Cardioprotective Potential of Human Endothelial-Colony Forming Cells from Diabetic and Nondiabetic Donors
Abstract
1. Background
2. Methods
2.1. Isolation and Culture of ECFCs
2.2. Animal Model
2.3. Cell Delivery
2.4. Invasive Evaluation of Cardiac Function
2.5. Flow Cytometry of Nonmyocyte Cardiac Cells
2.6. Histology and Immunohistochemical Analyses
2.7. Statistical Analyses
3. Results
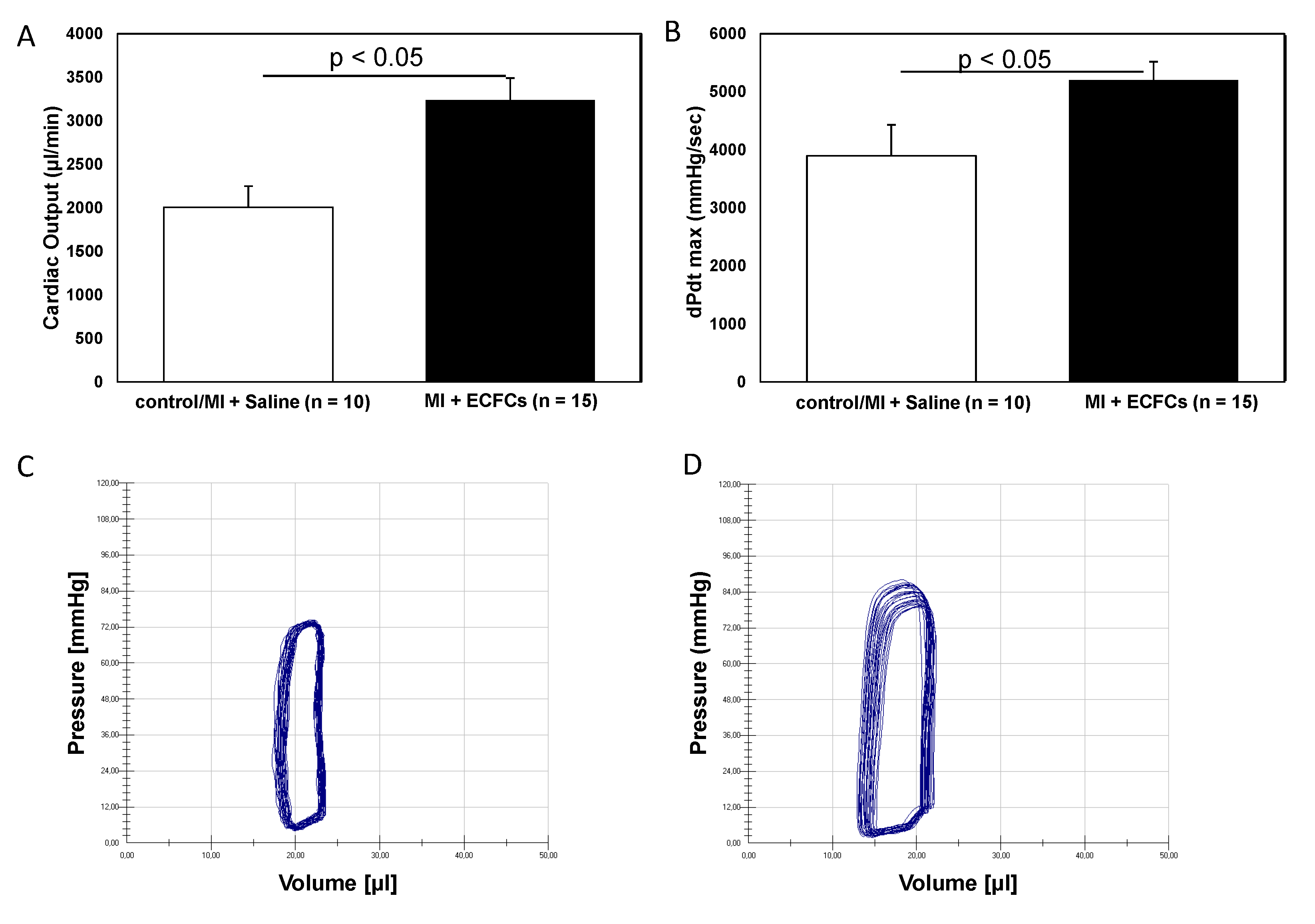
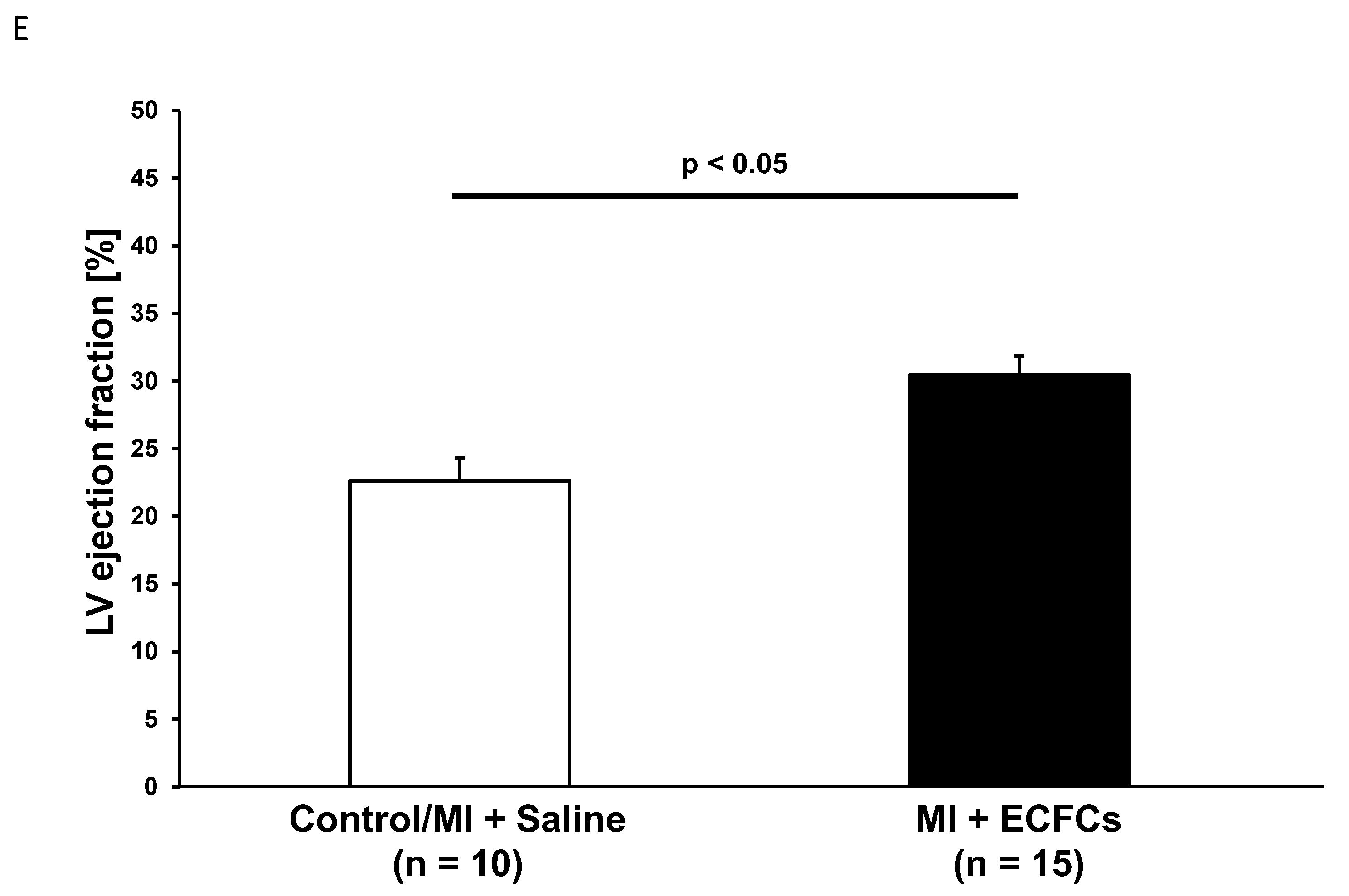
3.1. Improvement of Cardiac Function after Intramyocardial Injection of Human ECFCS into Ischemic Myocardium
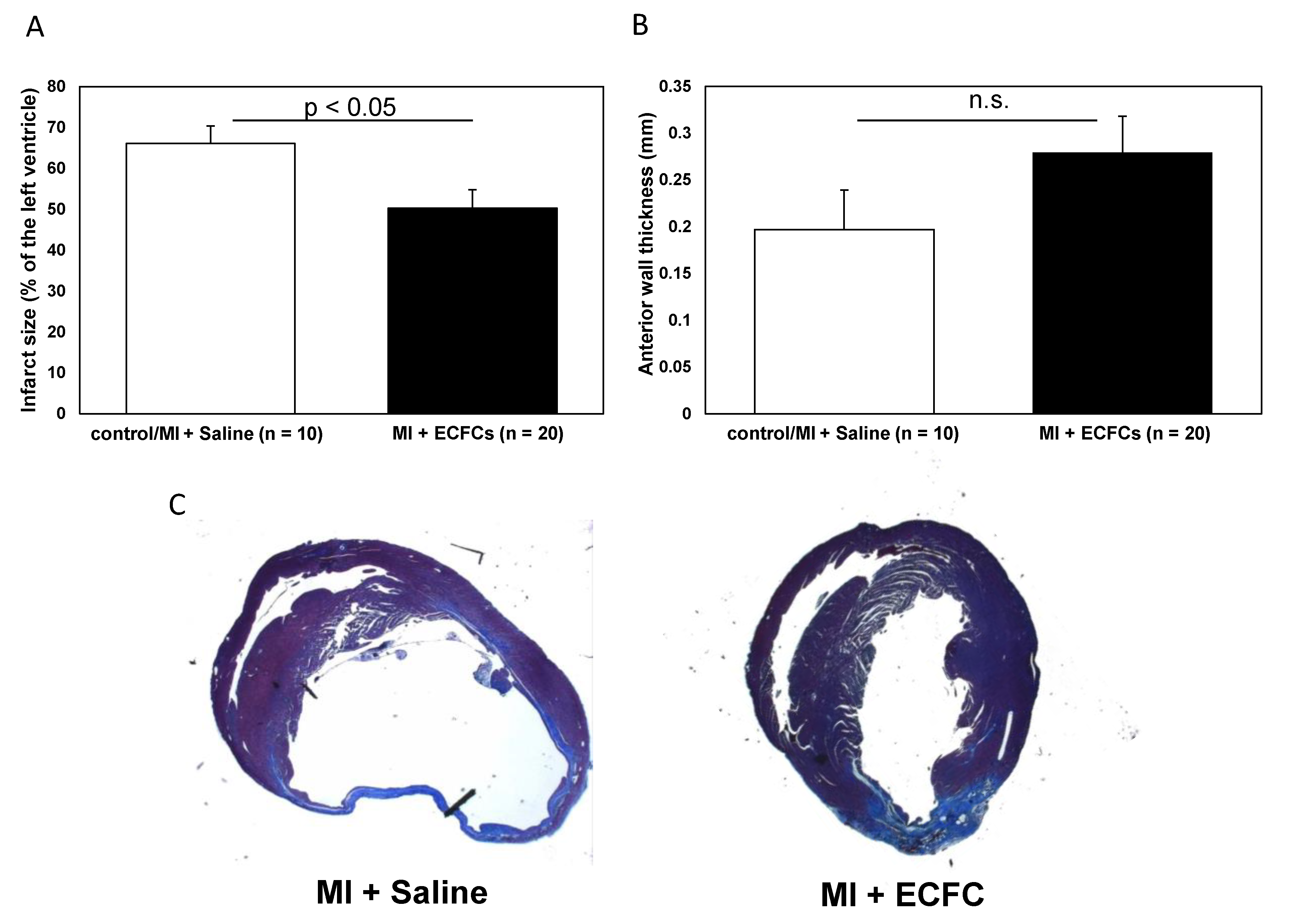
3.2. Attenuated Infarct Remodeling after Transplantation of Human ECFCS into Ischemic Myocardium
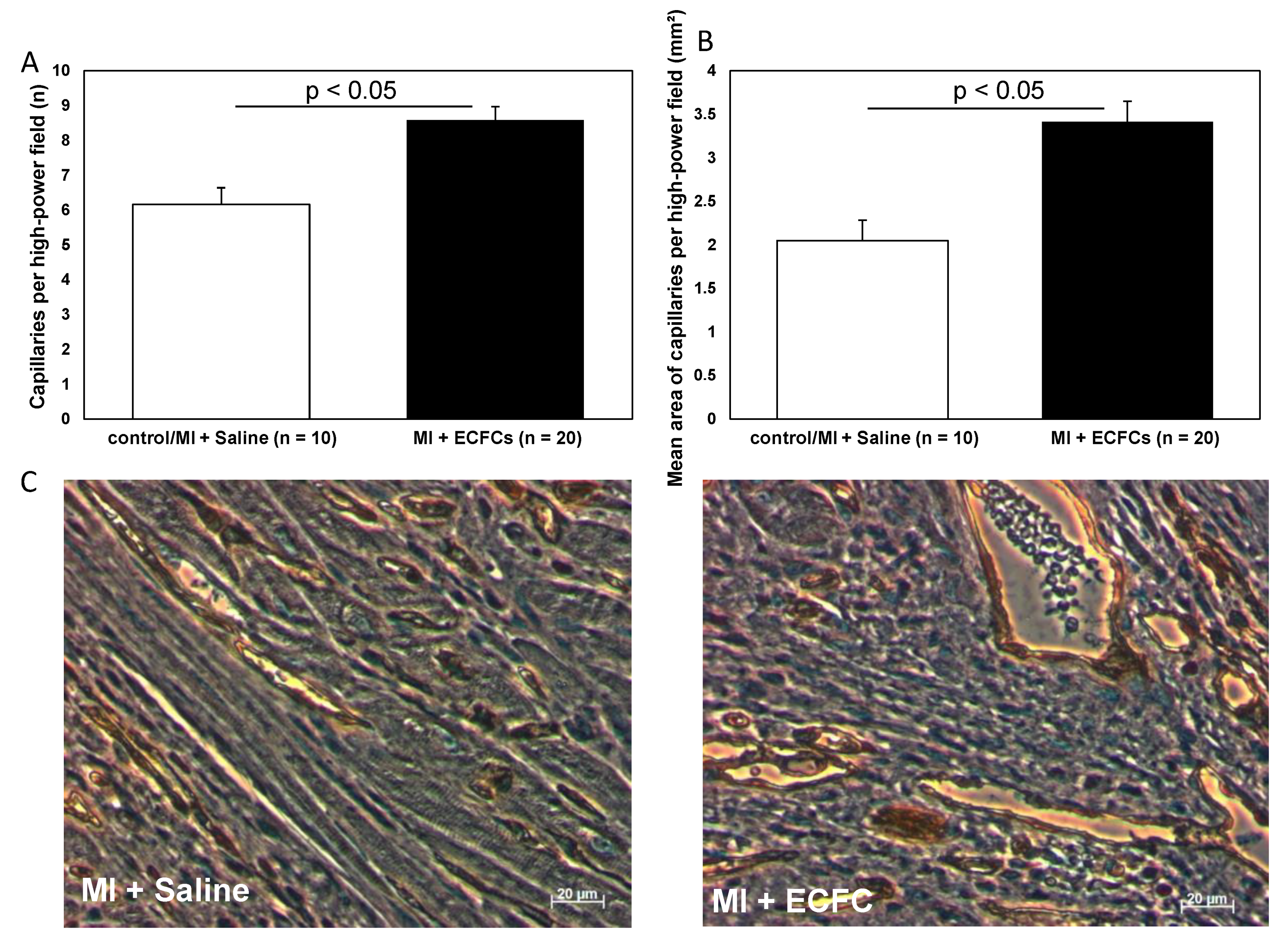
3.3. Increased Neovascularization after Intramyocardial Injection of Human ECFCS into Ischemic Myocardium
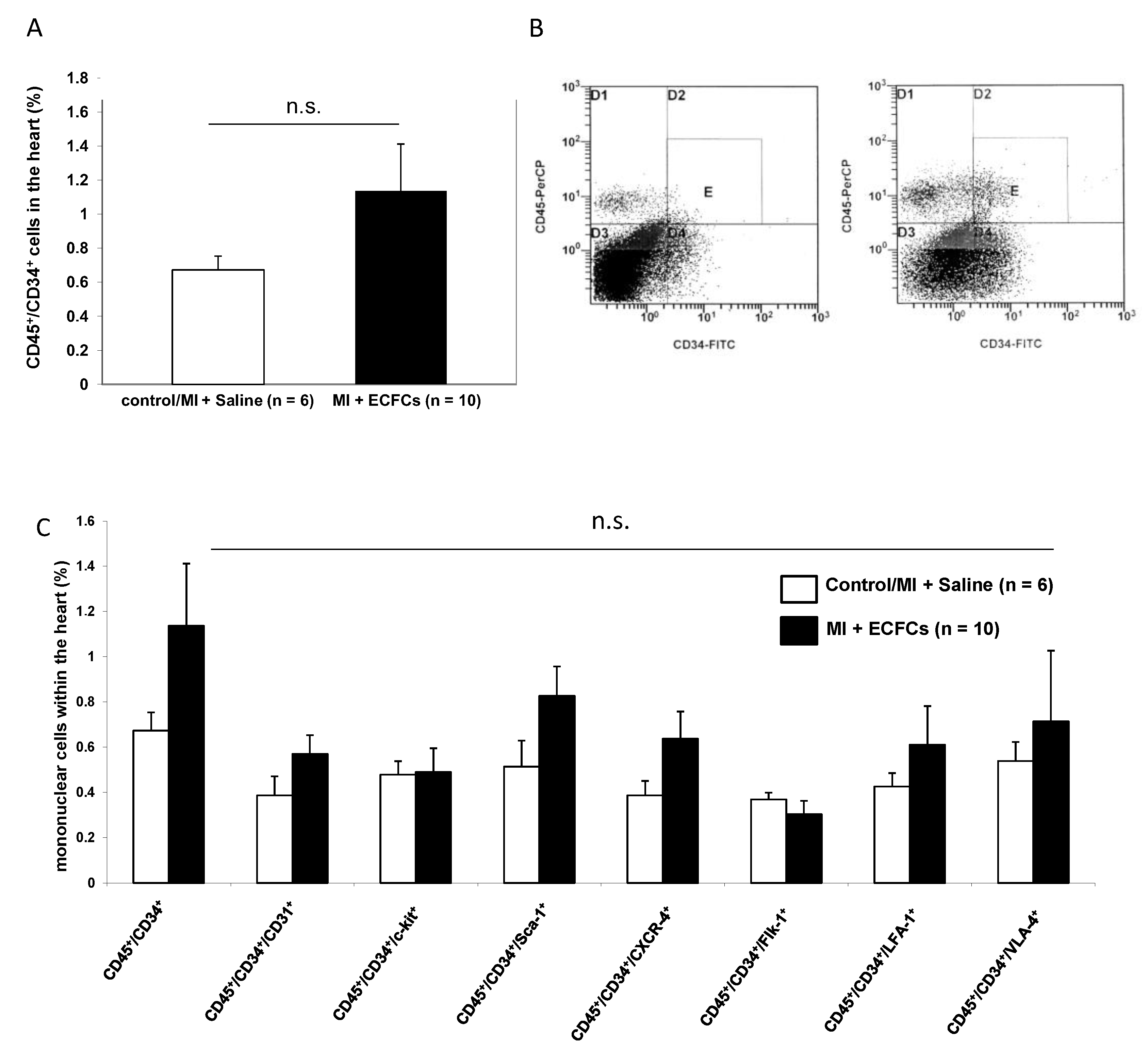
3.4. No Enhanced Homing of BM-Derived Progenitor Cells after Human ECFC Injection into Ischemic Myocardium
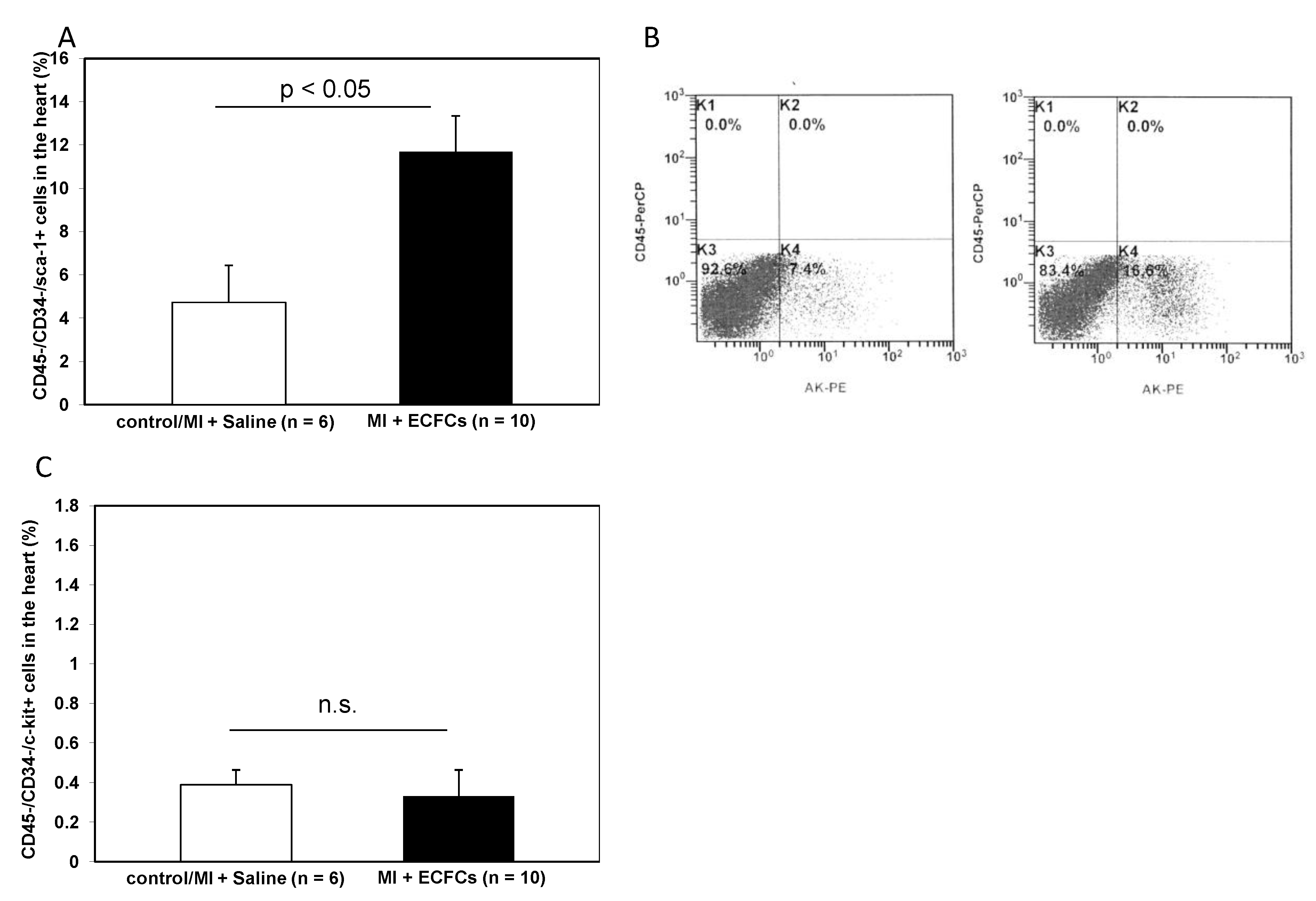
3.5. Increased Numbers of Sca-1 Positive Resident Cardiac Stem Cells after ECFCS Injection into Ischemic Myocardium
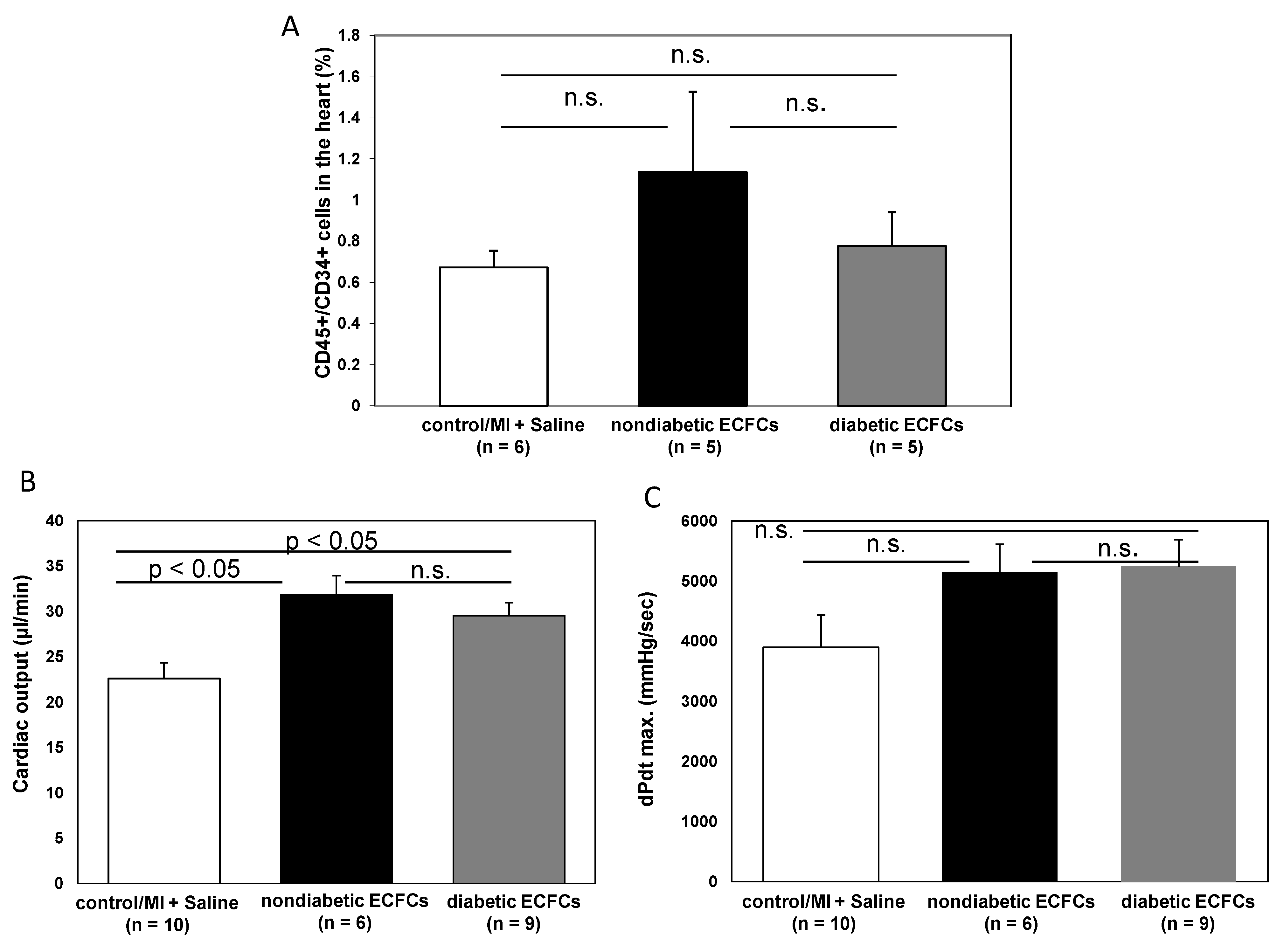
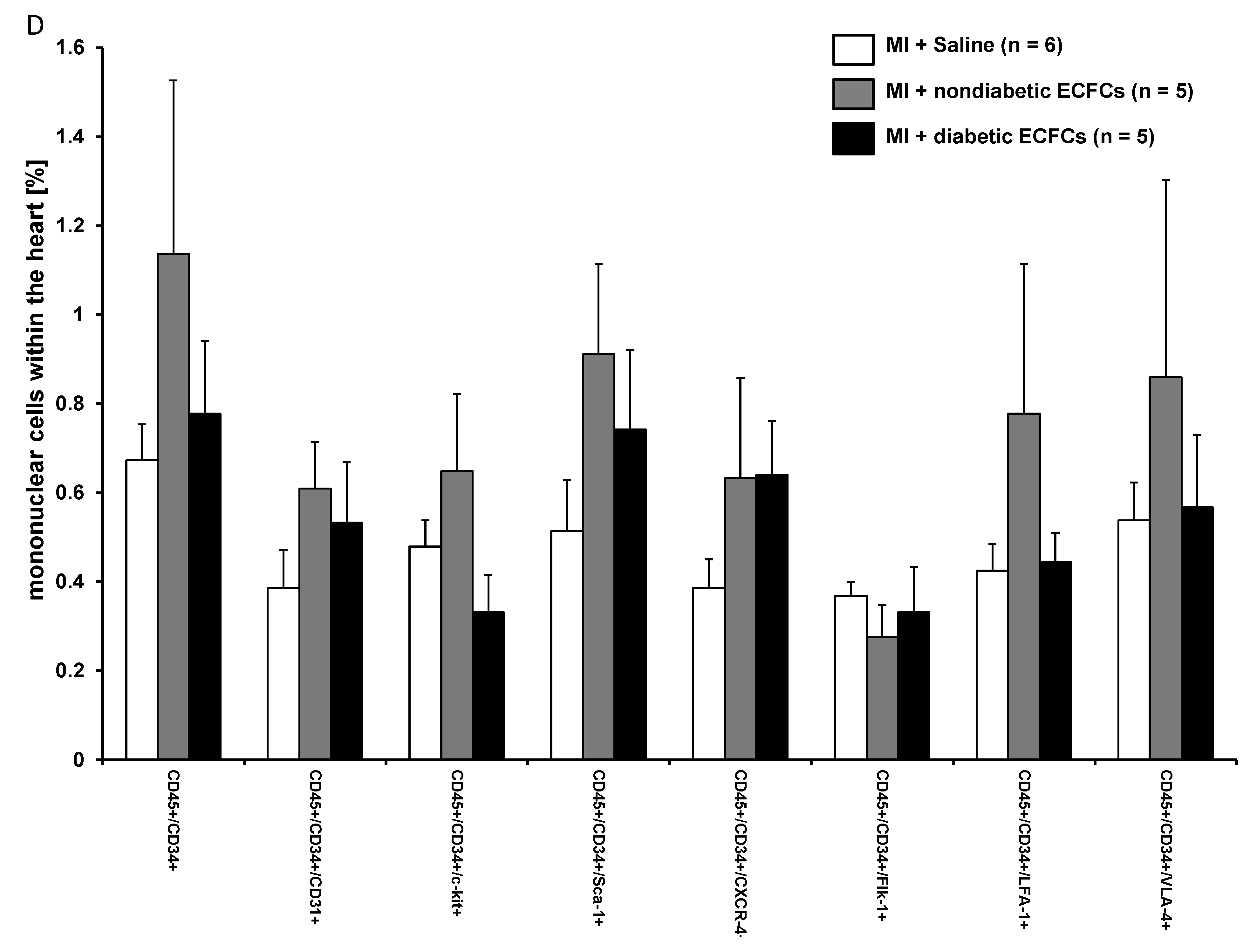
3.6. No Difference in Therapeutic Effectiveness of ECFCS Derived from Diabetic or Nondiabetic Donors after Transplantation into Ischemic Myocardium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart Disease and Stroke Statistics—2010 Update: A report from the American Hear Association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Velagaleti, R.S.; Pencina, M.J.; Murabito, J.M.; Wang, T.J.; Parikh, N.I.; D’Agostino, R.B.; Levy, D.; Kannel, W.B.; Vasan, R.S. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 2008, 118, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Zeiher, A.M.; Schneider, M.D. Unchain my heart: The scientific foundations of cardiac repair. J. Clin. Investig. 2005, 115, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Drexler, H. Cell therapy for the treatment of coronary heart disease: A critical appraisal. Nat. Rev. Cardiol. 2010, 7, 204–215. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Haller, P.M.; Blake, D.J.; Martin-Rendon, E. Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Huber, B.C.; Narsinh, K.H.; Spin, J.M.; Van Der Bogt, K.; De Almeida, P.E.; Ransohoff, K.J.; Kraft, D.L.; Fajardo, G.; Ardigo, D.; et al. In Vivo Functional and Transcriptional Profiling of Bone Marrow Stem Cells after Transplantation into Ischemic Myocardium. Arterioscler. Thromb. Vasc. Boil. 2012, 32, 92–102. [Google Scholar] [CrossRef]
- Fadini, G.P.; Losordo, U.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Ingram, D.A.; Yoder, M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Boil. 2008, 28, 1584–1595. [Google Scholar] [CrossRef]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- O’Neill, C.L.; McLoughlin, K.J.; Chambers, S.E.J.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. The Vasoreparative Potential of Endothelial Colony Forming Cells: A Journey Through Pre-clinical Studies. Front. Med. 2018, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Moubarik, C.; Guillet, B.; Youssef, B.; Codaccioni, J.-L.; Piercecchi, M.-D.; Sabatier, F.; Lionel, P.; Dou, L.; Foucault-Bertaud, A.; Velly, L.; et al. Transplanted Late Outgrowth Endothelial Progenitor Cells as Cell Therapy Product for Stroke. Stem Cell Rev. Rep. 2011, 7, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Keller, U.; Knoedler, M.; Götze, K.S.; Doss, K.; Fischer, P.; Urlbauer, K.; Debus, G.; Von Bubnoff, N.; Rudelius, M.; et al. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005, 19, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Gehling, U.M.; Ergun, S.; Schumacher, U.; Wagener, C.; Pantel, K.; Otte, M.; Schuch, G.; Schafhausen, P.; Mende, T.; Kilic, N.; et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000, 95, 3106–3112. [Google Scholar] [CrossRef]
- Deindl, E.; Zaruba, M.-M.; Brunner, S.; Huber, B.; Mehl, U.; Assmann, G.; E Hoefer, I.; Mueller-Hoecker, J.; Franz, W.-M. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006, 20, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.C.; Fischer, R.; Brunner, S.; Groebner, M.; Rischpler, C.; Segeth, A.; Zaruba, M.M.; Wollenweber, T.; Hacker, M.; Franz, W.-M. Comparison of parathyroid hormone and G-CSF treatment after myocardial infarction on perfusion and stem cell homing. Am. J. Physiol. Circ. Physiol. 2010, 298, H1466–H1471. [Google Scholar] [CrossRef]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef]
- Lips, D.J.; Nagel, T.V.D.; Steendijk, P.; Palmen, M.; Janssen, B.J.; Dantzig, J.-M.V.; Windt, L.; Doevendans, P. Left ventricular pressure?volume measurements in mice: Comparison of closed-chest versus open-chest approach. Basic Res. Cardiol. 2004, 99, 351–359. [Google Scholar] [CrossRef]
- Huber, B.C.; Brunner, S.; Segeth, A.; Nathan, P.; Fischer, R.; Zaruba, M.M.; Vallaster, M.; Theiss, H.D.; David, R.; Gerbitz, A.; et al. Parathyroid hormone is a DPP-IV inhibitor and increases SDF-1-driven homing of CXCR4+ stem cells into the ischaemic heart. Cardiovasc. Res. 2011, 90, 529–537. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E. Ventricular enlargement following infarction is a modifiable process. Am. J. Cardiol. 1991, 68, 127–131. [Google Scholar] [CrossRef]
- Noseda, M.; Paiva, M.S.A.; Schneider, M. The Quest for the Adult Cardiac Stem Cell. Circ. J. 2015, 79, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Anton, M.; Dumler, K.; Seidl, S.; Pelisek, J.; Saraste, A.; Welling, A.; Hofmann, F.; Oostendorp, R.A.; Gansbacher, B.; et al. Combined Reporter Gene PET and Iron Oxide MRI for Monitoring Survival and Localization of Transplanted Cells in the Rat Heart. J. Nucl. Med. 2009, 50, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Tkebuchava, T.; Yamaguchi, J.-I.; Nishimura, H.; Yoon, Y.; Milliken, C.; Uchida, S.; Masuo, O.; Iwaguro, H.; Ma, H.; et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003, 107, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell. Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef]
- Cho, H.-J.; Lee, N.; Lee, J.Y.; Choi, Y.J.; Ii, M.; Wecker, A.; Jeong, J.-O.; Curry, C.; Qin, G.; Yoon, Y. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007, 204, 3257–3269. [Google Scholar] [CrossRef]
- Yang, Y.; Min, J.-Y.; Rana, J.S.; Ke, Q.; Cai, J.; Chen, Y.; Morgan, J.P.; Xiao, Y.-F. VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC-differentiated cells. J. Appl. Physiol. 2002, 93, 1140–1151. [Google Scholar] [CrossRef]
- Dubois, C.; Liu, X.; Claus, P.; Marsboom, G.; Pokreisz, P.; Vandenwijngaert, S.; Dépelteau, H.; Streb, W.; Chaothawee, L.; Maes, F.; et al. Differential Effects of Progenitor Cell Populations on Left Ventricular Remodeling and Myocardial Neovascularization After Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2232–2243. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jin, H.L.; Kang, S.-M.; Kim, S.; Yoo, K.-J.; Jang, Y.; Kim, H.O.; Yoon, Y. Therapeutic effects of late outgrowth endothelial progenitor cells or mesenchymal stem cells derived from human umbilical cord blood on infarct repair. Int. J. Cardiol. 2016, 203, 498–507. [Google Scholar] [CrossRef][Green Version]
- Soonpaa, M.H.; Lafontant, P.J.; Reuter, S.; Scherschel, J.A.; Srour, E.F.; Zaruba, M.-M.; Der Lohe, M.R.-V.; Field, L.J. Absence of Cardiomyocyte Differentiation Following Transplantation of Adult Cardiac-Resident Sca-1 + Cells Into Infarcted Mouse Hearts. Circulation 2018, 138, 2963–2966. [Google Scholar] [CrossRef]
- Neidig, L.E.; Weinberger, F.; Palpant, N.J.; Mignone, J.; Martinson, A.M.; Sorensen, D.W.; Bender, I.; Nemoto, N.; Reinecke, H.; Pabon, L.; et al. Evidence for Minimal Cardiogenic Potential of Stem Cell Antigen 1–Positive Cells in the Adult Mouse Heart. Circulation 2018, 138, 2960–2962. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, Y.; Huang, X.; He, L.; Zhang, L.; Wang, H.; Yu, W.; Pu, W.; Tian, X.; Nie, Y.; et al. Fate Mapping of Sca1+ Cardiac Progenitor Cells in the Adult Mouse Heart. Circulation 2018, 138, 2967–2969. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, R.J.; Sargent, M.A.; Lin, S.-C.J.; Palpant, N.J.; Murry, C.E.; Molkentin, J.D. Genetic Lineage Tracing of Sca-1+ Cells Reveals Endothelial but Not Myogenic Contribution to the Murine Heart. Circulation 2018, 138, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Qiu, L.; Li, G.; Li, C.; Zheng, C.; Meng, H.; Yang, W. Transplantation of healthy but not diabetic outgrowth endothelial cells could rescue ischemic myocardium in diabetic rabbits. Scand. J. Clin. Lab. Investig. 2010, 70, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Vaynrub, M.; Masuda, H.; Ito, R.; Kobori, M.; Miyasaka, M.; Mizuno, H.; Warren, S.M.; Asahara, T. Quality-Control Culture System Restores Diabetic Endothelial Progenitor Cell Vasculogenesis and Accelerates Wound Closure. Diabetes 2013, 62, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Langford-Smith, A.W.W.; Hasan, A.; Weston, R.; Edwards, N.; Jones, A.M.; Boulton, A.J.M.; Bowling, F.L.; Rashid, S.T.; Wilkinson, F.L.; Alexander, M.Y. Diabetic endothelial colony forming cells have the potential for restoration with glycomimetics. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Huber, B.C.; Ransohoff, J.D.; Ransohoff, K.J.; Riegler, J.; Ebert, A.; Kodo, K.; Gong, Y.; Sanchez-Freire, V.; Dey, D.; Kooreman, N.G.; et al. Costimulation-adhesion blockade is superior to cyclosporine A and prednisone immunosuppressive therapy for preventing rejection of differentiated human embryonic stem cells following transplantation. Stem Cells. 2013, 31, 2354–2363. [Google Scholar] [CrossRef]
- Kooreman, N.G.; Ransohoff, J.D.; Wu, J.C. Tracking gene and cell fate for therapeutic gain. Nat. Mater. 2014, 13, 106–109. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deutsch, M.-A.; Brunner, S.; Grabmaier, U.; David, R.; Ott, I.; Huber, B.C. Cardioprotective Potential of Human Endothelial-Colony Forming Cells from Diabetic and Nondiabetic Donors. Cells 2020, 9, 588. https://doi.org/10.3390/cells9030588
Deutsch M-A, Brunner S, Grabmaier U, David R, Ott I, Huber BC. Cardioprotective Potential of Human Endothelial-Colony Forming Cells from Diabetic and Nondiabetic Donors. Cells. 2020; 9(3):588. https://doi.org/10.3390/cells9030588
Chicago/Turabian StyleDeutsch, Marcus-André, Stefan Brunner, Ulrich Grabmaier, Robert David, Ilka Ott, and Bruno C. Huber. 2020. "Cardioprotective Potential of Human Endothelial-Colony Forming Cells from Diabetic and Nondiabetic Donors" Cells 9, no. 3: 588. https://doi.org/10.3390/cells9030588
APA StyleDeutsch, M.-A., Brunner, S., Grabmaier, U., David, R., Ott, I., & Huber, B. C. (2020). Cardioprotective Potential of Human Endothelial-Colony Forming Cells from Diabetic and Nondiabetic Donors. Cells, 9(3), 588. https://doi.org/10.3390/cells9030588



