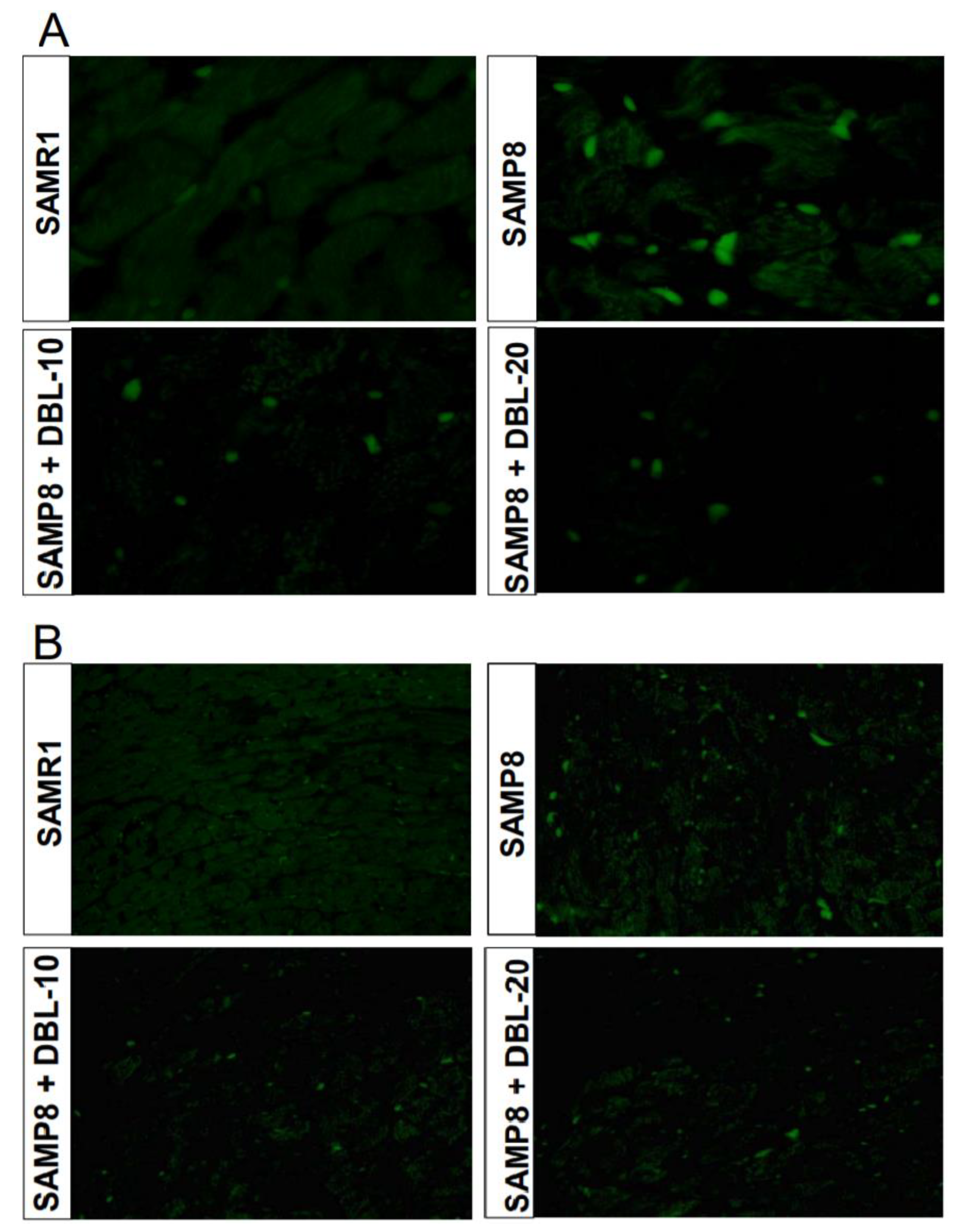
3.4. DBL Prevents Myocardial Apoptosis in the SAMP8 Mice Hearts
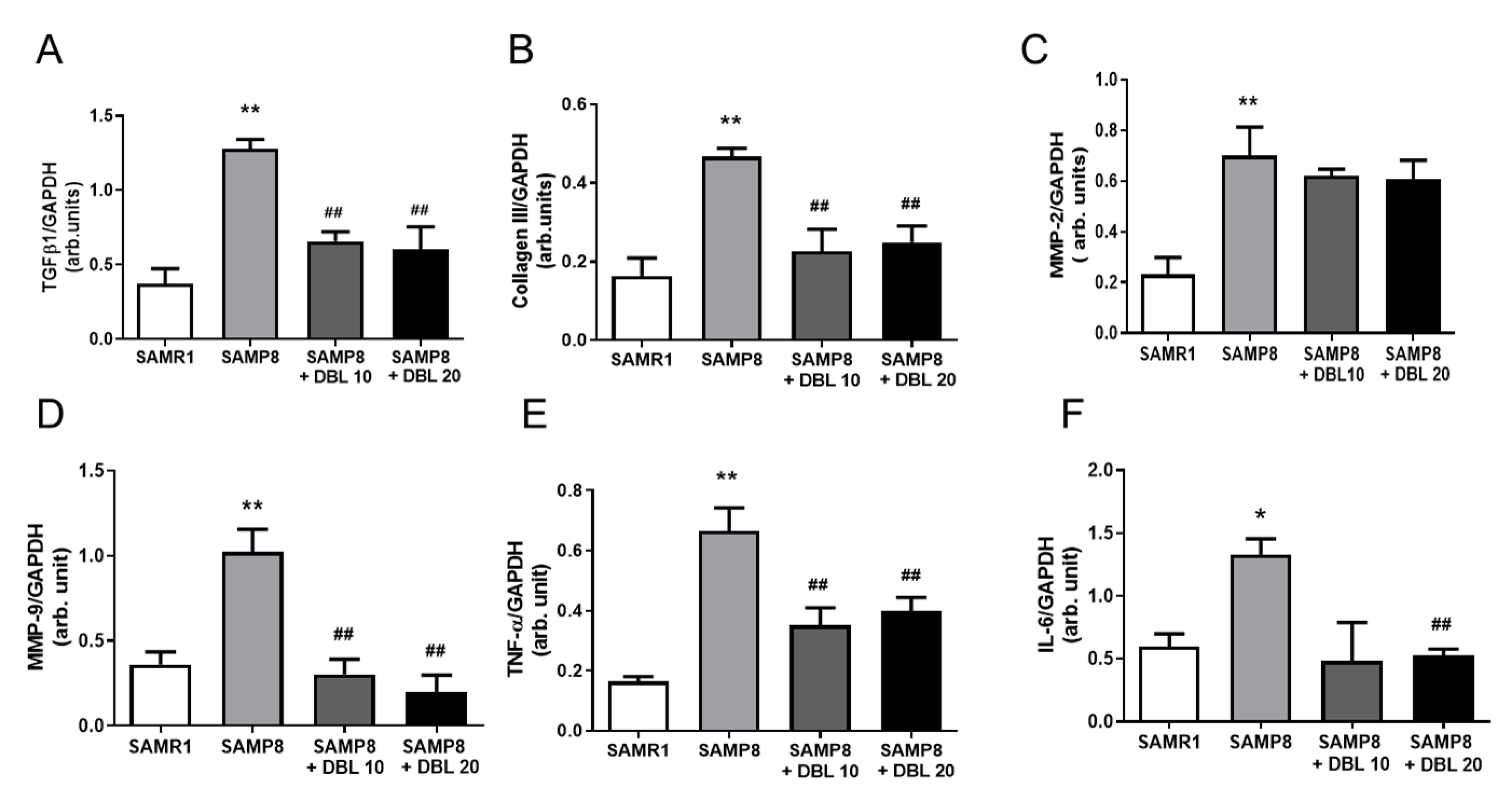
3.5. DBL Modulates Myocardial Inflammatory Cytokines and Cardiac Remodeling Gene Expression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and Autophagy in the Heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef]
- Gude, N.A.; Broughton, K.M.; Firouzi, F.; Sussman, M. Cardiac ageing: Extrinsic and intrinsic factors in cellular renewal and senescence. Nat. Rev. Cardiol. 2018, 15, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-W.; Hur, H.; Chang, K.-C.; Lee, T.-S.; Ka, K.-H.; Jankovsky, L. Introduction to Distribution and Ecology of Sterile Conks of Inonotus obliquus. Mycobiology 2008, 36, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, I.; Nakajima, Y.; Nishida, H.; Konishi, T.; Takeuchi, T.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Inhibitory effects of low molecular weight polyphenolics from Inonotus obliquus on human DNA topoisomerase activity and cancer cell proliferation. Mol. Med. Rep. 2013, 8, 535–542. [Google Scholar] [CrossRef]
- Gunjima, K.; Tomiyama, R.; Takakura, K.; Yamada, T.; Hashida, K.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-Dihydroxybenzalacetone Protects Against Parkinson’s Disease-Related Neurotoxin 6-OHDA Through Akt/Nrf2/Glutathione Pathway. J. Cell. Biochem. 2014, 115, 151–160. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyúl-Tóth, Á.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. GeroScience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Tomiyama, R.; Takakura, K.; Takatou, S.; Le, T.M.; Nishiuchi, T.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester induce preconditioning ER stress and autophagy in SH-SY5Y cells. J. Cell. Physiol. 2017, 233, 1671–1684. [Google Scholar] [CrossRef]
- Nakajima, Y.; Nishida, H.; Nakamura, Y.; Konishi, T. Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone isolated from Chaga (Inonotus obliquus (persoon) Pilat). Free. Radic. Boil. Med. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Forman, K.; Vara, E.; García, C.; Kireev, R.; Cuesta, S.; Escames, G.; Tresguerres, J.A.F. Effect of a Combined Treatment With Growth Hormone and Melatonin in the Cardiological Aging on Male SAMP8 Mice. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2011, 66, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Giridharan, V.V.; Arumugam, S.; Sreedhar, R.; Palaniyandi, S.S.; Krishnamurthy, P.; Quevedo, J.; Watanabe, K.; Konishi, T.; Thandavarayan, R.A. Modulation of Macrophage Polarization and HMGB1-TLR2/TLR4 Cascade Plays a Crucial Role for Cardiac Remodeling in Senescence-Accelerated Prone Mice. PLoS ONE 2016, 11, e0152922. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, R.; Giridharan, V.V.; Arumugam, S.; Karuppagounder, V.; Palaniyandi, S.S.; Krishnamurthy, P.; Quevedo, J.; Watanabe, K.; Konishi, T.; Thandavarayan, R.A. Role of MAPK-mediated endoplasmic reticulum stress signaling in the heart during aging in senescence-accelerated prone mice. BioFactors 2016, 42, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Babu, S.S.; Palaniyandi, S.S.; Watanabe, K.; Cooke, J.P.; Thandavarayan, R.A. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res. Rev. 2017, 35, 291–296. [Google Scholar] [CrossRef]
- OECD. Acute Oral Toxicity—Acute Toxic Class Method organization for Economic Co-operation and Development Publishing. In Test No 423; OECD: Paris, France, 2001. [Google Scholar]
- Uesugi, D.; Hamada, H.; Shimoda, K.; Kubota, N.; Ozaki, S.-I.; Nagatani, N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci. Biotechnol. Biochem. 2017, 81, 226–230. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Arumugam, S.; Mizuno, M.; Nawa, H.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T. Schisandrin B Ameliorates ICV-Infused Amyloid β Induced Oxidative Stress and Neuronal Dysfunction through Inhibiting RAGE/NF-κB/MAPK and Up-Regulating HSP/Beclin Expression. PLoS ONE 2015, 10, e0142483. [Google Scholar] [CrossRef]
- Chaubey, R.C.; Bhilwade, H.N.; Rajagopalan, R.; Bannur, S.V. Gamma ray induced DNA damage in human and mouse leucocytes measured by SCGE-Pro: A software developed for automated image analysis and data processing for Comet assay. Mutat. Res. Mol. Mech. Mutagen. 2001, 490, 187–197. [Google Scholar] [CrossRef]
- Thandavarayan, R.A.; Watanabe, K.; Sari, F.R.; Ma, M.; Lakshmanan, A.P.; Giridharan, V.V.; Gurusamy, N.; Nishida, H.; Konishi, T.; Zhang, S. Modulation of doxorubicin-induced cardiac dysfunction in dominant-negative p38α mitogen-activated protein kinase mice. Free. Radic. Boil. Med. 2010, 49, 1422–1431. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Bhilwade, H.N.; Ko, K.M.; Watanabe, K.; Konishi, T. Schisandrin B, attenuates cisplatin-induced oxidative stress, genotoxicity and neurotoxicity through modulating NF-κB pathway in mice. Free. Radic. Res. 2012, 46, 50–60. [Google Scholar] [CrossRef]
- Wold, L.E.; Aberle, N.S.; Ren, J. Doxorubicin induces cardiomyocyte dysfunction via a p38 MAP kinase-dependent oxidative stress mechanism. Cancer Detect. Prev. 2005, 29, 294–299. [Google Scholar] [CrossRef]
- Kuchařová, M.; Hronek, M.; Rybáková, K.; Zadák, Z.; Štětina, R.; Josková, V.; Patková, A. Comet assay and its use for evaluating oxidative DNA damage in some pathological states. Physiol. Res. 2019, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.F.P.; Cortinhas, A.; Bento, T.; Leitão, J.; Collins, A.; Gaivão, I.; Mota, M.P. Aging and DNA damage in humans: A meta-analysis study. Aging 2014, 6, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar] [PubMed]
- Kang, P.M.; Izumo, S. Apoptosis in heart: Basic mechanisms and implications in cardiovascular diseases. Trends Mol. Med. 2003, 9, 177–182. [Google Scholar] [CrossRef]
- Kwak, H.-B. Effects of aging and exercise training on apoptosis in the heart. J. Exerc. Rehabilitation 2013, 9, 212–219. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Disease. Available online: www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 23 May 2016).
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Butterfield, D.; Poon, H. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Forman, K.; Vara, E.; García, C.; Kireev, R.; Cuesta, S.; Acuña-Castroviejo, D.; Tresguerres, J. Influence of aging and growth hormone on different members of the NFkB family and IkB expression in the heart from a murine model of senescence-accelerated aging. Exp. Gerontol. 2016, 73, 114–120. [Google Scholar] [CrossRef]
- Rui, Y.; Cheng, J.; Qin, L.; Shan, C.; Chang, J.; Wang, G.; Wan, Z. Effects of vitamin D and resveratrol on metabolic associated markers in liver and adipose tissue from SAMP8 mice. Exp. Gerontol. 2017, 93, 16–28. [Google Scholar] [CrossRef]
- Puig, Á.; Rancan, L.; Paredes, S.D.; Carrasco, A.; Escames, G.; Vara, E.; Tresguerres, J.A. Melatonin decreases the expression of inflammation and apoptosis markers in the lung of a senescence-accelerated mice model. Exp. Gerontol. 2016, 75, 1–7. [Google Scholar] [CrossRef]
- Tresguerres, J.A.; Kireev, R.; Forman, K.; Cuesta, S.; Tresguerres, A.F.; Vara, E. Effect of chronic melatonin administration on several physiological parameters from old Wistar rats and SAMP8 mice. Curr. Aging Sci. 2012, 5, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative Stress in Aging-Matters of the Heart and Mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Pole, A.; Dimri, M.; Dimri, G.P. Oxidative stress, cellular senescence and ageing. AIMS Mol. Sci. 2016, 3, 300–324. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.-K.; Yun, B.-S. Phenolic compounds from the fungus Inonotus obliquus and their antioxidant properties. J. Antibiot. 2016, 69, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Reed, A.L.; Tanaka, A.; Sorescu, D.; Liu, H.; Jeong, E.M.; Sturdy, M.; Walp, E.R.; Dudley, S.C., Jr.; Sutliff, R.L. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H824–H831. [Google Scholar] [CrossRef]
- Meschiari, C.A.; Ero, O.K.; Pan, H.; Finkel, T.; Lindsey, M. The impact of aging on cardiac extracellular matrix. GeroScience 2017, 39, 7–18. [Google Scholar] [CrossRef]
- Toba, H.; Cannon-Stewart, P.; Yabluchanskiy, A.; Iyer, R.P.; D’Armiento, J.; Lindsey, M. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am. J. Physiol. Circ. Physiol. 2017, 312, H375–H383. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Chen, K.; Li, D.; Tang, B.; Peng, K.; Wang, Z.; Yang, P.; Yang, D.; Yang, Y. Dietary Menthol Attenuates Inflammation and Cardiac Remodeling After Myocardial Infarction via the Transient Receptor Potential Melastatin 8. Am. J. Hypertens. 2019. [Google Scholar] [CrossRef]
- Yadav, Y.C.; Pattnaik, S.; Swain, K. Curcumin loaded mesoporous silica nanoparticles: Assessment of bioavailability and cardioprotective effect. Drug Dev. Ind. Pharm. 2019, 45, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Consoli, C.; Gatta, L.; Iellamo, F.; Molinari, F.; Rosano, G.M.; Marlier, L.N. Severity of left ventricular dysfunction in heart failure patients affects the degree of serum-induced cardiomyocyte apoptosis. Importance of inflammatory response and metabolism. Int. J. Cardiol. 2013, 167, 2859–2866. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giridharan, V.V.; Karupppagounder, V.; Arumugam, S.; Nakamura, Y.; Guha, A.; Barichello, T.; Quevedo, J.; Watanabe, K.; Konishi, T.; Thandavarayan, R.A. 3,4-Dihydroxybenzalacetone (DBL) Prevents Aging-Induced Myocardial Changes in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice. Cells 2020, 9, 597. https://doi.org/10.3390/cells9030597
Giridharan VV, Karupppagounder V, Arumugam S, Nakamura Y, Guha A, Barichello T, Quevedo J, Watanabe K, Konishi T, Thandavarayan RA. 3,4-Dihydroxybenzalacetone (DBL) Prevents Aging-Induced Myocardial Changes in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice. Cells. 2020; 9(3):597. https://doi.org/10.3390/cells9030597
Chicago/Turabian StyleGiridharan, Vijayasree V., Vengadeshprabhu Karupppagounder, Somasundaram Arumugam, Yutaka Nakamura, Ashrith Guha, Tatiana Barichello, Joao Quevedo, Kenichi Watanabe, Tetsuya Konishi, and Rajarajan A. Thandavarayan. 2020. "3,4-Dihydroxybenzalacetone (DBL) Prevents Aging-Induced Myocardial Changes in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice" Cells 9, no. 3: 597. https://doi.org/10.3390/cells9030597
APA StyleGiridharan, V. V., Karupppagounder, V., Arumugam, S., Nakamura, Y., Guha, A., Barichello, T., Quevedo, J., Watanabe, K., Konishi, T., & Thandavarayan, R. A. (2020). 3,4-Dihydroxybenzalacetone (DBL) Prevents Aging-Induced Myocardial Changes in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice. Cells, 9(3), 597. https://doi.org/10.3390/cells9030597






